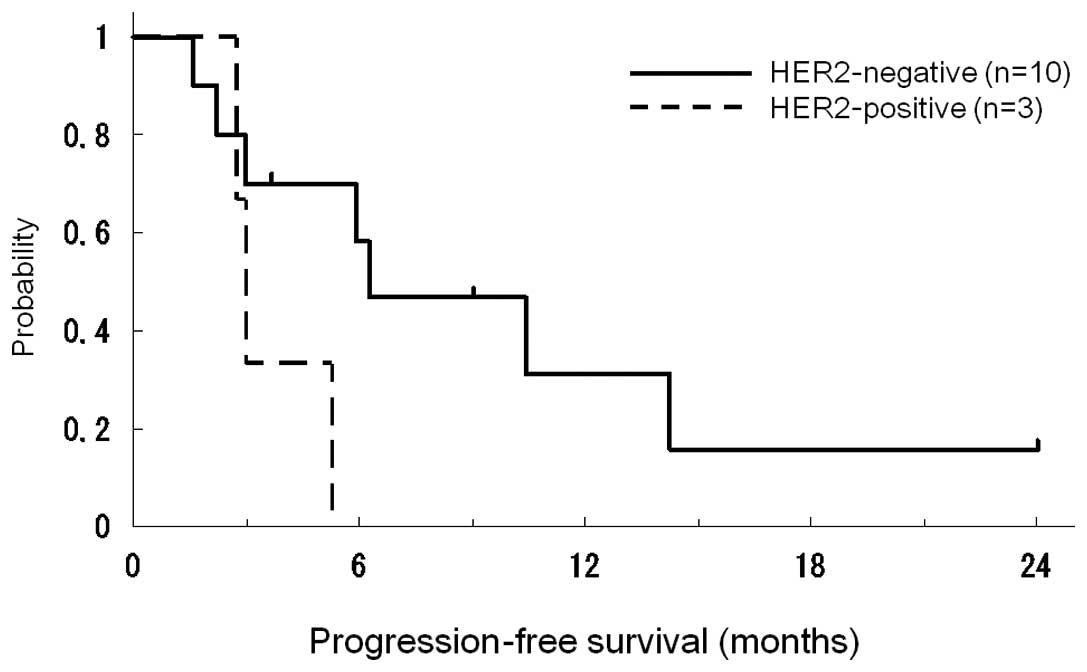
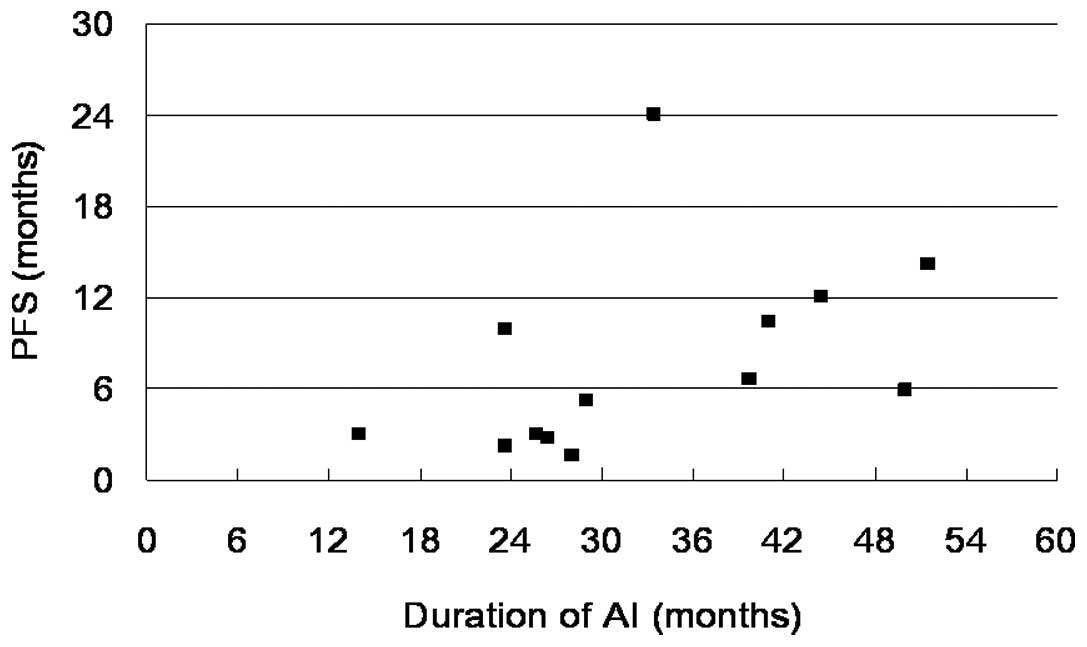
|
1
|
Baum M, Budzar AU, Cuzick J, Forbes J,
Houghton JH, Klijn JG and Sahmoud T: Anastrozole alone or in
combination with tamoxifen versus tamoxifen alone for adjuvant
treatment of postmenopausal women with early breast cancer: first
results of the ATAC randomised trial. Lancet. 359:2131–2139. 2002.
View Article : Google Scholar
|
|
2
|
Howell A, Cuzick J, Baum M, Buzdar A,
Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY and
Tobias JS: Results of the ATAC (Arimidex, Tamoxifen, Alone or in
Combination) trial after completion of 5 years’ adjuvant treatment
for breast cancer. Lancet. 365:60–62. 2005.
|
|
3
|
Thurlimann B, Keshaviah A, Coates AS,
Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch
M, Gelber RD, Rabaglio M, et al: A comparison of letrozole and
tamoxifen in postmenopausal women with early breast cancer. N Engl
J Med. 353:2747–2757. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winer EP, Hudis C, Burstein HJ, Wolff AC,
Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J,
et al: American Society of Clinical Oncology technology assessment
on the use of aromatase inhibitors as adjuvant therapy for
postmenopausal women with hormone receptor-positive breast cancer:
status report 2004. J Clin Oncol. 23:619–629. 2005. View Article : Google Scholar
|
|
5
|
Thurlimann B, Robertson JF, Nabholtz JM,
Buzdar A and Bonneterre J: Efficacy of tamoxifen following
anastrozole (‘Arimidex’) compared with anastrozole following
tamoxifen as first-line treatment for advanced breast cancer in
postmenopausal women. Eur J Cancer. 39:2310–2317. 2003.
|
|
6
|
Lonning PE, Bajetta E, Murray R,
Tubiana-Hulin M, Eisenberg PD, Mickiewicz E, Celio L, Pitt P, Mita
M, Aaronson NK, et al: Activity of exemestane in metastatic breast
cancer after failure of nonsteroidal aromatase inhibitors: a phase
II trial. J Clin Oncol. 18:2234–2244. 2000.PubMed/NCBI
|
|
7
|
Ingle JN, Suman VJ, Rowland KM,
Mirchandani D, Bernath AM, Camoriano JK, Fishkin PA, Nikcevich DA
and Perez EA: Fulvestrant in women with advanced breast cancer
after progression on prior aromatase inhibitor therapy: North
Central Cancer Treatment Group Trial N0032. J Clin Oncol.
24:1052–1056. 2006. View Article : Google Scholar
|
|
8
|
Perey L, Paridaens R, Hawle H, Zaman K,
Nole F, Wildiers H, Fiche M, Dietrich D, Clement P, Koberle D, et
al: Clinical benefit of fulvestrant in postmenopausal women with
advanced breast cancer and primary or acquired resistance to
aromatase inhibitors: final results of phase II Swiss Group for
Clinical Cancer Research Trial (SAKK 21/00). Ann Oncol. 18:64–69.
2007. View Article : Google Scholar
|
|
9
|
Lonning PE: Additive endocrine therapy for
advanced breast cancer – back to the future. Acta Oncol.
48:1092–1101. 2009.
|
|
10
|
Lewis JD, Chagpar AB, Shaughnessy EA,
Nurko J, McMasters K and Edwards MJ: Excellent outcomes with
adjuvant toremifene or tamoxifen in early stage breast cancer.
Cancer. 116:2307–2315. 2010.PubMed/NCBI
|
|
11
|
Pyrhonen S, Ellmen J, Vuorinen J,
Gershanovich M, Tominaga T, Kaufmann M and Hayes DF: Meta-analysis
of trials comparing toremifene with tamoxifen and factors
predicting outcome of antiestrogen therapy in postmenopausal women
with breast cancer. Breast Cancer Res Treat. 56:133–143. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gams R: Phase III trials of toremifene vs
tamoxifen. Oncology (Williston Park). 11:23–28. 1997.PubMed/NCBI
|
|
13
|
Holli K, Valavaara R, Blanco G, Kataja V,
Hietanen P, Flander M, Pukkala E and Joensuu H: Safety and efficacy
results of a randomized trial comparing adjuvant toremifene and
tamoxifen in postmenopausal patients with node-positive breast
cancer. Finnish Breast Cancer Group. J Clin Oncol. 18:3487–3494.
2000.
|
|
14
|
Iino Y, Takai Y, Ando T, Sugamata N,
Maemura M, Takeo T, Ohwada S and Morishita Y: Effect of toremifene
on the growth, hormone receptors and insulin-like growth factor-1
of hormone-dependent MCF-7 tumors in athymic mice. Cancer Chemother
Pharmacol. 32:353–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruohola JK, Valve EM, Karkkainen MJ,
Joukov V, Alitalo K and Harkonen PL: Vascular endothelial growth
factors are differentially regulated by steroid hormones and
antiestrogens in breast cancer cells. Mol Cell Endocrinol.
149:29–40. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stenbygaard LE, Herrstedt J, Thomsen JF,
Svendsen KR, Engelholm SA and Dombernowsky P: Toremifene and
tamoxifen in advanced breast cancer – a double-blind cross-over
trial. Breast Cancer Res Treat. 25:57–63. 1993.
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
18
|
Burstein HJ, Prestrud AA, Seidenfeld J,
Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis
CA, Malin J, et al: American Society of Clinical Oncology clinical
practice guideline: update on adjuvant endocrine therapy for women
with hormone receptor-positive breast cancer. J Clin Oncol.
28:3784–3796. 2010. View Article : Google Scholar
|
|
19
|
Yamamoto Y, Masuda N, Ohtake T, Yamashita
H, Saji S, Kimijima I, Kasahara Y, Ishikawa T, Sawaki M, Hozumi Y
and Iwase H: Clinical usefulness of high-dose toremifene in
patients relapsed on treatment with an aromatase inhibitor. Breast
Cancer. 17:254–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jonsson PE, Malmberg M, Bergljung L,
Ingvar C, Ericsson M, Ryden S, Nilsson I and Terje IJ: Phase II
study of high dose toremifene in advanced breast cancer progressing
during tamoxifene treatment. Anticancer Res. 11:873–875.
1991.PubMed/NCBI
|
|
21
|
Vogel CL, Shemano I, Schoenfelder J, Gams
RA and Green MR: Multicenter phase II efficacy trial of toremifene
in tamoxifen-refractory patients with advanced breast cancer. J
Clin Oncol. 11:345–350. 1993.PubMed/NCBI
|
|
22
|
Pyrhonen S, Valavaara R, Vuorinen J and
Hajba A: High dose toremifene in advanced breast cancer resistant
to or relapsed during tamoxifen treatment. Breast Cancer Res Treat.
29:223–228. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaufman B, Mackey JR, Clemens MR, Bapsy
PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova
A, et al: Trastuzumab plus anastrozole versus anastrozole alone for
the treatment of postmenopausal women with human epidermal growth
factor receptor 2-positive, hormone receptor-positive metastatic
breast cancer: results from the randomized phase III TAnDEM study.
J Clin Oncol. 27:5529–5537. 2009. View Article : Google Scholar
|
|
24
|
Kato S, Endoh H, Masuhiro Y, Kitamoto T,
Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H,
et al: Activation of the estrogen receptor through phosphorylation
by mitogen-activated protein kinase. Science. 270:1491–1494. 1995.
View Article : Google Scholar : PubMed/NCBI
|