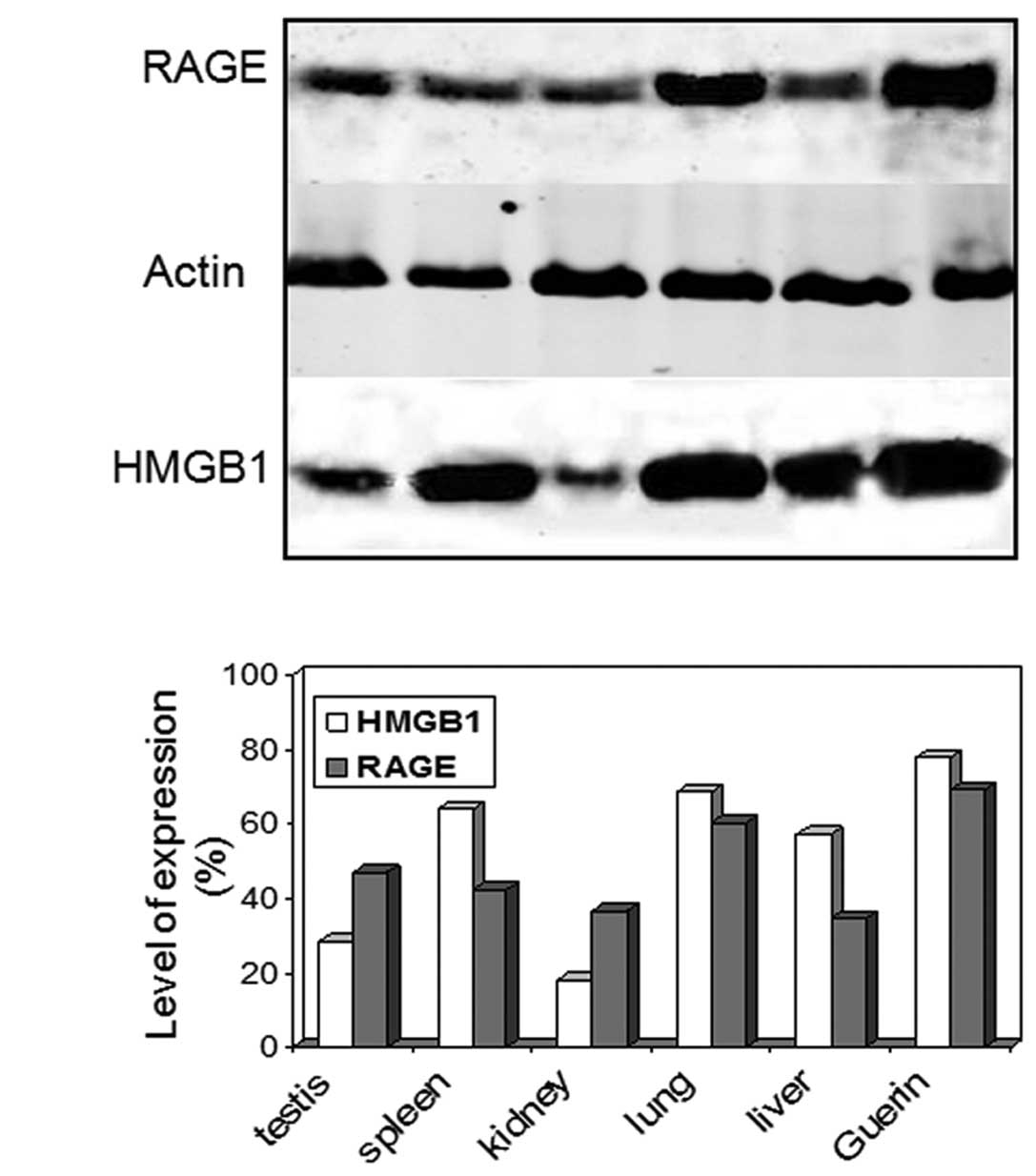
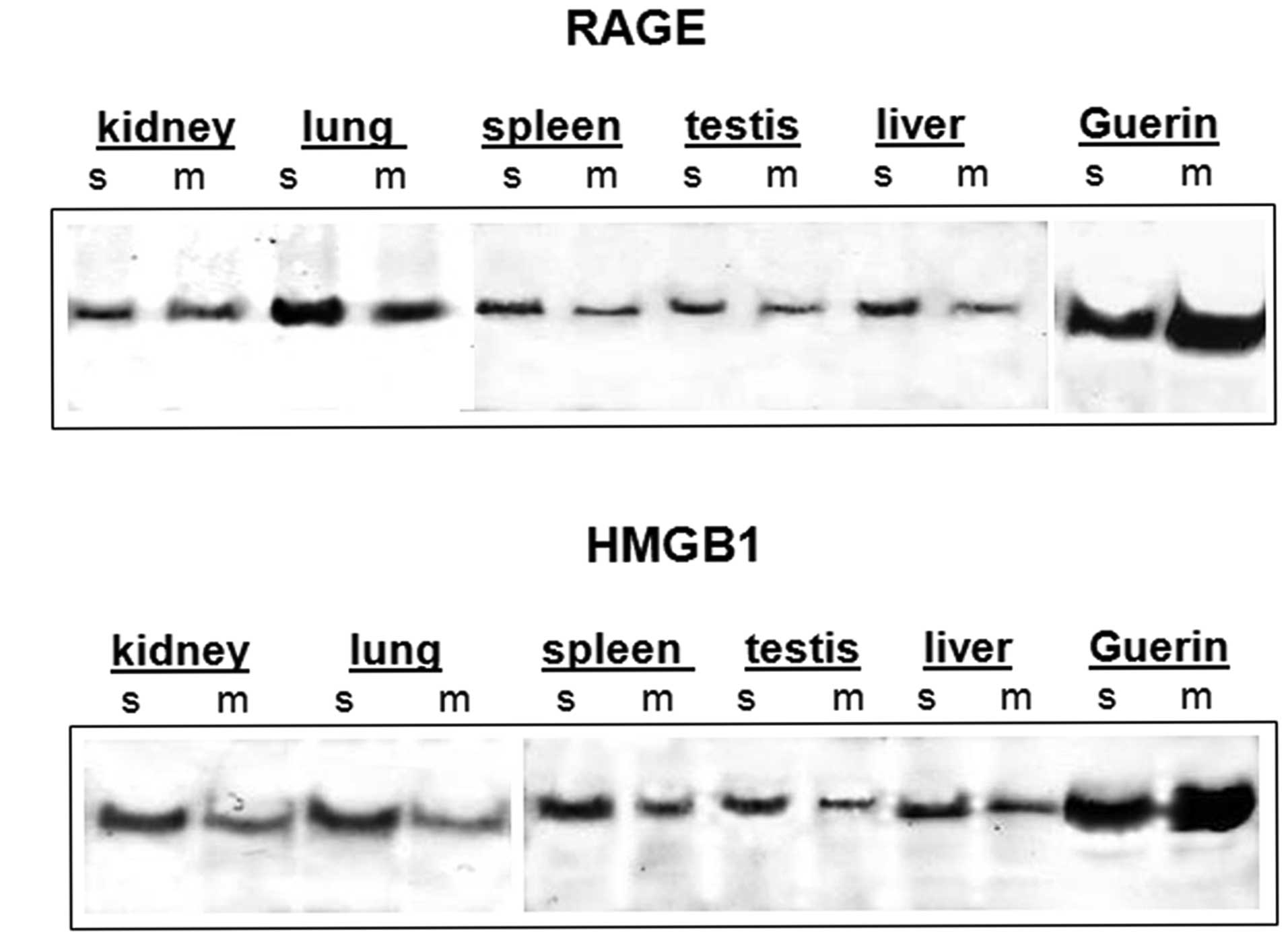
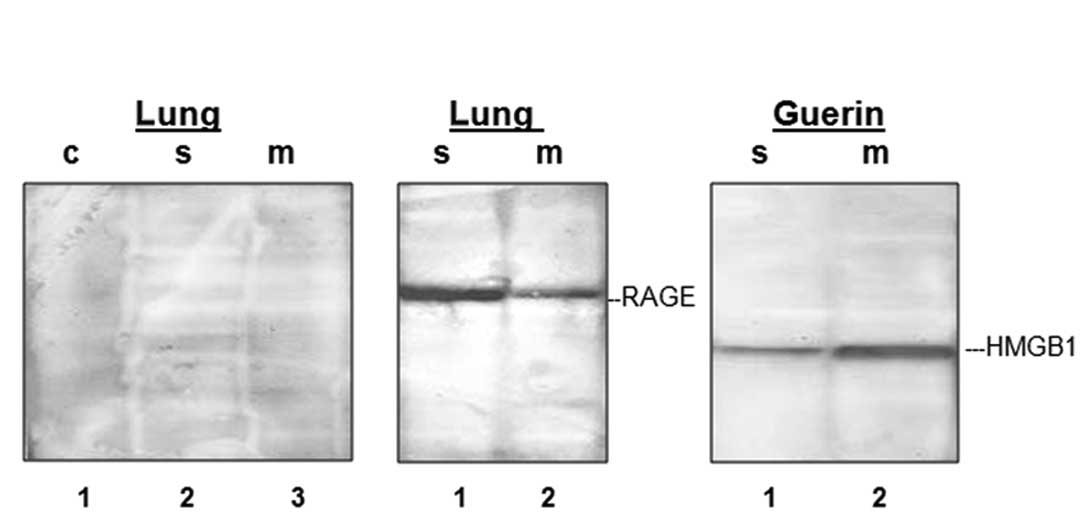
|
1
|
Thomas JO and Travers A: HMG1 and 2, and
related ‘architectural’ DNA-binding proteins. Trends Biochem
Science. 26:167–174. 2001.
|
|
2
|
Bianchi ME, Bertrame M and Paonessa G:
Specific recognition of cruciform DNA by nuclear protein HMG1.
Science. 243:1056–1059. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pil PM and Lippard SJ: Specific binding of
chromosomal protein HMG1 to DNA damaged by the anticancer drug
cisplatin. Science. 256:234–237. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pasheva EA, Pashev IG and Favre A:
Preferential binding of high mobility group 1 protein to UV-damaged
DNA. Role of the COOH-terminal domain. J Biol Chem.
273:24730–24736. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paull T, Hakynson MJ and Johnson RC: The
nonspecific DNA-binding and bending proteins HMG1 and HMG2 promote
the assembly of complex nucleoprotein structures. Genes Dev.
7:1521–1534. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onate SA, Prendergast P, Wagner JP, Nissen
M, Reeves R, Pettijhon DE and Edwards DP: The DNA-bending protein
HMG-1 enhances progesterone receptor binding to its target DNA
sequences. Mol Cell Biol. 14:3375–3391. 1994.PubMed/NCBI
|
|
7
|
Ugrinova I, Zlateva S, Pashev IG and
Pasheva EA: Native HMGB1 protein inhibits repair of
cisplatin-damaged nucleosomes in vitro. Int J Biochem Cell Biol.
41:1556–1562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Topalova D, Ugrinova I, Pashev IG and
Pasheva EA: HMGB1 protein inhibits DNA replication in vitro: a role
of the acetylation and the acidic tail. Int J Biochem Cell Biol.
40:1536–1542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonaldi T, Langst G, Strohner R, Becker PB
and Bianchi ME: The DNA chaperone HMGB1 facilitates ACF/CHRAC
dependent nucleosome sliding. EMBO J. 21:6865–6873. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ugrinova I, Pashev IG and Pasheva EA:
Nucleosome binding properties and co-remodeling activities of
native and in vivo acetylated HMGB-1 and HMGB-2 proteins.
Biochemistry. 48:6502–6507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L,
Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina
PE, Abumrad NN, Sama A and Tracey KJ: HMG-1 as a late mediator of
endotoxin lethality in mice. Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andersson U, Wang H, Palmblad K, Aveberger
AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M,
Yang H and Tracey KJ: High mobility group 1 protein (HMG-1)
stimulates proinflammatory cytokine synthesis in human monocytes. J
Exp Med. 192:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Degryse B and De Virgilio M: The nuclear
protein HMGB1, a new kind of chemokine. FEBS Lett. 553:11–17. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonaldi T, Talamo F, Scaffidi P, Ferrera
D, Porto A, Bachi A, Rubartelli A, Agresti A and Bianchi ME:
Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect
it towards secretion. EMBO J. 22:5551–5560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youn JH and Shin JS: Nucleocytoplasmic
shuttling of HMGB1 is regulated by phosphorylation that redirects
it toward secretion. J Immunol. 177:7889–7897. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito I, Fukuzawa J and Yoshida M:
Post-translational methylation of high mobility group box 1 (HMGB1)
causes its cytoplasmic localization in neutrophils. J Biol Chem.
282:16336–16344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ditsworth D, Zong WX and Thompson CB:
Activation of poly(ADP)-ribosepolymerase (PARP-1) induces release
of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol
Chem. 282:17845–17854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ellerman JE, Brown CK, De Vera M, Zeh HJ,
Billiar T, Rubartelli A and Lotze M: Masquerader: high mobility
group box-1 and cancer. Clin Cancer Res. 13:2836–2848. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasahira T, Kirita T, Oue N, Bhawal UK,
Yamamoto K, Fujii K, Ohmori H, Luo Y, Yasui W, Bosserhoff AK and
Kuniyasu H: High mobility group box-1-inducible melanoma inhibitory
activity is associated with nodal metastasis and lymphangiogenesis
in oral squamous cell carcinoma. Cancer Sci. 99:1806–1812.
2008.PubMed/NCBI
|
|
21
|
Fages C, Nolo R, Huttunen H, Eskelinen E
and Rauvala H: Regulation of cell migration by amphoterin. J Cell
Science. 113:611–620. 2000.
|
|
22
|
Taguchi A, Blood DC, Del Toro G, Canet A,
Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger
T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM and Schmidt
AM: Blockade of RAGE-amphoterin signalling suppresses tumour growth
and metastases. Nature. 405:354–360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuniyasu H, Chihara Y and Takahashi T:
Co-expression of receptor for advanced glycation end products and
the ligand amphoterin associates closely with metastasis of
colorectal cancer. Oncol Rep. 10:445–448. 2003.PubMed/NCBI
|
|
24
|
Bierhaus A, Humpert P, Morcos M, Wendt T,
Chavakis T, Arnold B, Stern D and Nawroth P: Understanding RAGE,
the receptor for advanced glycation end products. J Mol Med.
83:876–886. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sasahira T, Akama Y, Fujii K and Kuniyasu
H: Expression of receptor for advanced glycation end products and
HMGB1/amphoterin in colorectal adenomas. Virchows Arch.
446:411–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kostova N, Zlateva S, Ugrinova I and
Pasheva E: The expression of HMGB1 protein and its receptor RAGE in
human malignant tumors. Mol Cell Biochem. 337:251–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dignam JD: Preparation of extracts from
higher eukaryotes. Methods Enzymol. 182:194–203. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prasad S and Thakur MK: Distribution of
high mobility group proteins in different tissues of rats during
aging. Biochem Int. 20:687–695. 1990.PubMed/NCBI
|
|
29
|
Muller S, Ronfani L and Bianchi M:
Regulated expression and subcellular localization of HMGB1, a
chromatin protein with a cytokine function. J Int Med. 255:332–343.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flohr A, Rogalla P, Meiboom M, Borrmann L,
Krohn M, Thode-Halle B and Bullrdiek J: Variation of HMGB1
expression in breast cancer. Anticancer Res. 21:3881–3885.
2001.PubMed/NCBI
|
|
31
|
Bartling B, Hofmann H, Weiglel B, Silber S
and Simm A: Down-regulation of the receptor for advanced glycation
end-products (RAGE) supports non-small cell lung carcinoma.
Carcinogenesis. 26:293–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Merenmies J, Pihlaskari R, Laitinen J,
Wartiovaarall J and Rauvala H: 30-kDa heparin-binding protein of
brain (amphoterin) involved in neurite outgrowth. J Biol Chem.
266:16722–16729. 1991.PubMed/NCBI
|
|
33
|
Sparvero L, Asafu-Adjei D, Kang R, Tang D,
Amin N, Im J, Rutledge R, Lin B, Amoscato A, Zeh H and Lotze M:
RAGE (Receptor for Advanced Glycation End products), RAGE ligands
and their role in cancer and inflammation. J Transl Med. 7:17–39.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rouhiainen A, Imai S, Rauvala H and
Parkkinen J: Occurrence of amphoterin (HMG1) as an endogenous
protein of human platelets that is exported to the cell surface
upon platelet activation. Thromb Haemost. 84:1087–1094.
2000.PubMed/NCBI
|
|
35
|
Passalacqua M, Zicca A, Sparatore B,
Patrone M, Melloni E and Pontremoli S: Secretion and binding of
HMG1 protein to the external surface of the membrane are required
for murine erythroleukemia cell differentiation. FEBS Lett.
400:275–279. 1997. View Article : Google Scholar : PubMed/NCBI
|