1. Introduction
Ovarian cancer is the most lethal gynecological
malignancy. Efforts at early detection and new therapeutic
approaches to reduce mortality have largely been unsuccessful,
since the origin and pathogenesis of epithelial ovarian cancer are
poorly understood (1). Transitional
cell carcinoma (TCC), a recently recognized subtype, resembles
urothelium rather than ovarian surface epithelium (methothelium)
(2,3). A small percentage of ovarian cancer
types are accounted for by TCC, which has proven to be a distinct
group with various histological and immunohistological patterns.
Patients with TCC had better prognoses compared to patients with
all other types of ovarian carcinomas following standardized
chemotherapy (4). The aim of this
review was to describe our typical cases of primary TCC, and to
review the medical literature for information on TCC management in
order to determine appropriate diagnostic methods and therapy.
2. General considerations
TCC of the ovary is a recently recognized subtype of
ovarian surface epithelial cancer. TCC has been described as a
primary ovarian carcinoma in which definite urothelial features are
present, but no benign, metaplastic and/or proliferating Brenner
tumor can be identified. TCC of the ovary was initally defined by
Austin and Norris (5). These
investigators reported a group of patients who had ovarian tumors
presenting with histologic features similar to those observed in a
malignant Brenner tumor, but the tumors lacked the associated
benign Brenner tumor component. Pure TCC was thus distinguished
from malignant Brenner tumor. In addition to not having a benign
Brenner tumor component, TCC lacks the prominent stromal
calcification (5–7). Since TCC of the ovary has close
morphological similarities to TCC of the bladder and it behaves
more aggressively than malignant Brenner tumors, Austin and Norris
(5) concluded that ovarian TCC
arises directly from the pluripotent surface epithelium of the
ovary and from cells with urothelial potential, rather than from a
benign or proliferative Brenner tumor precursor.
3. Incidence
The true incidence of TCC of the ovary remains
unknown. Transitional cell tumors, including TCC, and benign and
malignant Brenner tumors of the ovary represent ~2% of all ovarian
tumors. Moreover, according to the World Heath Organization (WHO),
depending on the histological pattern, these tumors are classified
as benign, borderline or malignant Brenner tumors and TCC (8). Silva et al (9) observed the focal or diffuse TCC
pattern in 88 of 934 ovarian cancers (9%).
4. Diagnosis
The common presenting symptoms of TCC of the ovary
are abdominal pain, abdominal swelling or distension and weight
loss. Occasionally, the patient may present with uterine bleeding,
back pain, bowel or urinary symptoms, as shown in our cases
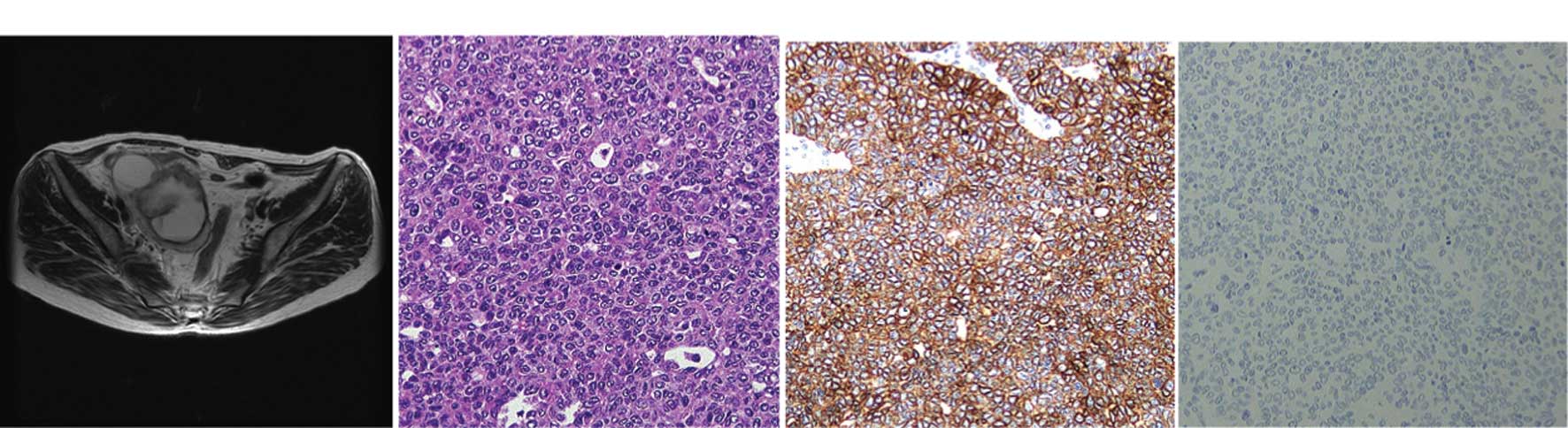
(Figs. 1 and 2). However, the clinical presentation is
indistinguishable from other types of ovarian carcinoma (5,10). As
described in detail by Eichhorn and Young (10), ovarian TCC typically shows
undulating, diffuse, insular and trabecular growth patterns. The
tumor cell nuclei were oblong or round, often exhibiting nucleoli
with longitudinal grooves. The cytoplasm was often pale and
granular, and was rarely clear or eosinophilic (Figs. 1 and 2).
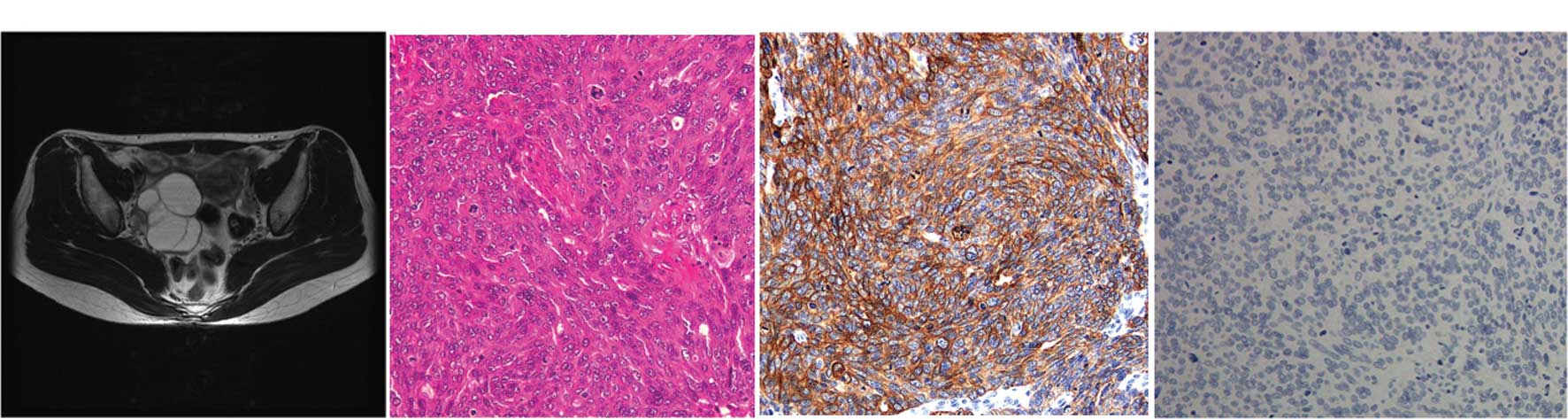 | Figure 2Primary TCC of the ovary (case 2). A
44-year-old woman with a right ovarian cyst was referred to the
gynecology department. (A) The MRI (T2-weighted, horizontal)
revealed a 5×6 cm multiple cystic mass in the right adenexa,
suggestive of an ovarian tumor. There was no obvious metastatic
foci in other organs. The levels of serum CA72-4 and CA125 were 7.7
U/ml (normal, <10.0 U/ml) and 12.9 U/ml (normal, <10 U/ml),
respectively. (B) The right ovarian cyst was laparoscopically
resected and diagnosed as TCC, grade 3 with serosal involvement.
(C) Immunohistochemical stains for CK7 were positive; (D) but
staining for CK20 was negative. The ascites cytology was reported
to be positive. The patient was staged as FIGO stage Ic. One month
following adenectomy, the patient was submitted for exploratory
staging procedures including total abdominal hysterectomy,
bilateral salpingo-oophorectomy, infracolic omentectomy and pelvic
lymph node dissection. The extensively sampled adnexal tissue was
histologically uninvolved by the tumor. After recovering from
surgery, the patient received six cycles of chemotherapy with
paclitaxel-carboplatin. The patient is presently doing well without
any recurrent disease for 2 years. |
CA125 is clinically useful as a serum marker of
tumor progression and recurrence, although early stages may be
CA125-negative (Fig. 2). In the
study by Ceauşu et al (11),
13 archived formalin-fixed paraffin-embedded samples of
transitional cell tumors of the ovary were assessed using standard
hematoxylin-eosin staining and the indirect tristadial ABC
peroxidase immunohistochemistry method for 11 antibodies including
CA125, cytokeratin (CK) 7 and CEA. Over 50% of the samples were
malignant Brenner tumors, CA125 was positive in all malignant
tumors (of Brenner type and TCCs), but not in the benign and
borderline tumors, while CK7 was positive in approximately 70% of
all cases. The two antibodies have shown a high sensitivity and low
specificity, but do not correlate with each other. Recent findings
(11,12) have shown that p63 is expressed in
benign and borderline Brenner tumors, but not in malignant
counterparts and TCCs of the ovary, suggesting that this antigen is
a marker for the differential diagnosis of malignant Brenner tumors
and TCCs, and may also play a role in Brenner carcinogenesis. The
aim of these studies was to detect tumors when they are still
confined to the ovaries, thereby increasing the likelihood of cure
and reducing the mortality of the disease. The modalities that are
currently in use to screen women are pelvic examination, imaging
modality and measurement of serum CA125 (1) (Figs. 1
and 2), although case 2 was
CA125-negative.
6. Treatment
Optimal surgical resectability followed by
cisplatin-based chemotherapy may contribute to the survival benefit
(4,6,7). The
estimated 5-year survival following surgery for 88 patients was
37%, whereas for patients who received chemotherapy, survival was
at 41% (6,7). Factors associated with survival for
patients who received chemotherapy were the clinical stage, the
percentage of the TCC component in the primary tumor and the
results of second-look surgery. The predominance of TCC was a
favorable prognostic factor and patients with higher clinical
stages had poorer prognoses.
7. Prognosis
The relative effects of tumor biology and treatment
strategies remain undetermined (6,7).
Gershenson et al (13,14)
concluded that advanced-stage ovarian TCC was significantly more
chemosensitive and associated with better prognosis than poorly
differentiated serous carcinoma. Kommoss et al (4) also documented that patients with TCC
had better prognoses compared to patients with all other types of
ovarian carcinomas following standardized chemotherapy. The
metastatic pathways of the tumor simulate TCC of the bladder, which
implicates a loss of the integrity of E-cadherin (5–7).
8. Metastatic TCC to the ovaries
The ovaries are common sites for intra-abdominal
metastasis (15). Approximately 6%
of ovarian cancers found at laparotomy are secondary tumors from
other sites (15,16). Metastatic TCC from the urinary
bladder, or elsewhere within the urinary system, involving the
ovary is extremely rare (16).
There have been six cases reported thus far, as described by Lee
et al (17). In all cases,
secondary ovarian tumors are unilateral. The time interval to the
appearance of ovarian metastases varied from synchronous to 4
years. In the study by Lee et al (17), all cases received surgery, with the
overall survival ranging from 3 months to 7 years. These cases
favor metastatic ovarian tumors for the following reasons: definite
histological evidence of a primary renal tumor, and deep stromal
invasion. The origin of primary lesions has prognostic significance
as TCC of the ovary has a modest response to chemotherapy (10) and metastatic TCC from the renal
pelvis results in mortality (17).
Microscopically, metastatic TCC of the ovary
resembles a primary ovarian TCC. Primary TCC accounts for 1–2% of
all ovarian tumors (10,17). TCC of the ovary is a recently
recognized subtype of ovarian surface epithelial-stromal cancer,
and studies of its morphology are rare. The presence of a component
of benign or borderline Brenner tumor confirms an ovarian primary
tumor. TCC of the ovaries has mucin pools and thick papillae with
smooth luminal borders, in contrast to the pseudo-papillae of tumor
cell necrosis that is common in metastatic TCC (10,17).
Another study has shown that the morphological similarity between
transitional cell carcinoma of the ovary and its counterpart from
the urinary bladder does not indicate any histogenic similarity,
but CK7 and CK20, together with uroplakin III and WT1 may prove
useful in distinguishing primitive TCCs of the ovary, and
metastases from invasive TCC of the bladder to the ovary, the
former being a variant morphology in the spectrum of surface
epithelial carcinomas (11,18).
9. Conclusion
Microscopic examination remains the first tool in
the diagnosis of these heterogeneous tumors and in the separation
of closely related tumors. Primary TCC of the ovary is a relatively
rare subtype of epithelial ovarian cancer. Surgical resection is
the primary therapeutic approach, and patient outcomes following
chemotherapy are better than for other types of ovarian
cancers.
References
|
1
|
Kurman R and Shih I-M: The origin and
pathogenesis of epithelial ovarian cancer: A proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cuatrecasas M, Catasus L, Palacios J and
Prat J: Transitional cell tumors of the ovary: A comparative
clinicopathologic, immunohistochemical, and molecular genetic
analysis of Brenner tumors and transitional cell carcinomas. Am J
Surg Pathol. 33:556–567. 2009. View Article : Google Scholar
|
|
3
|
Oh S, Rha S, Jung S, Lee Y, Choi B, Byun
J, Ku Y and Jung C: Transitional cell tumor of the ovary: computed
tomographic and magnetic resonance imaging features with
pathological correlation. J Comput Assist Tomogr. 33:106–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kommoss F, Kommoss S, Schmidt D, Trunk M,
Pfisterer J and du Bois A: Survival benefit for patients with
advanced-stage transitional cell carcinomas vs. other subtypes of
ovarian carcinoma after chemotherapy with platinum and paclitaxel.
Gynecol Oncol. 97:195–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Austin R and Norris H: Malignant Brenner
tumor and transitional cell carcinoma of the ovary: A comparison.
Int J Gynecol Pathol. 6:29–39. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tazi E, Lalya I, Tazi M, Ahellal Y,
M’rabti H and Errihani H: Transitional cell carcinoma of the ovary:
a rare case and review of literature. World J Surg Oncol. 8:98–101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin C, Liu F and Ho E: Transitional cell
carcinoma of the ovary. Taiwan J Obstet Gynecol. 45:268–271. 2006.
View Article : Google Scholar
|
|
8
|
World Health Organization. Classification
of tumours: Pathology and genetics of tumors of the breast and
female genital organs. IARC Press; Lyon: pp. 140–143. 2003
|
|
9
|
Silva E, Robey-Cafferty S, Smith T and
Gershenson D: Ovarian carcinomas with transitional cell carcinoma
pattern. Am J Clin Pathol. 93:457–465. 1990.PubMed/NCBI
|
|
10
|
Eichhorn J and Young R: Transitional cell
carcinoma of the ovary: a morphologic study of 100 cases with
emphasis on differential diagnosis. Am J Surg Pathol. 28:453–463.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ceauşu M, Terzea D, Georgescu A, Dobrea C,
Mihai M, Iosif C, Vasilescu F and Ardeleanu C: Transitional cell
tumors of the ovary: a compact group with a heterogeneous
histological and immunophenotypical pattern. Rom J Morphol Embryol.
49:513–516. 2008.PubMed/NCBI
|
|
12
|
Liao X, Xue W, Shen D, Ngan H, Siu M and
Cheung A: P63 expression in ovarian tumours: a marker for Brenner
tumours but not transitional cell carcinomas. Histopathology.
51:477–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gershenson D, Morris M, Burke T, Levenback
C, Kavanagh J, Fromm G, Silva E, Warner D and Wharton J: Combined
cisplatin and carboplatin chemotherapy for treatment of advanced
epithelial ovarian cancer. Gynecol Oncol. 58:349–355. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gershenson D, Silva E, Mitchell M,
Atkinson E and Wharton J: Transitional cell carcinoma of the ovary:
a matched control study of advanced-stage patients treated with
cisplatin-based chemotherapy. Am J Obstet Gynecol. 168:1178–1185.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valappil S, Toon P and Anandaram P:
Ovarian metastasis from primary renal cell carcinoma: report of a
case and review of literature. Gynecol Oncol. 94:846–849. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Skírnisdóttir I, Garmo H and Holmberg L:
Non-genital tract metastases to the ovaries presented as ovarian
tumors in Sweden 1990–2003: occurrence, origin and survival
compared to ovarian cancer. Gynecol Oncol. 105:166–171.
2007.PubMed/NCBI
|
|
17
|
Lee M, Jung Y, Kim S, Kim S and Kim Y:
Metastasis to the ovaries from transitional cell carcinoma of the
bladder and renal pelvis: a report of two cases. J Gynecol Oncol.
21:59–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Logani S, Olova E, Amin M, Folpe A, Cohen
C and Young R: Immunoprofile of ovarian tumors with putative
transitional cell (urothelial) differentiation using novel
urothelial markers: histogenetic and diagnostic implications. Am J
Surg Pathol. 27:1434–1441. 2003. View Article : Google Scholar
|