Introduction
Type IV scirrhous gastric cancer (SGC) is
characterized by diffuse infiltration and proliferation of poorly
differentiated gastric cancer cells accompanied by marked stromal
fibrosis (1). Linitis plastica
(LP), also known as leather bottle stomach, is believed to be a
typical case of SGC, usually involving the whole stomach and
characterized by a grossly thickened wall. Despite recent advances
in the diagnosis and treatment of gastric cancer, the majority of
cases of SGC are not detected at an early stage since tumor cells
migrate throughout the submucosa without severely affecting the
mucosal lining of the stomach. This causes difficulty in detecting
cancer cells by gastrointestinal series or endoscopy (2–5).
In LP, the cancer is poorly differentiated and
originates in the gastric fundic gland area as a small IIc lesion,
which shows extensive submucosal invasion without obvious
concavities or recesses and a leather bottle-like appearance with
giant folded walls. These walls are occasionally accompanied by
peritoneal dissemination, considerable lymph node metastasis and
direct invasion into the surrounding organs (6–8).
Therefore, LP is recognized as a far-advanced gastric cancer in
most cases, or as an early cancer that is accidentally detected in
that it is difficult to diagnose the disease during progression
from a small early stage lesion to leather bottle stomach at a
stage known as the prelinitis condition (9).
Only a few studies regarding the prelinitis
condition are available (5,7,9). By
contrast, numerous studies have commented on the definition
(1–4), incidence (10), significance (11), pathology (12), prognosis (10–12)
and management strategy (13–16) of
SGC pertaining solely to the early and the final phase of the
disease.
Although the prelinitis condition lacks an
established definition, it can be clinically identified as type IV
SGCs, other than LP, which do not involve the whole stomach, with
lesions localized in part of the stomach, which are sometimes
referred to as localized or partial SGC. Localized SGC is believed
to be better than LP in terms of surgical curability and
postsurgical prognosis, but a definitive report has not yet been
provided.
The present study aimed to investigate the
biological significance of localized SGC, to elucidate the
progression of SGC to LP and to determine whether localized SGC has
a prelinitis condition.
Patients and methods
Patients
A total of 509 consecutive patients with primary
advanced gastric cancer who had undergone gastrectomy at the
Department of Surgery, Center of Gastroenterology, National Kyushu
Medical Center (Japan) between January 1994 and December 2004 were
included in this study. The patients were divided into three groups
comprising 19 patients with type IV scirrhous lesions invading the
whole stomach (LP), 60 patients with type IV scirrhous lesions
localized in less than two thirds of the stomach (LSGC) and the
remaining 430 patients with all other types of gastric cancer
(OGC).
Total gastrectomy was performed for 205 patients and
distal or proximal gastrectomy for the remaining 304 patients; all
resection margins had a macroscopically normal appearance. Cases
with a macroscopic positive stump, due to obvious invasion of the
esophagus and duodenum, were excluded from this study.
SGC was diagnosed by the macroscopic appearance of
the surgical specimen and postoperative histological examination.
The study population comprised 340 males and 169 females, whose
ages ranged from 23 to 92 years, with a mean age of 66. The
patients were followed up, and only those who succumbed to gastric
cancer were regarded as having succumbed to tumor-related causes.
The follow-up interval after surgery ranged from 2 days to 10 years
and 11 months, with a mean interval of 4 years and 2 months.
Clinicopathological results were assessed according to the general
rules for clinical and pathological studies on gastric cancers
based on the Japanese classification of gastric carcinoma (17) and tumor-node-metastasis staging
criteria (18). In particular, the
curative potential of gastric resection was adopted for its
curability in the present study (17).
Definition of localized scirrhous gastric
cancer
As mentioned previously, 79 SGC patients were
divided into two groups of 19 patients with LP and 60 patients with
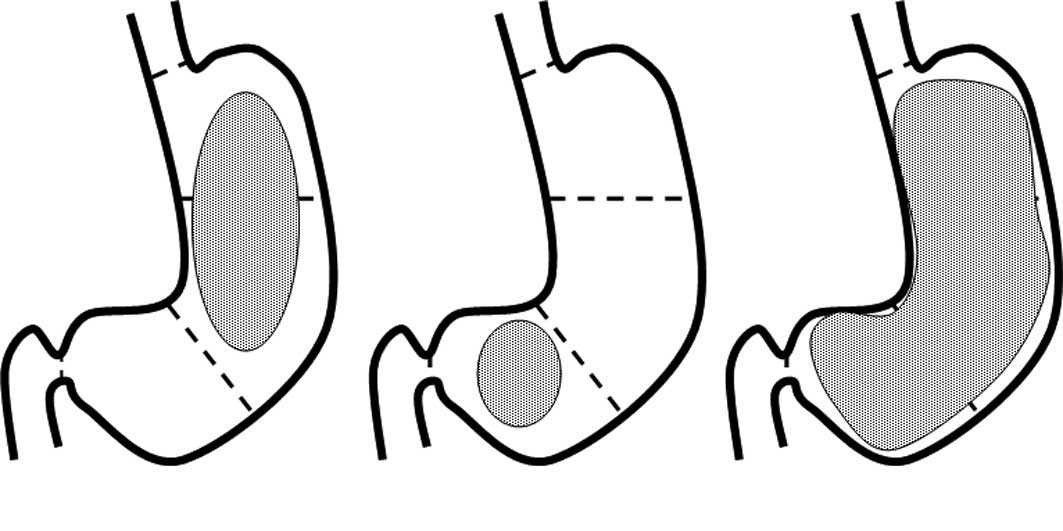
LSGC. The stomach was anatomically divided into three sections:
upper (U), middle (M) and lower (L) (Fig. 1) (17). Cases with lesions that invaded the
duodenum or esophagus and those with a macroscopically positive
stump were excluded. First, we reviewed the cases of 84 SGC
patients who underwent gastrectomy, 5 of whom were excluded,
leaving a total of 79 patients who were included in this study. Of
the 79 SGC patients, total gastrectomy was performed in 51 and
distal gastrectomy in the remaining 28 patients. The study
population consisted of 50 males and 29 females, whose ages ranged
from 29 to 87 years with a mean age of 65. The follow-up interval
after surgery ranged from 23 days to 10 years and 1 month with a
mean interval of 2 years and 5 months.
Statistical analysis
The clinicopathological characteristics of the three
groups were compared using the Chi-square test and Student’s
t-test. Cumulative survival rates were evaluated by the
Kaplan-Meier method and the survival curves were tested by the
Mantel-Cox method. Multivariate survival analysis was performed
according to Cox’s proportional hazards model in a forward stepwise
manner. P<0.05 was considered to be statistically
significant.
Results
Comparison of clinicopathological
findings among OGC, LSGC and LP
Of the 509 patients with advanced gastric cancer
included in the study, 60 (11.8%) were classified as LSGC, 19
(3.7%) as LP and the remaining 430 (84.5%) as OGC. The relationship
between LP, LSGC and the clinicopathological characteristics of the
patients are shown in Table I.
 | Table IClinicopathological characteristics of
LSGC and LP. |
Table I
Clinicopathological characteristics of
LSGC and LP.
| Variables | LSGC (n=60) | LP (n=19) | P-value |
|---|
| Gender | | | N.S. |
| Male | 39 (65%) | 11 (57.9%) | |
| Female | 21 (35%) | 8 (42.1%) | |
| Age (years) | 63.1±12.5 | 69.4±7.7 | 0.04 |
| Histology | | | N.S. |
| Tub1 | 2 (3.3%) | 0 (0%) | |
| Tub2 | 3 (5%) | 4 (21.1%) | |
| Por1 | 9 (15%) | 1 (5.2%) | |
| Por2 | 37 (61.7%) | 11 (57.9%) | |
| Signet | 9 (15%) | 3 (15.8%) | |
| Tumor depth | | | 0.006 |
| T2 | 20 (33.3%) | 2 (10.5%) | |
| T3 | 39 (65%) | 13 (68.4%) | |
| T4 | 1 (1.7%) | 4 (21.1%) | |
| Lymph node
metastasis | | | N.S. |
| Negative | 15 (25%) | 2 (10.5%) | |
| Positive | 45 (75%) | 17 (89.5%) | |
| Lymphatic
invasion | | | N.S. |
| Negative | 1 (1.7%) | 0 (0%) | |
| Positive | 59 (98.3%) | 19 (100%) | |
| Venous invasion | | | N.S. |
| Negative | 19 (31.6%) | 3 (15.8%) | |
| Positive | 41 (68.4%) | 16 (84.2%) | |
| Peritoneal
dissemination | | | 0.01 |
| Negative | 50 (83.3%) | 10 (52.6%) | |
| Positive | 10 (16.7%) | 9 (47.4%) | |
| Peritoneal
cytology | | | 0.018 |
| Negative | 47 (78.3%) | 9 (47.4%) | |
| Positive | 13 (21.7%) | 10 (52.6%) | |
| Curability | | | 0.03 |
| A, B | 40 (66.7%) | 7 (36.8%) | |
| C | 20 (23.3%) | 12 (63.2%) | |
| TNM stage | | | N.S. |
| I | 9 (15%) | 1 (5.3%) | |
| II | 9 (15%) | 1 (5.3%) | |
| III | 36 (60%) | 11 (57.8%) | |
| IV | 6 (10%) | 6 (31.6%) | |
The LP group showed lower curability (p=0.03),
higher mean age (p=0.04) and deeper invasion to the gastric wall
(p=0.006) compared with the LSGC group. Peritoneal dissemination
and positive signs of peritoneal cytology (CY) were frequent in the
LP group. No significant differences were noted in the distribution
of gender, histological differentiation, frequency of venous and
lymphatic invasion and in the incidence of lymph node metastasis.
The relationship between LSGC and OGC is shown in Table II.
 | Table IIClinicopathological characteristics of
OGC and LSGC. |
Table II
Clinicopathological characteristics of
OGC and LSGC.
| Variables | LSGC (n=60) | OGC (n=430) | P-value |
|---|
| Gender | | | N.S. |
| Male | 39 (65%) | 290 (67.4%) | |
| Female | 21 (35%) | 140 (32.6%) | |
| Age (years) | 63.1±12.5 | 66.4±11.5 | 0.04 |
| Tumor size
(mm) | 78.9±34.8 | 56.4±29.6 | <0.0001 |
| Histology | | | <0.0001 |
| Tub1, Tub2 | 5 (8.3%) | 198 (46.0%) | |
| Por1, Por2 | 46 (76.7%) | 169 (39.4%) | |
| Signet | 9 (15%) | 41 (9.5%) | |
| Others | 0 (0%) | 22 (5.1%) | |
| Tumor depth | | | <0.0001 |
| T2 | 20 (33.3%) | 258 (60.0%) | |
| T3 | 39 (65%) | 155 (36.0%) | |
| T4 | 1 (1.7%) | 17 (4.0%) | |
| Lymph node
metastasis | | | N.S. |
| Negative | 15 (25%) | 145 (33.7%) | |
| Positive | 45 (75%) | 285 (66.3%) | |
| Lymphatic
invasion | | | N.S. |
| Negative | 1 (1.7%) | 34 (7.9%) | |
| Positive | 59 (98.3%) | 396 (92.1%) | |
| Venous
invasion | | | 0.048 |
| Negative | 19 (31.6%) | 197 (45.8%) | |
| Positive | 41 (68.4%) | 233 (54.2%) | |
| Peritoneal
dissemination | | | 0.0006 |
| Negative | 50 (83.3%) | 413 (96.0%) | |
| Positive | 10 (16.7%) | 17 (4.0%) | |
| Liver
metastasis | | | N.S. |
| Negative | 59 (98.3%) | 414 (96.3%) | |
| Positive | 1 (1.7%) | 16 (3.7%) | |
| Curability | | | <0.0001 |
| A, B | 40 (66.7%) | 380 (88.4%) | |
| C | 20 (23.3%) | 50 (11.6%) | |
| TNM stage | | | 0.005 |
| I | 9 (15%) | 118 (27.5%) | |
| II | 9 (15%) | 108 (25.1%) | |
| III | 36 (60%) | 158 (36.7%) | |
| IV | 6 (10%) | 46 (10.7%) | |
Significant differences were observed between the
LSGC and OGC groups with regard to tumor size, stage, venous
involvement, curability, depth of invasion, histological
differentiation and peritoneal dissemination. The LSGC group showed
significantly larger tumor size (p<0.0001), more advanced tumor
stage (p=0.005), lower curability (p<0.0001), deeper invasion
into the gastric wall (p<0.0001) and poorer histological
differentiation (p<0.0001) compared with the OGC group.
Peritoneal dissemination and venous involvement were more frequent
in the LSGC group (p<0.0001 and p<0.05) than in the OGC
group. Severe lymphatic invasion was frequent in the LSGC group
(p=0.002) (data not shown). Therefore, these factors suggest that
LSGC is associated with more advanced and unfavorable
clinicopathological findings compared with OGC but fewer compared
with LP.
Survival comparison between OGC, LSGC and
LP
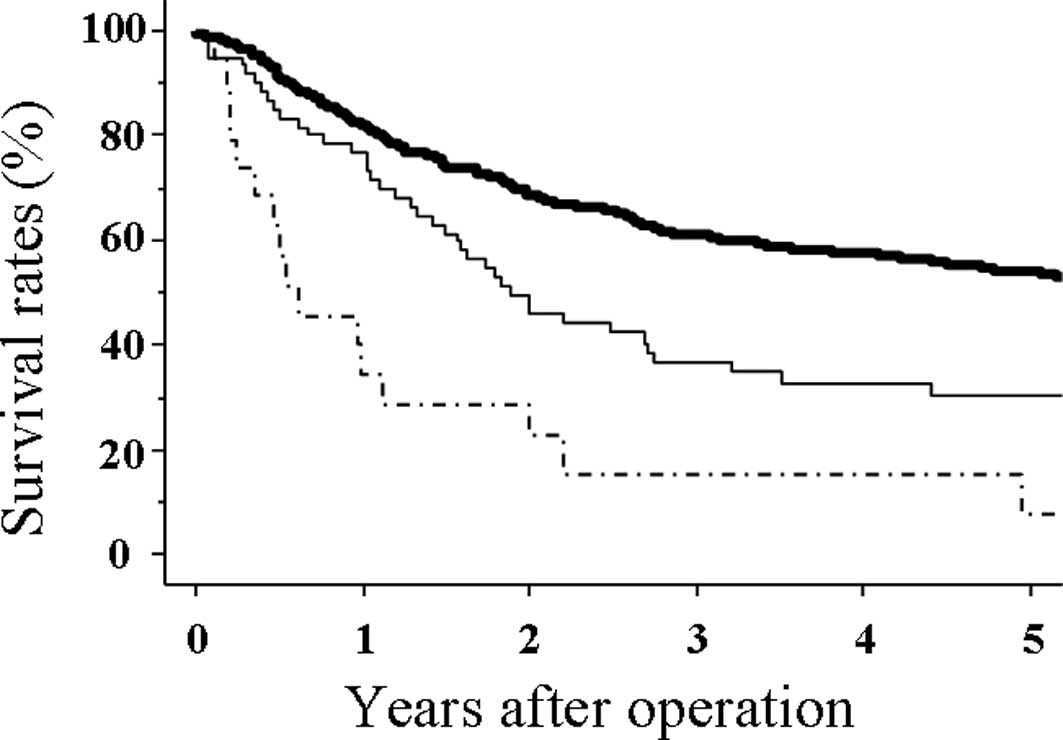
The 1-, 3- and 5-year survival rates of patients
with OGC were 81.7, 61.1 and 53.8%, respectively, which were
significantly higher than those of patients with LSGC (73.2, 36.5
and 29.8%, respectively; p=0.0015) (Fig. 2). However, the 1-, 3- and 5-year
survival rates of patients with LP were 26.3, 21.1 and 7%,
respectively, which were significantly lower than those of LSGC
(p=0.002) (Fig. 2). Of the 19
patients with LP, only 2 patients survived >3 years and only 1
patient survived >5 years.
Prognostic factors in patients with
SGC
Multivariate analysis revealed that CY and
curability were independently associated prognostic factors in
patients with SGC (Table III),
whereas SGC, lymph node metastasis, curability and TNM stage were
independently associated prognostic factors in patients with
advanced gastric cancer (Table
IV).
 | Table IIIFactors independently associated with
prognosis in SGC. |
Table III
Factors independently associated with
prognosis in SGC.
| Variables | Standard error | Odds ratio | CI 95% | P-value |
|---|
| Peritoneal
cytology | 0.456 | 2.207 | 0.150–0.894 | 0.027 |
| LSGC (LP/LSGC) | 0.312 | 1.103 | 0.385–1.306 | 0.269 |
| Serosal
invasion | 0.385 | 0.900 | 0.333–1.504 | 0.368 |
| Curability (A,
B/C) | 0.431 | 2.885 | 0.124–0.671 | 0.004 |
| Lymph node
metastasis | 0.460 | 1.900 | 0.169–1.028 | 0.057 |
 | Table IVFactors independently associated with
prognosis in advanced gastric cancer. |
Table IV
Factors independently associated with
prognosis in advanced gastric cancer.
| Variables | Standard error | Odds ratio | CI 95% | P-value |
|---|
| SGC (SGC/OGC) | 0.156 | 2.242 | 0.520–0.957 | 0.025 |
| Lymph node
metastasis | 0.212 | 3.669 | 0.302–0.696 | 0.0002 |
| Curability (A,
B/C) | 0.150 | 9.095 | 0.190–0.343 | <0.0001 |
| Stage (I, II/III,
IV) | 0.173 | 2.994 | 0.425–0.836 | 0.003 |
Discussion
The classification of advanced gastric cancer into
type I through IV by Borrmann has been globally accepted (19). The definition of SGC in this study
is the same as that of Borrmann type IV and includes a number of
grades of disease from LP to scirrhous pattern carcinoma. When the
entire stomach wall is invaded by carcinoma cells, the stomach has
a leather bottle-like appearance and the disease condition is known
as LP, which is the typical and complete case of SGC. However, not
all SGCs are defined as LP with a typical appearance. When only a
section of the stomach is invaded by scirrhous pattern carcinoma
cells, the case is also defined as SGC, although the stomach does
not have a leather bottle-like appearance. Such atypical and
localized cases of SGC (LSGC) have been classified in the same
category as LP. However, the differences between LP and LSGC have
not been studied. In the present study, we investigated the
differences in malignant behavior between LP and LSGC using several
clinical factors.
Our data demonstrated that LP is more aggressive in
terms of peritoneal dissemination, depth of invasion, curability
and peritoneal cytology than LSGC, and that the 5-year survival
rate of patients with LSGC was significantly better than that of
patients with LP.
Only 36.8% (7/19) of patients with LP underwent
resection A or B compared with 66.7% (40/60) of patients with LSGC.
Of the 19 patients with LP, 16 suffered from peritoneal recurrence
and only one patient with LP survived for more than 5 years
following surgery. Lymph node metastasis and venous invasion were
frequent and TNM staging was more advanced in LP compared with
LSGC, although no significant differences were observed.
These data suggest that LP is definitely a more
advanced stage cancer with higher malignant potential than LSGC,
and that surgical treatment for LP may not be effective in
improving long-term survival. However, LSGC was also not easily
treated. The data presented in this study also indicate that LSGC
was aggressive in a number of prognostic factors, and that the
5-year survival rate of patients with LSGC was significantly worse
than that of patients with OGC. SGC was an independent prognostic
factor in advanced gastric cancer, as indicated by several previous
studies (20–23). The malignant grade of LSGC is
intermediate between OGC and LP.
SGC has been shown to possess unique
clinicopathological characteristics and a poor survival outcome
despite curative gastrectomy (10–16,20–23).
One reason for the poor prognosis specific to SGC is the difficulty
in early detection. Poorly differentiated adenocarcinoma,
developing from the fundic gland mucosa around the greater
curvature of the stomach, are known to infiltrate submucosal tissue
prior to ulceration of the primary lesion. This infiltration
represents the initial lesions of LP, which are difficult to detect
even by modern endoscopic examination (24). Furthermore, Oguro reported that the
development of SGC was divided into both stable and rapid phases
(7). SGC undergoes almost no change
in the stable phase and then suddenly moves into the rapid phase.
In the rapid phase, the development of SGC is extremely fast and is
detected with difficulty, and early cancer or LP is usually
identified as the disease condition of SGC (6,7),
rather than the prelinitis condition. Certain studies have
elucidated the mechanisms underlying the development of SGC and
identified the prelinitis condition (5,9).
However, the definition of the prelinitis condition has yet to be
established. In this study, LSGC may correspond to the prelinitis
condition of the disease.
A new therapeutic strategy for SGC has been
considered in order to improve its poor prognosis and determine the
mechanisms and clinical course of SGC development. Clinical studies
have demonstrated that preoperative chemotherapy, close staging by
peritoneal cytology with exploratory laparoscopy, and strong
chemotherapy, including S-1, may be a more favorable option than
aggressive surgery or palliative gastrectomy (2,3,13,16).
In the present study, peritoneal cytology was an
independent prognostic factor in SGC. LP versus LSGC was not a
prognostic factor, but surgical treatment for LP was not effective
in prolonging patient survival, whereas a longer survival rate was
expected in patients with LSGC who underwent curative resection. A
consistent systematic chemotherapy was not performed for the 79
patients with SGC in this study, but the survival rate of patients
who received chemotherapy was better than that of those who did not
receive chemotherapy (data not shown), indicating that adequate
systematic chemotherapy is essential for improving the outcome of
SGC treatment.
In conclusion, we found that the clinicopathological
characteristics and surgical outcomes of LSGC are different from
those of LP and that its malignant grade is intermediate between
OGC and LP. LP appears not to be a surgical disease, whereas
long-term survival is expected in patients with LSGC who underwent
curative resection. Furthermore, we believe that LSGC may represent
the prelinitis condition.
Acknowledgements
The authors wish to express their gratitude to Drs
S. Momosaki and Y. Nakayama of the Department of Pathology,
National Kyushu Medical Center, Japan, for discussions related to
pathology.
References
|
1
|
Takemura S, Yashiro M, Sunami T, et al:
Novel models for human scirrhous gastric carcinoma in vivo. Cancer
Sci. 95:893–900. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeguchi M, Miyake T, Matsunaga T, et al:
Recent results of therapy for scirrhous gastric cancer. Surg Today.
39:290–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki T, Koizumi W, Tanabe S, et al: TS-1
as first-line therapy for gastric linitis plastica: historical
control study. Anticancer Drugs. 17:581–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodera Y, Nakanishi H, Ito S, et al:
Detection of disseminated cancer cells in linitis plastica-type
gastric carcinioma. Jpn J Clin Oncol. 34:525–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morita K, Fujimori T, Ono Y, et al:
Identification of the prelinitis condition in gastric cancer and
analysis of TGF-b, TGF-b RII and pS2 expression. Pathobiology.
69:321–328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsukawa M, Kurihara M, Hirashima M, et
al: Radiological findings of gastric scirrhous cancer. Gan To
Kagaku Ryoho (in Japanese with English abstract). 21:2378–2383.
1994.PubMed/NCBI
|
|
7
|
Oguro Y: Endoscopic diagnosis of scirrhous
gastric carcinoma. Gan To Kagaku Ryoho (in Japanese with English
abstract). 21:2384–2391. 1994.PubMed/NCBI
|
|
8
|
Kohri Y, Takeda S and Kawai K: Earlier
diagnosis of gastric infiltrating carcinoma (Scirrhous cancer). J
Clin Gastroenterol. 3:17–20. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ichikawa Y, Koshikawa N, Hasegawa S, et
al: Marked increase of trypsin(ogen) in serum of linitis plastica
(gastric cancer, Borrmann 4) patients. Clin Cancer Res.
6:1385–1388. 2000.PubMed/NCBI
|
|
10
|
Kitamura K, Beppu R, Anai H, et al:
Clinicopathological study of patients with Borrmann type IV gastric
cancer. J Surg Oncol. 58:112–117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
An JY, Kang TH, Choi MG, et al: Borrmann
Type IV: An independent prognostic factor for survival in gastric
cancer. J Gastrointest Surg. 12:1364–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naito Y and Kino I: Pathogenesis and
progression of scirrhous carcinoma. Gan To Kagaku Ryoho (in
Japanese with English abstract). 21:2364–2370. 1994.PubMed/NCBI
|
|
13
|
Ikeguchi M, Yamamoto O and Kaibara N:
Management protocol for scirrhous gastric cancer. In Vivo.
18:577–580. 2004.PubMed/NCBI
|
|
14
|
Otsuji E, Kuriu Y, Okamoto K, et al:
Outcome of surgical treatment for patients with scirrhous carcinoma
of the stomach. Am J Surg. 188:327–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunisaki C, Shimada H, Nomura M, et al:
Therapeutic strategy of scirrhous type gastric cancer.
Hepatogastroenterology. 52:314–318. 2005.PubMed/NCBI
|
|
16
|
Ikeguchi M, Matsumoto S, Yoshioka S, et
al: Laparoscopic assisted intraperitoneal chemotherapy for patients
with scirrhous gastric cancer. Chemotherapy. 51:15–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Gastric Cancer Association.
Japanese Classification of Gastric Carcinoma – 2nd English edition.
Gastric Cancer. 1:10–24. 1998.
|
|
18
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th edition. A John
Wiley & Sons, Ltd; NY: pp. 1–310. 2009
|
|
19
|
Borrmann R: Geschwulstic des Magen und des
Deudenums. Henke F and Lubarsch O: Handbuch der Speziellen
Pathologischen Anatomic und Histologie. IV(part 1)Springer; Berlin:
pp. 812–1054. 1926
|
|
20
|
Nakamura R, Saikawa Y, Wada N, et al:
Retrospective analysis of prognosis for scirrhous-type gastric
cancer: one institution’s experience. Int J Clin Oncol. 12:291–294.
2007.PubMed/NCBI
|
|
21
|
Kodera Y, Yamamura Y, Ito S, et al: Is
Borrmann type IV gastric carcinoma a surgical disease? An old
problem revisited with reference to the result of peritoneal
washing cytology. J Surg Oncol. 78:175–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Oh SJ, Kim S, et al: Macroscopic
Borrmann type as a simple prognostic indicator in patients with
advanced gastric cancer. Oncology. 77:197–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen CY, Wu CW, Lo SS, et al: Peritoneal
carcinomatosis and lymph node metastasis are prognostic indicators
in patients with Borrmann type IV gastric carcinoma.
Hepatogastroenterology. 49:874–877. 2002.PubMed/NCBI
|
|
24
|
Park MS, Ha HK, Choi BS, et al: Scirrhous
gastric carcinoma: endoscopy versus upper gastrointestinal
radiolography. Radiology. 231:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|