Introduction
Stem cells are unspecialized cells capable of
self-renewal in a living organism through cell division, and the
production of specialized cells. These cells are capable of
multi-differentiation and possess the ability to become transferred
tissue cells (1). Stem cells are
obtained from two different sources: i) embryonic stem cells are
obtained from an internal cell population of blastocysts in the
earlier stages of embryonic development, and ii) postnatal stem
cells are obtained from non-embryonic (organ-specific,
tissue-specific or in mature stem cells) sources (2). Cells obtained from embryonic stem
cells are capable of differentiating into various cell types
originating from the ectoderm, mesoderm and endoderm layers,
besides the placenta (3). Stem
cells in mature tissues are unspecialized cells that usually
generate the cell types which belong to the tissue in which they
occur. These cells are also capable of regeneration, providing
replenishment and repair to the tissues to which they belong.
Mature stem cells differentiate into multi-cell types and this
status is known as ‘transdifferentiation’ or ‘plasticity’ (4,5). At
present, the most commonly referenced method for identifying stem
cell types is based on the use of stem cell markers. The majority
of these markers, which appear on the surface of the cells and play
a role in cell signaling pathways or cell-cell adhesion molecules,
are known as ‘clusters of differentiation’ (CD), and are found
specifically or commonly for the cell type (6). Hematopoietic stem cells (HSCs) are
found in bone marrow and cord blood, and have in vivo
engraftment potential. Peripheral blood (PB) stem-progenitor cells
mobilize with growth factors. These two cell types form
progressively more mature hematopoietic progenitor cells (HPCs).
Due to their ability to regenerate and differentiate into all blood
cells, HSCs are regarded as blood cell precursors (7). By contrast, HPCs are differentiated
into various blood cells, i.e., myeloid cells (monocytes,
macrophages, basophils, eosinophils, erythrocytes,
megakaryocytes/platelets and some dendritic cells) and lymphocytes
[T cells, B cells, natural killer (NK) cells and some dendritic
cells]. The primary surface markers of these cells are CD34, CD14,
CD38, CD45 and CD133. In humans, the majority of HSCs and HPCs
carry CD34 phosphoglycoprotein and its mRNA. A substantial
proportion of CD34+/CD38−/Lin−
cells occur within HSCs (8–10). Moreover, fluorescent aldehyde
dehydrogenase (ALDH)-substrates have been used to identify and
isolate human and mouse hematopoietic cells by
fluorescence-activated cell sorting (FACS) (11).
In a previous study, CD38 activity was investigated
in erythrocytes obtained from different patient groups, including
cancer patients and patients with systemic diseases, and was found
to be higher when compared to the control group. This increased
activity in cancer patient erythrocytes is significant.
Additionally, in cancer cases with high carcino-embryonic antigen
(CEA) values, the erythrocyte protein band corresponding to 45 kDa
molecular weight had an increased signal in the Western blot
analysis, and this indicates that CD38 expression was induced in
cancer (12).
Human CD38, a type II surface antigen expressed by
immature hematopoietic cells, is rearranged at high levels by
activated lymphocytes, such as T cells, B cells, dendritic cells
and NK cells. In bone marrow, CD38 is expressed in primitive cells,
and it is known that 50–80% of the mononuclear cells in cord blood
are CD38-positive (13). Cell
surface antigen CD38 is an enzyme with a number of functions and is
also expressed in hematopoietic cells through differentiation. CD38
is a multifunctional ectoenzyme that exhibits NAD glycohydrolase,
ADP ribosyl cyclase and ADP ribosyl hydrolase activities. A number
of studies indicated that a high level of CD38 expression is a sign
of malignancy (14,15). CD38 also has receptor activity in
addition to its enzymatic features. This activity is thought to
play a role in cell proliferation and differentiation processes
through in vivo signal transmission. By contrast, the
correlation between the enzymatic features of CD38 and its
receptor-like behavior remains to be determined. CD38 is considered
valuable to research due to its effectiveness and physiological
function. Primitive/progenitor hematopoietic cells
[CD34+/CD38−], which are obtained from cord
blood, persist in the erythroid development process until the
nucleus loss stage, in addition to favorable factors [such as stem
cell factor (SCF)/erythropoietin] in serum-free culture medium.
Therefore, the present study aimed to investigate changes in
primitive hematopoietic cells through CD38 expression, and identify
the stage at which erythrocyte differentiation CD38 gains activity
and the effects of serum factors on this expression in the
erythroid development process. To achieve this aim, a HSC system
was established.
Materials and methods
CD34+ cell isolation from cord
blood
CD34+ cells were isolated by a magnetic
cell-sorting system. Cord blood (10 ml) was diluted 1:1 with
phosphate-buffered saline (PBS). Ficoll (5 ml) was added to a 15 ml
tube into which diluted cord blood was gradually layered. Samples
were centrifuged at 3000 rpm for 20 min. The buffy coat was
transferred to polystyrene tubes and 1 ml of medium [2% fetal
bovine serum (FBS) + 1 mM EDTA-containing PBS] was added to the
tubes. EasySep antibody cocktail (100 μl) was added following cell
suspension. The mixture was then pipetted and incubated for 15 min
at room temperature. Subsequently, 50 μl of mixture containing
magnetic nanoparticles was added, and the mixture was then
incubated for 10 min at room temperature following 4–5 more
pipettes. A total volume of 2500 ml was obtained with medium and
the tube that was placed inside the magnet was incubated for 5 min.
Without removing the tube from the magnet, the content of the tube
was emptied following a 2–3-sec wait. The tube was removed from the
magnet and washed a further four times by adding 2.5 ml PBS. The
tube content (CD34+ cells) became isolated (16). CD34+ cells were examined
under an inverted microscope and, in terms of CD34+ cell
rate, were examined by flow cytometry.
Isolation of
ALDH+CD38− cells with FACs after selecting
CD34+ cells from cord blood
CD34+ cells from cord blood were isolated
by immunomagnetic labeling. For ALDH labeling, which is a stem cell
marker, 50 μl activator was added to the reagent tube and the tube
was incubated for 20 min at room temperature. A 1.5 ml
neutralization buffer was also added to the tube. Simultaneously, 5
μl diethylaminobenzaldehyde (DEAB, Sigma, St. Louis, MO, USA) was
added to the control tube. A 500 ml cell sample (obtained from cord
blood) was transferred to the reagent tube, and then a 500 μl
sample was transferred to the control tube. The sample was
incubated for 30 min at 37°C. A 1 ml sample was collected from the
reagent tube and transferred to a separate flow tube. CD34
allophycocyanin (APC) (5 μl) and CD38 phycoerythrin (PE) (5 μl)
were added. The tubes were incubated for 20 min at 37°C.
Supernatants were removed following centrifugation for 5 min at
2000 rpm. Following the addition of 0.5 ml measurement buffer,
analysis was carried out using a FACSDiva Software 6.1.2.
Culture of phase I and II
ALDH+CD34+CD38− Lin−
cells from which erythroid primitives developed
ALDH+CD34+ CD38−
Lin− cells were plated at 1×106 cell/ml in
the 5 ml voluminous wells. Erythropoietin (Epo) [recombinant human
(rH)] (10 μg/ml), 100 U/ml penicillin-streptomycin, 10 ng/ml IL-3
and IL-6, and 100 ng/ml SCF were added to CD34+ cells
(1×105 cell/ml) and 2 mM L-glutamine-enriched Iscove’s
modified Dulbecco’s medium (IMDM) and then incubated in 5%
CO2 at 37°C. Cell counts were diluted in fresh medium
(1×105/ml) and maintained for 10 days. Ten days
following the SCF/Epo incubation, the process was continued into
the second phase of erythroid culture in 20% bovine serum albumin,
insulin and transferrin (BIT) and 1 U/ml human Epo-containing
medium. Transforming growth factor (TGF-β1) (5 ng/ml) was added to
the cultures on days 0, 5 and 10 of phase II. Cells, which had been
followed on different days (0, 4, 8, 12 and 16) of the maturation
(normoblast/erythroblast) by flow cytometry, were evaluated by
staining with a Megacult-c staining kit.
Assay for NAD glycohydrolase activity of
hematopoietic progenitor cells
The NAD glycohydrolase enzyme activity of
ALDH+CD34+CD38− cells cultured
with SCF was examined by supernatant samples obtained on different
days (0, 4, 8, 12 and 16) (cell counts were maintained as a
constant).
NAD+ glycohydrolase activity of
ALDH+CD34+CD38− cells cultured
with SCF was determined by the separation of
[carbonyl-14C] nicotinamide released from
[carbonyl-14C] NAD+ in BioRad AG1×4 anion
exchange resin (BioRad, Hercules, CA, USA) (17). Reaction mixtures (20 μl, pH 9.0)
containing 12 μl serum, 10 mM NaCl, 500 μM ZnCl2, 50 μM
CaCl2, 20 mM Tris-HCl and 5 μM [carbonyl-14C]
NAD+ were incubated for 30 min at 37°C (12). Reactions were stopped with 1 ml 0.1%
sodium dodecyl sulfate (SDS). The samples were then applied to a
BioRad AG1×4 column and [carbonyl-14C] nicotinamide was
eluted with H2O, whereas unhydrolised
[carbonyl-14C] NAD was retained on the column and then
eluted with 0.5 M NaCl. The radioactivity was determined by
counting aliquots from the eluate in a liquid scintillation counter
(Packard Tri-Carb 1000 TK; Meriden, CT, USA), with a counting
efficiency of 90% for 14C.
SDS-PAGE and Western blotting
SDS-PAGE and Western blotting using CD38-specific
goat polyclonal IgG (M-19) were performed as previously described
(18,19). Bovine serum albumin (BSA) (66 kDa),
ovalbumin (45 kDa) and carbonic anhydrase (29 kDa) were used as
standards.
SDS-PAGE analyses of stem cell culture supernatant
liquids were made by using the Laemmli method (18). Stem cell culture supernatant liquids
were solubilized in 1 M Tris-HCl, pH 6.8, 2% glycerin, 10% SDS, 5%
2-mercaptoethanol and 0.1 % bromphenol blue, and subjected to
SDS-PAGE. The separated proteins were then transferred
electrophoretically onto nitrocellulose membranes (Schleicher &
Schuell BioScience, Dassel, Germany). The blots were blocked by 1 h
incubation with 0.5% BSA in TBST (10 mM Tris-HCl, pH 8.0, 150 mM
NaCl and 0.05% Tween-20) followed by successive 1 h incubations
with CD38-specific goat polyclonal IgG (M-19). Detection of
immunocomplexes was achieved using alkaline phophatase-conjugated
bovine anti-goat antibody (Sigma) [1:1000 in TBST and
nitrotetrazolium blue chloride/5 bromo-4 chloro-3′-indolyl
phosphate (BCIP/NBP)] tablets as the substrate (12).
Statistical analysis
Each experiment was repeated at least three times.
Results were expressed as the mean value ± standard deviation (SD).
A paired t-test was used for statistical analyses of data.
P<0.05 was considered to be statistically significant and tests
were two-tailed. Statistical analyses were performed using the
statistical package SPSS 10.1 (SPSS, Chicago, IL, USA).
Results
Isolation of hematopoietic progenitor
cells
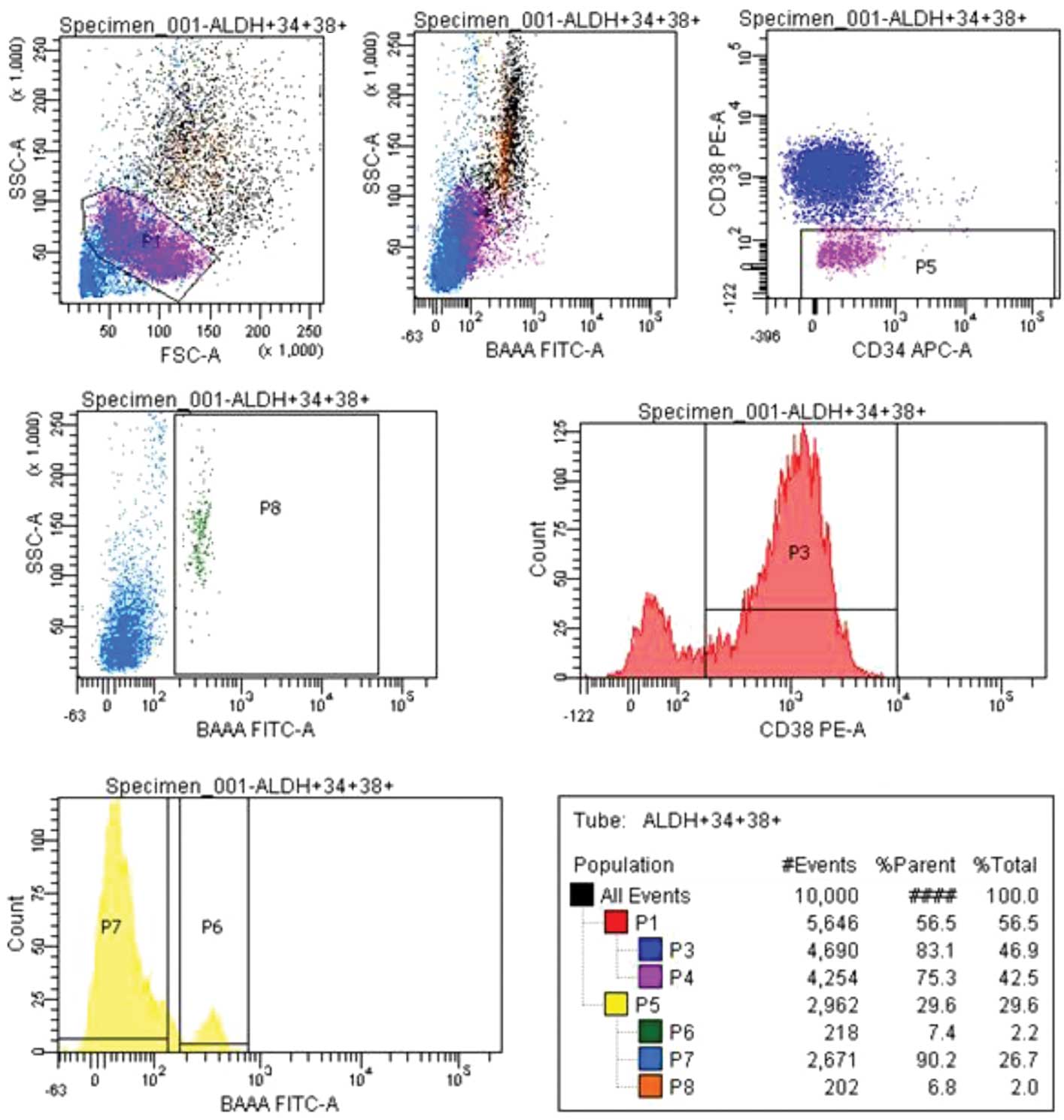
CD34+ cells from cord blood were selected
by immunomagnetic labeling and sorted with FACS. With the P1 gate,
cells showing lymphocyte morphology were gated; a total of 10.000
cell were gated. From the selected cells, a
CD34+CD38− cell group was gated, (P5) 29.6%,
and an ALDH rate was obtained from this cell group. From the
ALDH+, (P6) 7.4%, and ALDH−, (P7) 90.2%,
populations, the CD34+CD38− ALDH+,
(P8) 6.8%, section was sorted (Fig.
1).
Generation of erythroid progenitors from
primitive hematopoietic cells in liquid culture (phase I)
Essential cytokines and factors [Epo (1 μl/ml), SCF
(100 ng/ml), IL-3 (10 ng/ml), and IL-6 (10 ng/ml)] were added to
20% BIT 9500, 100 U/ml penicillin-streptomycin and 15%
FBS-containing medium, and collected hematopoietic progenitor
(ALDH+CD34+CD38−) cells were
cultured in 2 mM L-glutamin-enriched IMDM. Cell counts were
maintained <1×106/ml by repeated cell dilutions in
fresh medium.
Terminal erythroid differentiation in
liquid culture (phase II)
Following 10 days of SCF/Epo stimulation, the washed
cells were transferred into the second phase of the erythroid
culture in 20% BIT (2×105/ml) (phase II, 14 days),
10−5 M β-mercaptoethanol (β-ME), 10−5 M
dexamethasone and 1 U/ml rH Epo-containing IMDM. TGF-β1 (5 ng/ml)
was added on days 0, 5 and 10 of phase II. Cells which had been
followed on different days (0, 4, 8, 12 and 16) of maturation
(normoblast/erythroblast) were assessed by staining with a
Megacult-c staining kit (Fig.
2).
The cell count resulted in significant
cell proliferation in Epo/SCF-stimulated cultures
This proliferation was correlated to erythroid cell
generation in SCF/Epo-stimulated cultures. In the second
differentiation phase on different days (0, 4, 8, 12 and 16), flow
cytometric analyses of cells were carried out. Results showed that
CD38 activities of cells increased over time, whereas CD34
percentages decreased.
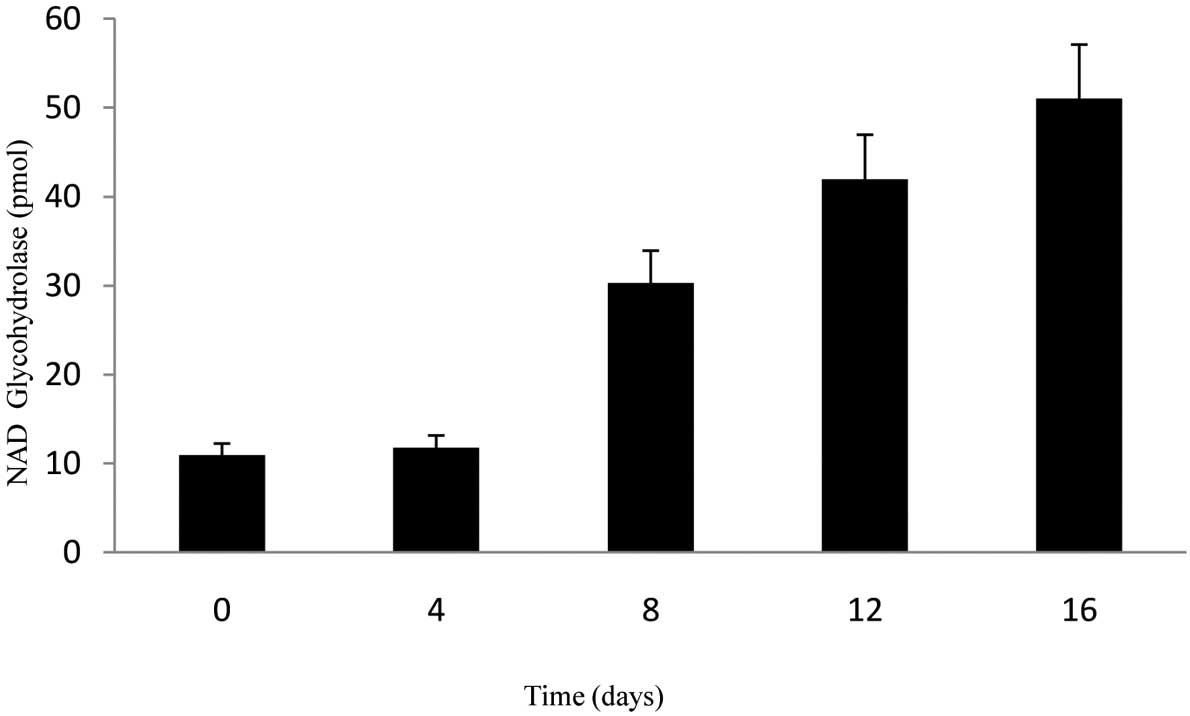
NAD glycohydrolase activity of
hematopoietic progenitor cells
CD34+CD38−ALDH+
cells cultured with SCF were transferred into Epo and
SCF-containing medium for phase I, which is the erythroid line
differentiation stage. Ten days later, the cells were transferred
into the second phase medium, which contained transforming growth
factor β1, mercaptoethanol and dexamethasone. A significant
increase was observed in the activity rates of NAD glycohydrolase
obtained from the supernatant samples on different days (0, 4, 8,
12 and 16) of the differentiation phase (Fig. 3). At the end of the 16th day,
activity rates were approximately 10 times more compared with days
0 and 4. The paired t-test was used for statistical analyses of
data. P<0.05 was considered to be statistically signficant and
tests were two-tailed.
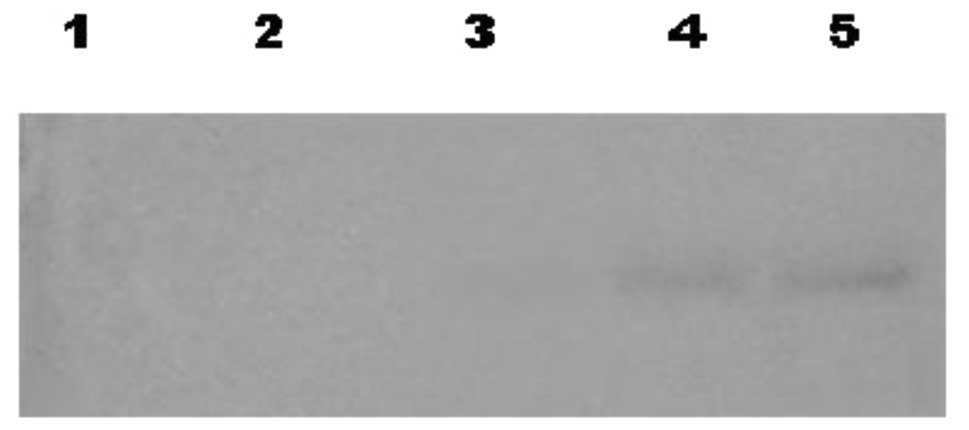
Second differentiation phase Western blot
analysis
On different days (0, 4, 8, 12 and 16) during the
second differentiation phase, Western blotting analyses of
supernatants cultured with SCF were performed following SDS-PAGE.
From the eighth day of differentiation, an increase over time was
determined on a signal correlated to a 45-kDa molecular weight
protein, which reacts with anti-CD38 (Fig. 4).
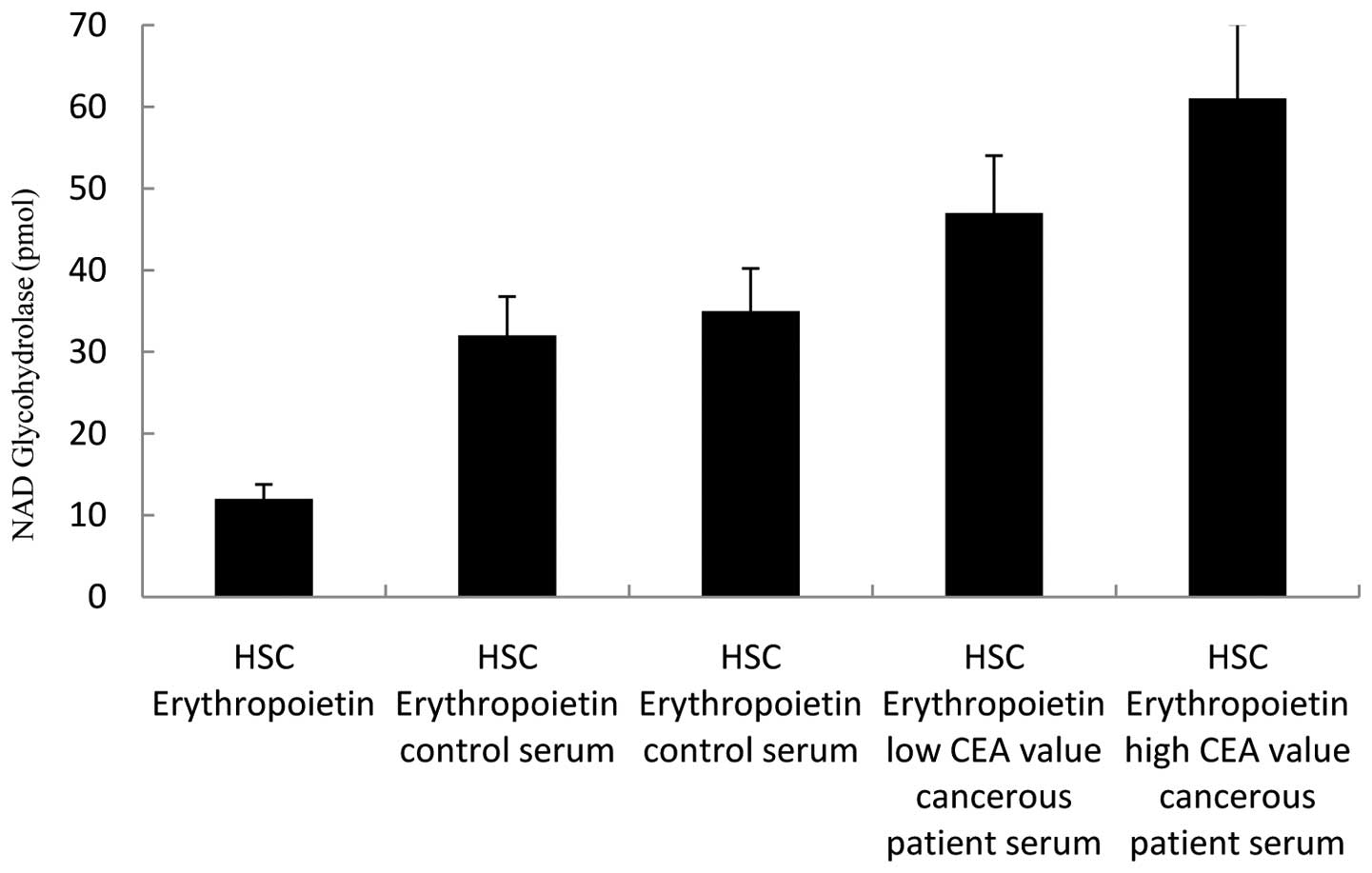
NAD glycohydrolase activity of HSCs in
the presence of serum factors
We have analyzed the effects of serum factors on
co-culture experiments and CD38 expression in cell culture studies
by using a NAD glycohydrolase activity test. The following media
were added in the experiments conducted on 5×105/ml
CD34+CD38−ALDH+ HSCs: only Epo was
added to the first well; control serum to the second and third
wells; patient serum with a low CEA value to the fourth well; and
patient serum with a high CEA value to the fifth well. Seven days
later, the NAD glycohydrolase activity of supernatants was
analyzed. A significant increase in NAD glycohydrolase activity, in
other words CD38 expression, compared to other wells was determined
in the well to which cancerous patient serum was added (Fig. 5). The paired t-test was used for
statistical analyses of data. P<0.05 was considered to be
statistically significant and the tests were two-tailed.
Discussion
In this study, CD34+ cells from cord
blood were labeled immunomagnetically and sorted with FACS. The
collected hematopoietic progenitor cells were transferred into
culture medium and erythroid progenitors were developed in both
phases I and II.
In their study, Storms et al have shown that
various primitive progenitor cells that are capable of generating
into a number of lines are more substantial than
ALDH+CD34+ cell lines and myeloid progenitors
(20). We observed that a number of
CD34+ cells were ALDH+ as a result of
fluorescent microscopic and the flow cytometry of ALDH-fluorescein
isothiocyanate (ALDH-FITCH)-labeled CD34+ cells isolated
from cord blood.
The cell counts resulted in significant cell
proliferation in Epo/SCF-stimulated cultures. This proliferation in
SCF/Epo-stimulated cultures is significantly correlated to
erythroid cell generation. CD34+CD38− cells
are primitive subpopulations of progenitor cells.
According to Hao et al,
CD34+CD38− cells in cord blood show the same
immunophenotype as mature bone marrow cells isolated from cord
blood that proliferate seven times more rapidly in the presence of
SCF and IL-6 compared to bone marrow (21).
ALDH+/CD34+/CD38−
cells were collected during progenitor cell sorting from cord blood
with FACS. Prior to the selection process they were observed using
fluorescent microscope images, from which cord blood leukocyte
fractions were analyzed, and cells with overall consumed antibodies
were present in this fraction.
Neildez-Nguyen et al identified an essential
medium required for HSCs originating in the cord blood in order to
differentiate into red blood cells (22).
By performing a more extended study in vitro,
stem cells were placed into a two-phased culture study. Cells that
were maintained in the first phase for 10 days were transferred
into the second phase, which is the differentiation period, and
maintained in this medium for 16 days. Through the erythropoietic
development process, hematopoietic cells were observed until the
erythroblast/normoblast stage. At the end of a 26-day process, by
staining cells with a Megacult c-staining kit, it was established
that progenitor cells nucleate and differentiate into 8–10 μm-sized
erythroid cell lines (22,23).
CD38 is an ectoenzyme that has NAD glycohydrolase,
ADP ribosyl cyclase and ADP ribosyl hydrolase activities.
NAD+ glycohydrolase is usually found partially in cell
and partially in nucleus (24).
CD38 has also been determined in erythrocytes, the liver, pancreas,
brain, muscle cells, plasma cells, monocyte/macrophages and HSCs
(24–25). CD38 has receptor activity in
addition to enzymatic functions. This activity is thought to play a
role in cell proliferation and differentiation processes through
in vivo signal transmission. However, the correlation
between the enzymatic characteristics of CD38 and its receptor-like
behavior remains unknown.
CD38 is a significant system that is worth
investigating in terms of its effect on systems and its
physiological function. In the study by Albeniz et al
(12), CD38 activity was
investigated in erythrocytes obtained from different patient
groups, and this activity in the erythrocytes of patients with
cancer and systemic diseases was found to be higher compared with
the control group. This increased activity in the cancer patient
erythrocytes was notably significant. Furthermore, in cancer cases
with a high CEA value, an erythrocyte protein band corresponding to
a 45-kDa molecular weight had an increased signal in the Western
blot analysis, and this indicates that CD38 expression was induced
in cancer (12).
In this study, as an indication of the stage at
which CD38 erythroid expression begins, supernatants obtained from
stem cells differentiating into an erythroid cell line increase NAD
glycohydrolase activity rates. This activity increase was also
observed in results obtained from the Western blot analysis carried
out following SDS-PAGE at a 45-kDa anti-CD38 reactive band as of
day 8.
Previous studies have shown that when significant
changes in NAD/ADP-ribose metabolism occur, CD38 expression
increases and significant associated enzymatic activities occur in
neoplastic development (15,26).
Finally, the effects of serum factors on CD38
expression were assessed in the experiments carried out on
CD34+/ALDH+/CD38− HSCs. Serum
samples from healthy individuals, and from cancer patients with
high CEA rates (>100 ng/ml) and low CEA rates (<100 ng/ml)
were suspended in vitro in medium and seven days later NAD
glychohydrolase activity was assessed in the supernatant samples.
Findings of the present study show an increase in NAD
glycohydrolase activity. These indications suggest that the
stimulative effect on CD38 expression may originate from certain
serum factors.
CD38 becomes a prominent system for research in
terms of its effect on form and physiological function. Cord blood
cell cultures were thought to be a new model system in the study of
CD38 expression in the erythrocyte development pathway.
Primitive/progenitor hematopoietic cells obtained from cord blood
[CD34+/CD38−] perpetuate the erythroid
development process in a serum-free culture medium with the
addition of favorable factors (such as SCF/Epo) until the nucleus
loss stage is achieved (6). CD38
induction occurs at a number of differentiation stages of
hematopoietic cells. The stimulative effect on CD38 expression
suggests certain serum factors responsible for the induction of
CD38. In the proliferation process, the possible induction of CD38
through certain serum factors suggests that it may be an event in
the proliferation and apoptotic processes. CD38 cells were
protected from apoptosis through their receptor functions and
cooperation with a number of signaling pathways. Thus, CD38 cells
exhibit a longer survival rate, an increased proliferation
potential and contribute to malignancy. This is a study in which
the effects of HSCs on CD38 expression were analyzed in the
erythroid development process, and it is a pre-study that
constitutes the first step for characterization studies of the
physiological function of CD38 for stem cell applications.
Abbreviations:
|
Epo
|
erythropoietin
|
|
SCF
|
stem cell factor
|
|
HSCs
|
hematopoietic stem cells
|
|
HPCs
|
hematopoietic progenitor cells
|
|
ALDH
|
fluorescent aldehyde dehydrogenase
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
APC
|
allophycocyanin
|
|
PE
|
phycoerythrin
|
|
IMDM
|
Iscove’s modified Dulbecco’s
medium
|
|
BCIP
|
5-bromo-4-chloro-3′-indolyl phosphate
p-toluidine
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
References
|
1
|
Verfaillie CM, Pera MF and Lansdorp PM:
Stem cells: hype and reality. Hematology. 1:369–391. 2002.
View Article : Google Scholar
|
|
2
|
Mitchell KE, Weiss ML, Mitchell BM and
Martin P: Matrix cells from Wharton’s jelly from neurons and glia.
Stem cells. 21:50–60. 2003.
|
|
3
|
Czyz J, Wiese C, Rolletschek A, Blyszczuk
P, Cross M and Wobus AM: Potential of embryonic and adult stem
cells in vitro. Biol Chem. 384:1391–1409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malik P, Fisher TC, Barsky LW, Zeng L,
Izadi P, Hiti AL, Weinberg KI, Coates TD, Meiselman HJ and Kohn DB:
An in vitro model of human red blood cell production from
hematopoietic progenitor cells. Blood. 91:2664–2671.
1998.PubMed/NCBI
|
|
5
|
Bickenbach JR and Stem MM: Plasticity of
epidermal stem cell: survival in various environments. Stem Cell
Rev. 1:71–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato N, Sanjuan IM, Heke M, Naef F and
Brivaniou AH: Molecular signature of human embryonic stem cells and
its comporation with the mouse. Dev Biol. 260:404–413. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Audet J, Miller CL, Rose-John S, Piret JM
and Eaves CJ: Distinct role of gp130 activation in promoting
self-renewal divisions by mitogenically stimulated murine
hematopoietic stem cells. Proc Natl Acad Sci USA. 98:1757–1762.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forsberg EC, Prohaska SS, Katzman S,
Heffner GC, Stuart JM and Weissman IL: Differential expression of
novel potential regulators in hematopoietic stem cells. PLoS Genet.
1:281–294. 2005. View Article : Google Scholar
|
|
9
|
Venezia TA, Merchant AA, Ramos CA,
Whitehouse NL, Young AS, Shaw CA and Goodell MA: Molecular
signatures of proliferation and quiescence in hematopoietic stem
cells. PLoS Biol. 2:1640–1651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steidl U, Kronenwett R, Rohr UP, Fenk R,
Kliszewski S, Maercker C, Neubert P, Aivado M, Koch J, Modlich O,
et al: Gene expression profiling identifies significant differences
between the molecular phenotypes of bone marrow-derived and
circulating human CD34+ hematopoietic stem cells. Blood.
99:2037–44. 2002.PubMed/NCBI
|
|
11
|
Wognum WA, Eaves CA and Thomas ET:
Identification and isolation of hematopoietic stem cells. Arch Med
Res. 34:461–475. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Albeniz I, Demir Ö, Türker-Şener L,
Yalçıntepe L, Nurten R and Bermek E: Erythrocyte CD38 as a
prognostic marker in cancer. Hematology. 12:409–414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Funaro A, Reinis M, Trubiani O, Santi S,
Di Primio R and Malavasi F: CD38 functions are regulated through an
internalization step. J Immunol. 160:2238–2247. 1998.PubMed/NCBI
|
|
14
|
Mainou-Fowler T, Dignum HM, Proctor SJ and
Summerfield GP: The prognostic value of CD38 expression and its
quantification in B cell chronic lymphocytic leukemia (B-CLL). Leuk
Lymphoma. 45:455–462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matrai Z: CD38 as a prognostic marker in
CLL. Hematology. 10:39–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akel S, Petrow-Sadowski C, Laughlin MJ and
Ruscett FW: Neutralization of autocrine transforming growth
factor-β in human cord blood
CD34+CD38−Lin− cell promotes stem
cell factor-mediated erythropoetin-independent early erythroid
progenitor development and reduces terminal differentiation. Stem
Cells. 21:557–567. 2003.
|
|
17
|
Kim H, Jacobson EL and Jacobson MK:
Synthesis and degradation of cyclic ADP-ribose by NAD
glycohydrolase. Science. 261:1330–1333. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gershoni JM and Palade GE: Protein
blotting: Principles and applications. Anal Biochem. 131:1–15.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Storms RW, Green PD, Safford KM,
Niedzwiecki D, Cogle CR, Colvin OM, Chao NJ, Rice HE and Smith CA:
Distinct hematopoietic progenitor compartments are delineated by
the expression of aldehyde dehydrogenase and CD34. Blood.
106:95–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao QL, Smogorzewska EM, Barsky LW and
Crooks GM: In vitro identification of single CD34+CD38− cells with
both lymphoid and myeloid potential. Blood. 91:4145–4151. 1998.
|
|
22
|
Neildez-Nguyen TM, Wajcman H, Marden MC,
Bensidhoum M, Moncollin V, Giarratana MC, Kobari L, Thierry D and
Douay L: Human erythroid cells produced ex vivo at large scale
differentiate into red blood cells in vivo. Nature Biotechnology.
20:467–472. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dorn I, Lazar-Karsten P, Boie S, Ribbat J,
Hartwig D, Driller B, Kirchner H and Schlenke P: In vitro
proliferation and differentiation of human CD34+ cells
from peripheral blood into mature red blood cells with two
different cell culture systems. Transplant Cell Eng. 48:1122–1132.
2008.PubMed/NCBI
|
|
24
|
Jacobson MK, Laurean DC, Strohm MS and
Jacobson EL: NAD glycohydrolase and the metabolism of cyclic
ADP-ribose. Biochimie. 77:341–344. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zocchi E, Franco L, Guida L, Benatti U,
Bargellesi A, Malavasi F, Lee HC and De Flora A: A single protein
immunologically identified as CD38 displays NAD+
glycohydrolase, ADP-ribosylcyclase and ADP-ribose hydrolase
activities at the outer surface of erythrocytes. Biochem Biophys
Res Commun. 196:1459–1465. 1993.PubMed/NCBI
|
|
26
|
Albeniz I, Demir Ö, Nurten R and Bermek E:
NAD glycohydrolase activities and ADP-ribose uptake in erythrocytes
from normal subject and cancer patients. Bioscience Rep. 24:41–53.
2004. View Article : Google Scholar : PubMed/NCBI
|