Introduction
Gastric cancer (GC) is the fourth most common cancer
and the second most common cause of cancer-associated death
worldwide. Approximately 10% of gastric cancers are hereditary and
a low percentage of these cases (1–3%) has been classified as a
single hereditary syndrome. Hereditary diffuse gastric cancer
(HDGC) (1) is an autosomal-dominant
inherited cancer syndrome in which affected individuals develop
diffuse-type gastric cancer at a young age (2). Approximately 30% of families
fulfilling the HDGC criteria possess a second allele of the
E-cadherin gene CDH1 inactivated due to methylation or mutation
events. CDH1 inactivation is currently the only genetic disorder
described as predisposing to the hereditary type of gastric cancers
(3,4).
Although the etiopathogenesis of GC is not fully
understood, it is evident that genetic factors increase the risk of
disease. In a previous study, we demonstrated that a family history
of gastric cancer (FHGC) predisposes patients towards the
development of gastric cancer (6,7). Our
data revealed that, for dyspeptic patients whose first degree
relative(s) died of GC, the expression of the fragile histidine
triad (FHIT) gene, a tumor suppressor gene (5), in gastric mucosa was inhibited
(6,7). A reduced expression of the FHIT
gene has been observed in the majority of gastric cancers and a
number of other organ tumors (8,9). The
FHIT protein is a member of the evolutionarily highly conserved HIT
protein superfamily. Another HIT protein is the histidine triad
nucleotide binding protein 1 (HINT1), a novel tumor suppressor that
plays an inhibitory role in controlling gene transcription. It was
previously revealed that the involvement of HINT1 in the regulation
of a number of apoptotic pathways is independent of its enzymatic
activity (10). HINT1 is deficient
in gastric (11) and colon cancers
(12) and in mammary and ovarian
tumors (13). Moreover,
down-regulation of the HINT1 gene may stimulate the
development of hepatocarcinoma (14). In this context, we investigated
whether FHGC correlates with the expression of HINT1 tumor
suppressor gene in the gastric mucosa of patients with dyspepsia.
Detailed analysis of the level of HINT1 expression in the
antrum and corpus of the stomach may aid in the elucidation of the
molecular basis for understanding of the gastric cancer
development.
Materials and methods
Patients
The study comprised 38 ethnically homogeneous
patients with dyspeptic symptoms, without concomitant chronic
diseases. The screened patients were divided into two groups: Group
I (control) comprising 18 patients (mean age 34.1±11.2) without
FHGC, and Group II including 20 patients (mean age 39.6±13.7) with
FHGC. FHGC was defined as patients having at least one first degree
relative who succumbed to gastric cancer prior to the age of 60,
and another family member who was diagnosed with cancer of any
organ. Data on the type of GC were not collected. Smokers, patients
receiving NSAIDs or PPIs 14 days prior to the study, as well as
Helicobacter pylori (H. pylori)-positive patients,
were not recruited to the study. Subjects underwent a gastroscopy
with biopsy procedure. The study was conducted in accordance with
the Declaration of Helsinki and the principles of Good Clinical
Practice. These studies were approved by the Ethical Commission of
the Medical University of Lodz, Poland. Each patient was acquainted
with the aim of the study prior to being recruited to the research
program and provided informed, written consent to participate in
the study. The characteristics of the patients are shown in
Table I.
 | Table ICharacteristics of patients enrolled
in the studies and the number of collected biopsy specimens from
the antrum and corpus. |
Table I
Characteristics of patients enrolled
in the studies and the number of collected biopsy specimens from
the antrum and corpus.
| Group | Selection
criterion | Gender (F/M) | No. of biopsy
specimens (A/C) | Age mean ± SD, range
(years) |
|---|
| I | No family history of
gastric cancer | 18 (6/12) | 108 (54/54) | 34.1±11.2
8–53 |
| II | Family history of
gastric cancer | 20 (7/13) | 120 (60/60) | 39.6±13.7
8–60 |
Bioptates
During endoscopic biopsy, 6 bioptates were collected
from each patient: 3 from the antrum and 3 from the corpus (in
total, 228 biopsy specimens). Of these, 2 samples from each
topographical site were used for molecular biology screenings (to
determine the level of expression of the selected genes) and the
remaining samples were used for histopathological assessment. The
gastric mucosa specimens were removed from the antrum at 3–5 cm
proximally from the pylorus and from the corpus at 5–8 cm distally
from the cardia.
Histopathological assessments
Histopathological assessments were performed using
hematoxylin-eosin and Giemsa staining. For a given patient, two
paraffin-embedded tissue blocks (from the antrum and corpus) were
used for a microscopic section preparation (76 samples). A total of
2 sections were obtained from each paraffin block. The microscopic
gastric mucosa assessment was based on a scale according to the
modified Sydney System classification (15).
Isolation of total RNA from biopsies
A biopsy sample was placed in a labeled sterile tube
and stored for further studies at -70°C for 2–4 weeks. Defrosted
material was washed three times with PBS buffer without
Ca2+ and Mg2+ ions. After washing, the
specimen was transferred to a manual homogenizer and 1 ml of lysing
reagent (TriPure Isolation Reagent, Mannheim, Germany) was added.
Total RNA was isolated from the homogenate according to the
manufacturer's instructions for TriPure Isolation Reagent. This
fraction was then used for determination of the level of expression
of the selected genes.
Real time RT-PCR
The level of HINT1 and GAPDH mRNAs in a biopsy
specimen was determined by a real time quantitative reverse
transcription polymerase chain reaction (qRT-PCR). Samples were
analyzed blind to the FHGC status. The primers (listed in Table II) were designed with the Primer3
program available at: http://frodo.wi.mit.edu/cgibin/primer3/primer3_www.egi.
Isolated total RNA (200 ng/2 μl), and each primer (1 μl of 3 μM
solution) was added to the enzymatic reaction (6 μl) containing LC
RT-PCR reaction mix SYBR-Green I (2 μl), LC RT-PCR enzyme mix (0.2
μl), MgCl2 (5 mM, 0.8 μl) stock solution and PCR-grade
water (Roche Applied Science, Mannheim, Germany). Amplification
conditions were as follows: reverse transcription of the RNA
template at 55°C for 10 min, deactivation of the reverse
transcriptase at 95°C for 30 sec; amplification of cDNA:
denaturation (95°C for 0 sec), annealing (56°C for 15 sec) and
extension (72°C for 13 sec). The products were identified by the
thermal dissociation method and electrophoresis in 2% agarose gel.
The melting temperatures (Tm ± SD) for the amplification products
of GAPDH and HINT1 genes were 84.86±0.27 and
80.42±0.29°C, respectively.
 | Table IIPrimers used for amplification of
HINT1 and GAPDH genes. |
Table II
Primers used for amplification of
HINT1 and GAPDH genes.
| Amplified gene | Accession number | Primer sequence | Amplicon size
(bp) |
|---|
| HINT1 | NM_005340.5 | F: 5′-CCG CAA GGA AAT
ACC AG-3′
R: 5′-GTG TCC AAG AAG ACT TTC ATC-3′ | 160 |
| GAPDH | NM_002046.3 | F: 5′-CAT CAT CTC TGC
CCC CTC TG-3′
R: 5′-TCC ACG ATA CCA AAG TTG TC-3′ | 159 |
Statistical analysis
The level of HINT1 gene expression was
normalized to the level of GAPDH mRNA. Data are given as a ratio of
HINT1 to GAPDH products (HINT1/). The Shapiro-Wilk W test
was used to analyze the normality of the data distribution. The
differences between two independent groups of data were calculated
using the non-parametric Mann-Whitney U test. Histopathological
assessment data were analyzed according to the Fisher's exact test.
Statistical analyses were carried out using Statistica ver. 8.0
software (StatSoft Inc., Tulsa, OK, USA). P<0.05 was considered
to be statistically significant.
Results
Histopathological analysis of biopsy
specimens
Histo- pathological microscopic examination of
hematoxylin-eosin- and Giemsa-stained samples from the antrum and
the corpus (taken from both groups of patients) was performed
according to the modified Sydney System scale. The results
(Table III) show that the samples
from patients without FHGC (the control group) predominantly
exhibited non-atrophic changes (approximately 90%). Atrophic
changes were observed only in four biopsy specimens obtained from
the antrum. None of the control patients had atrophic changes in
the corpus. Results of the statistical analysis with the Fisher's
exact test showed that atrophic changes occur more frequently in
patients with FHGC in the antrum (40%) and corpus (20%) samples
(p=0.0081 and p=0.0057, respectively).
 | Table IIIResults of histopathological
examination of 76 samples of gastric mucosa from 18 patients of
Group I and 20 patients of Group II a. |
Table III
Results of histopathological
examination of 76 samples of gastric mucosa from 18 patients of
Group I and 20 patients of Group II a.
| Selection
criterion | Number of bioptates
from antrum/corpus (%) |
|---|
|
|
|---|
| Non-atrophy | Atrophy |
|---|
| Group I (without
FHGC) | 16/18
(88.9/100.0) | 2/0 (11.1/0.0) |
| Group II (with
FHGC) | 12/16
(60.0/80.0) | 8/4 (40.0/20.0) |
Level of HINT1 mRNA in gastric mucosa
specimens
The level of HINT1 protein mRNA in biopsy specimens
(2 samples from the corpus and 2 from the antrum of each patient,
total 152 samples) was determined by the real time RT-PCR analysis.
GAPDH was used as a reference gene. The results are
expressed as a ratio of the amounts of HINT1 mRNA to GAPDH mRNA.
For each sample the amplification reaction was performed in
duplicate. The results were statistically analyzed in respect to
histopathological changes, stomach topography and FHGC.
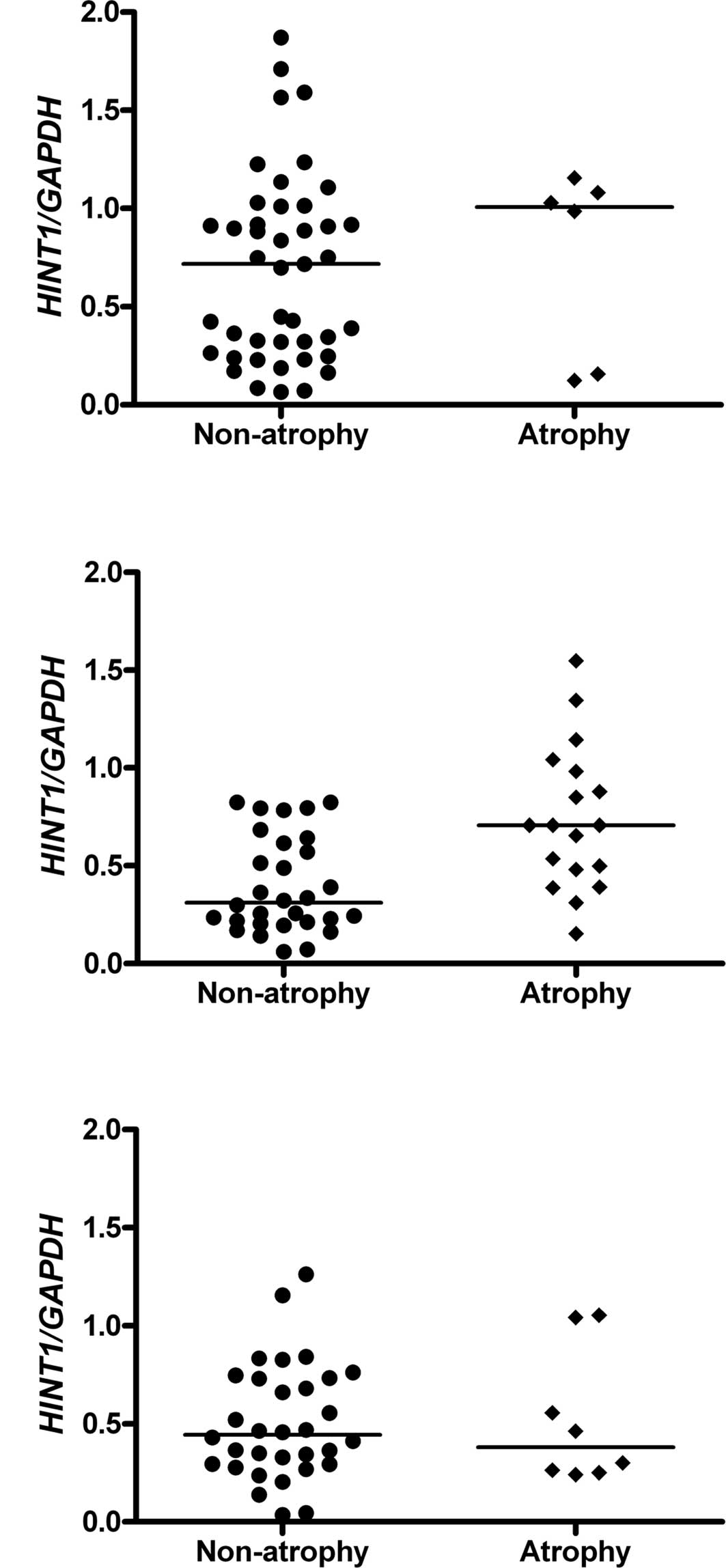
HINT1 mRNA level versus histopathological
changes
Only non-atrophic and atrophic changes as per the
Sydney System scale were considered. No statistically significant
differences were observed between the mRNA levels of HINT1 of the
non-atrophic and atrophic samples taken from the antrum of the
control patients (Fig. 1A) and the
corpus of the FHGC patients (Fig.
1C) due to the limited number of samples with atrophy. The
samples from the corpus of Group I patients were not analyzed due
to the lack of atrophic changes. Notably, the level of expression
of HINT1 gene was substantially higher in the atrophic
samples taken from the antrum of patients of Group II (with FHGC)
compared to the non-atrophic samples (Fig. 1B) (p=0.001040). The samples with
atrophic changes were excluded from further analysis due to the
statistically significant increase of their HINT1 mRNA levels.
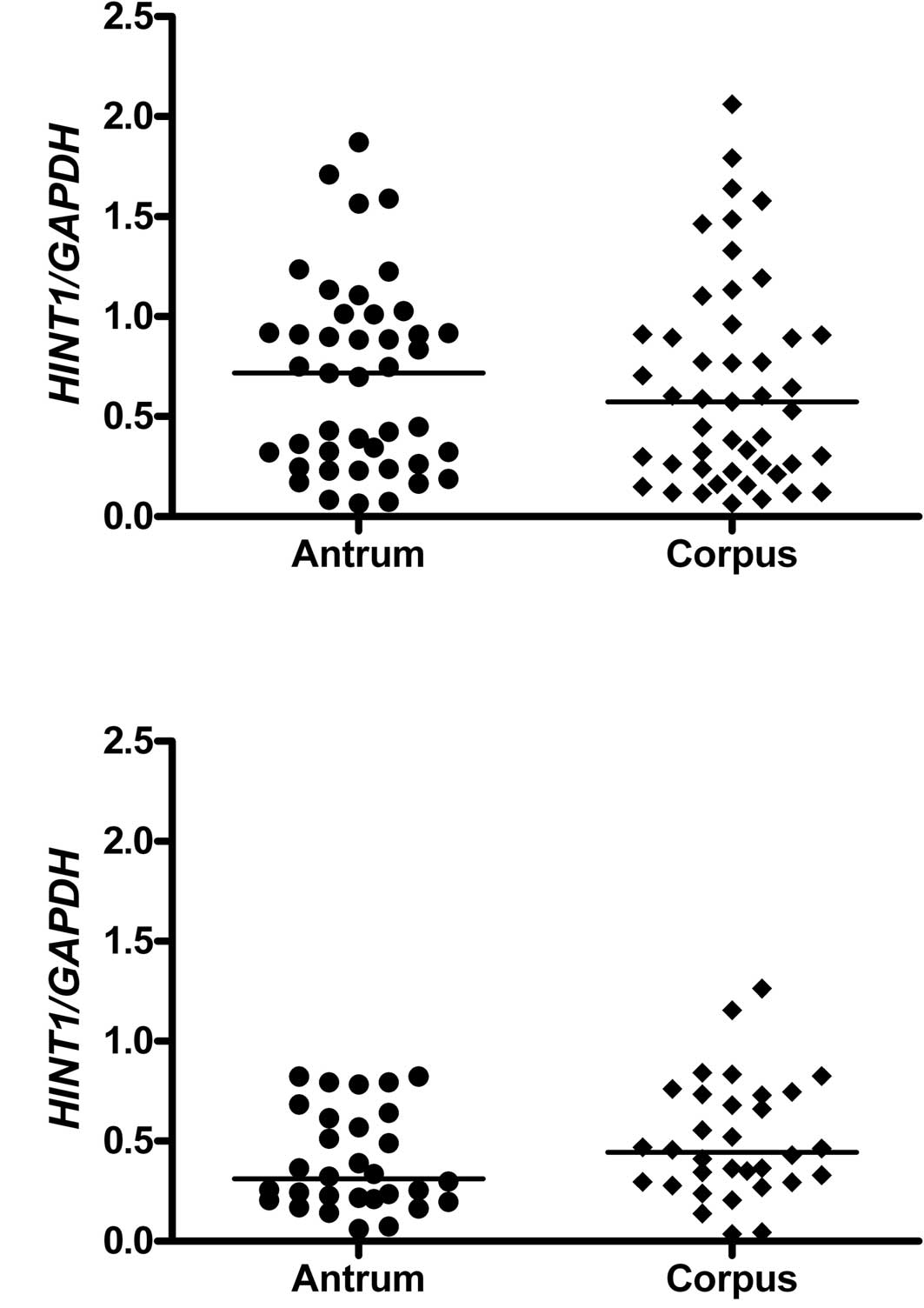
HINT1 mRNA level versus stomach
topography
Analysis of the samples with non-atrophic changes
(from both groups of patients) in respect to their site of origin
(antrum versus corpus) revealed that the level of HINT1 protein
mRNA was independent of the stomach topography (Fig. 2A and B, respectively).
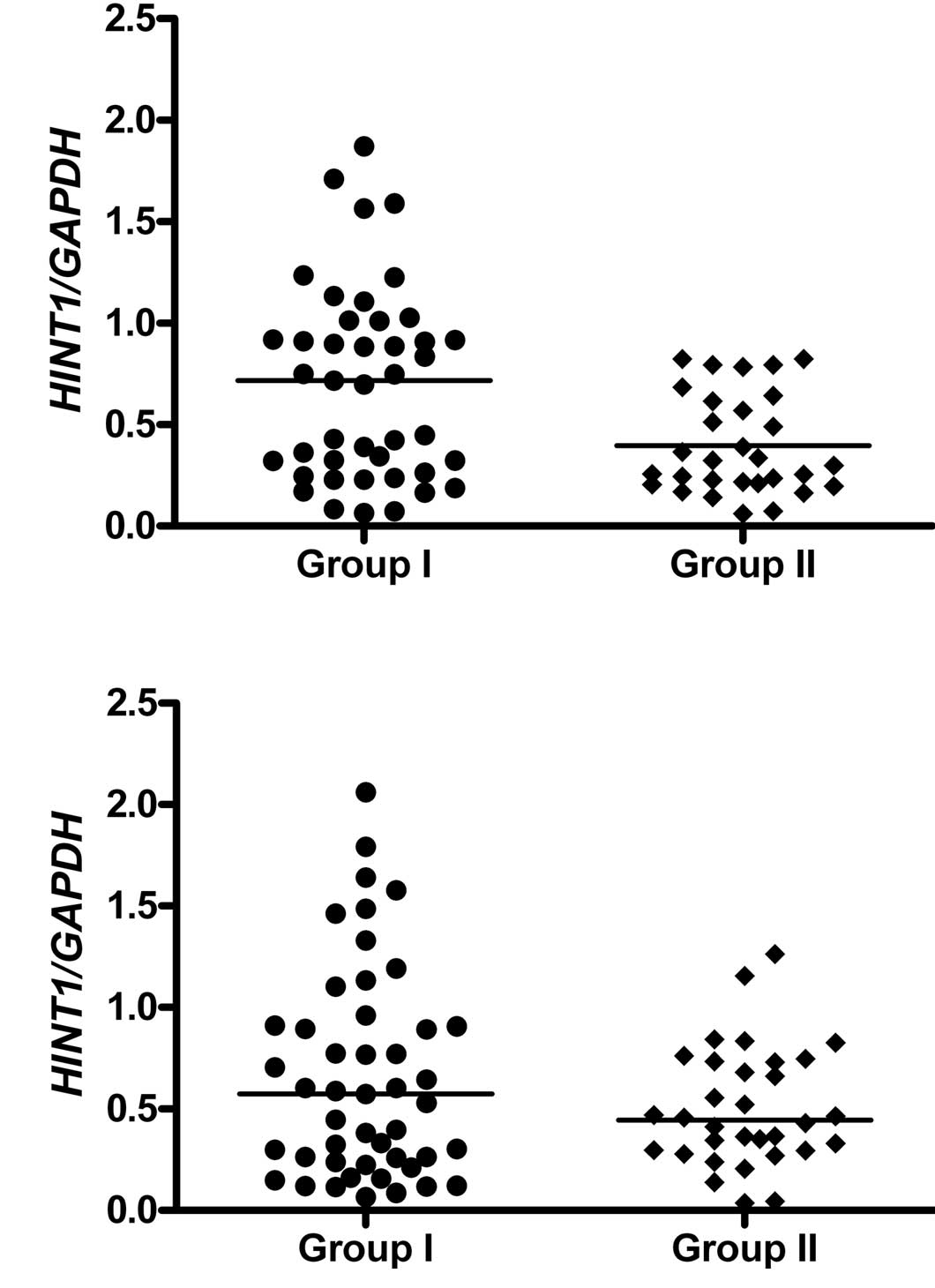
HINT1 mRNA level versus family history of
gastric cancer
Analysis of the samples from the antrum revealed
that the level of HINT1 mRNA was lower for patients with FHGC
compared to the control (p=0.003959) (Fig. 3A). Conversely, in samples from the
corpus the difference between levels of HINT1 mRNA between the two
groups was statistically non-significant (p=0.472116) (Fig. 3B).
Discussion
The HINT1 protein plays an inhibitory role in a
number of gene transcription control pathways. This protein
inhibits the activity of cyclin-dependent kinase 7,
microphthalmia-associated transcription factor, the transcription
factor USF2, the Pontin/Reptin/β-catenin/TCF4 complex and the AP-1
transcription factor (16–18).
In their study, Wang et al proposed that, the
HINT1 protein acts as a tumor suppressor due to the inhibitory
activity towards transcription factors (12). It has been demonstrated that after
association to complex of the plenty of SH3 domains protein (POSH)
and c-Jun NH(2)-terminal kinase
(POSH-JNK2), HINT1 inhibits activity of the AP-1 transcription
factor responsible for proliferation and angiogenesis of colon
cancer cells. A lower expression of HINT1 has been observed in
colon, breast, lung and liver cancers (12). In our study, the level of HINT1 mRNA
is statistically higher in gastric mucosa with atrophic changes in
comparison with non-atrophic changes, and only in samples from the
antrum, independently of FHGC (Fig.
1B). In the context of the widely accepted hypothesis that
atrophic changes described by the Correa cascade are critical in
gastric carcinogenesis, this observation indicates that elevated
levels of HINT1 are a response from gastric mucosa cells in defense
against the development of distal gastric cancer. Another study has
examined the pro-apoptotic function of HINT1 (10). Decreased HINT1 levels are observed
in breast and colon cancers, in which expression of the
pro-apoptotic Bax protein is up-regulated and that of
anti-apoptotic Bcl-2 protein is down-regulated, whereas silencing
of HINT1 results in decreased biosynthesis of Bax and p53 proteins.
An increased expression of HINT1 in human hepatoma cells, achieved
by inhibition of DNA methylation, has been shown to markedly
inhibit their growth (14).
Treatment of HINT1−/− mice with NBCA
(N-nitrosomethylbenzylamine), a known carcinogenic agent, induced
four adenomas and one adenocarcinoma of the glandular stomach. This
effect was not observed in HINT1+/+ mice (11).
Our analysis of the levels of HINT1 mRNA in the
corpus and antrum samples of patients with FHGC and the control
patients indicated a lack of dependence of HINT1 expression on
stomach topography (Fig. 2). On the
other hand, the level of HINT1 expression depends on the FHGC, and
the differences between the control and FHGC individuals reached
statistical significance for samples from the antrum, but not from
the corpus (Fig. 3). In this case,
the lowered level of HINT1 indicates increased susceptibility to
GC.
Since HINT1 expression is lowered in GC tissues,
etiological factors such as H. pylori infection or promoter
hypermethylation may play a role in gastric tumorigenesis by
regulation of the HINT1 gene expression (20). Increased spontaneous tumor formation
has been observed in mice bearing a deletion of the HINT
gene (11). Moreover, it has been
proposed that defects caused by the loss of HINT1 may occur only
when FHIT protein (another member of the HIT family, which may
recognize HINT1 substrates in vivo) is also inactivated
(21). In earlier studies, we
demonstrated that genetic and environmental factors, including
FHGC, affect the level of expression of FHIT tumor suppressor
(6,7). In gastric mucosa of patients with FHGC
the FHIT gene expression was down-regulated in comparison to the
controls. Moreover, an increased level of FHIT gene promoter
methylation was associated with hereditary (FHGC) and environmental
factors (infection with H. pylori) and resulted in the
down-regulation of the FHIT gene transcription (22).
Therefore, a lowered level of expression of HINT1, especially in
the stomach antrum, may predispose towards the development of GC.
Further studies are in progress to determine the clinical relevance
of HINT1 expression in the prevention of prepyloric gastric
carcinoma in patients with a family history of gastric cancer.
Acknowledgements
These studies were supported by the Ministry of
Science and Higher Education, Poland, during the period 2009–2012
(project N N402 3073 36 for B.N.).
References
|
1
|
Oliveira C, Seruca R and Carneiro F:
Hereditary gastric cancer. Best Pract Res Clin Gastroenterol.
23:147–157. 2009. View Article : Google Scholar
|
|
2
|
Cisco RM, Ford JM and Norton JA:
Hereditary diffuse gastric cancer: implications of genetic testing
for screening and prophylactic surgery. Cancer. 113:1850–1856.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fitzgerald RC, Hardwick R, Huntsman D,
Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K,
Van Krieken JH, et al: Hereditary diffuse gastric cancer: updated
consensus guidelines for clinical management and directions for
future research. International Gastric Cancer Linkage Consortium. J
Med Genet. 47:436–444. 2010. View Article : Google Scholar
|
|
4
|
Oliveira C, Bordin MC, Grehan N, Huntsman
D, Suriano G, Machado JC, Kiviluoto T, Aaltonen L, Jackson CE and
Caldas C: Screening E-cadherin in gastric cancer families reveals
germline mutations only in hereditary diffuse gastric cancer
kindred. Hum Mutat. 19:510–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishii H, Ozawa K and Furukawa Y:
Alteration of the fragile histidine triad gene early in
carcinogenesis: an update. J Exp Ther Oncol. 3:291–296. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stec-Michalska K, Antoszczyk S, Klupinska
G and Nawrot B: Loss of FHIT expression in the gastric mucosa of
patients with family history of gastric cancer and Helicobacter
pylori infection. World J Gastroenterol. 11:17–21. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stec-Michalska K, Peczek L, Michalski B,
Wisniewska-Jarosinska M, Krakowiak A and Nawrot B: Helicobacter
pylori infection and family history of gastric cancer decrease
expression of FHIT tumor suppressor gene in gastric mucosa of
dyspeptic patients. Helicobacter. 14:126–134. 2009. View Article : Google Scholar
|
|
8
|
Baffa R, Veronese ML, Santoro R, Mandes B,
Palazzo JP, Rugge M, Santoro E, Croce CM and Huebner K: Loss of
FHIT expression in gastric carcinoma. Cancer Res. 58:4708–4714.
1998.PubMed/NCBI
|
|
9
|
Lee SH, Kim WH, Kim HK, Woo KM, Nam HS,
Kim HS, Kim JG and Cho MH: Altered expression of the fragile
histidine triad gene in primary gastric adenocarcinomas. Biochem
Biophys Res Commun. 284:850–855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weiske J and Huber O: The histidine triad
protein HINT1 triggers apoptosis independent of its enzymatic
activity. J Biol Chem. 281:27356–27366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su T, Suzui M, Wang L, Lin CS, Xing WQ and
Weinstein IB: Deletion of histidine triad nucleotide-binding
protein 1/PKC-interacting protein in mice enhances cell growth and
carcinogenesis. Proc Natl Acad Sci USA. 100:7824–7829. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Zhang Y, Li H, Xu Z, Santella RM
and Weinstein IB: HINT1 inhibits growth and activator protein-1
activity in human colon cancer cells. Cancer Res. 67:4700–4708.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Zhang Y, Su T, Santella RM and
Weinstein IB: HINT1 is a haplo-insufficient tumor suppressor in
mice. Oncogene. 25:713–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YJ, Li H, Wu HC, Shen J, Wang L, Yu
MW, Lee PH, Weinstein IB and Santella RM: Silencing of HINT1, a
novel tumor suppressor gene, by promoter hypermethylation in
hepatocellular carcinoma. Cancer Lett. 275:277–284. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated Sydney
System International Workshop on the Histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996.PubMed/NCBI
|
|
16
|
Korsisaari N and Makela TP: Interactions
of Cdk7 and Kin28 with Hint/PKCI-1 and Hnt1 histidine triad
proteins. J Biol Chem. 275:34837–34840. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weiske J and Huber O: The histidine triad
protein HINT1 interacts with Pontin and Reptin and inhibits
TCF-beta-catenin- mediated transcription. J Cell Sci.
118:3117–3129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Li H, Zhang Y, Santella RM and
Weinstein IB: HINT1 inhibits beta-catenin/TCF4, USF2 and NFkappaB
activity in human hepatoma cells. Int J Cancer. 124:1526–1534.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Wei X, Su X, Qiao F, Xu Z, Gu D,
Fan H and Chen J: Clinical significance of expression of
Hint1 and potential epigenetic mechanism in gastric cancer.
Int J Oncol. 38:1557–1564. 2011.
|
|
20
|
Guranowski A, Wojdyła AM, Pietrowska-Borek
M, Bieganowski P, Khurs EN, Cliff MJ, Blackburn GM, Błaziak D and
Stec WJ: Fhit proteins can also recognize substrates other than
dinucleoside polyphosphates. FEBS Lett. 582:3152–3158. 2008.
View Article : Google Scholar
|
|
21
|
Stec-Michalska K: Clinical aspects of
changes of the level of expression of selected proapoptotic genes
in the gastric mucosa of patients with family history of gastric
cancer (habilitation dissertation). Medical University of Lodz;
Poland: 2010
|