Introduction
Epithelial ovarian cancer (EOC) (1) is the most common type of ovarian
cancer and the leading cause of gynecological cancer-related
mortality (2). It typically
develops as an insidious disease (1,3,4), with
few distinct symptoms until the tumor has become large or
disseminated (2). Thus, EOC is not
usually diagnosed prior to reaching an advanced stage, when the
five-year survival rate is poor (1). Currently, cytoreductive surgery
combined with platinum-based chemotherapy is the standard treatment
for patients with ovarian cancer (5). However, in patients of child-bearing
age, cytoreductive surgery for a malignant ovarian tumor frequently
results in the loss of ovarian function and menopausal symptoms.
The symptoms of iatrogenic menopause are usually significantly more
intense than those of natural menopause, due to the sudden onset of
symptoms at a younger age and their effects on common physical and
psychological problems of cancer therapy, including body image
issues and sexual dysfunction (6).
The oncologist may consider hormone replacement therapy (HRT) for
these patients, but concerns regarding the safety of HRT following
ovarian malignancy have rendered the advisability of its
post-surgical use controversial.
Concerns center primarily on the potential
stimulation of residual cancer by HRT and the induction of new
hormone-dependent disease (7).
Consistent with the female genital tract being a target organ of
hormones, epidemiological investigations have suggested that
malignancies of the genital tract may be associated with hormonal
stimuli, and significantly higher risks for breast, endometrial and
ovarian epithelium cancer have been observed in post-menopausal
women ingesting long-term oral estrogen (8–11).
In vitro experiments have yielded inconsistent results
regarding the estrogen stimulation of cancer cell proliferation.
Certain in vitro experiments have shown that estrogen is
capable of stimulating the proliferation of malignant cells
(12,13), whereas results of other studies
showed tumor cell growth inhibition by estrogen (14), and yet other authors found no effect
of estrogen on malignant cell growth (15,16).
Furthermore, current scientific evidence does not show HRT to
adversely affect outcome in patients following treatment for
ovarian malignancy (6,16).
Since maintaining quality of life and minimizing the
physical and psychological impacts of treatment side effects are
crucial factors in cancer care, it is imperative to provide
patients with unbiased information regarding whether their
individual cancer status allows them to use HRT without any
detrimental effects on their survival. However, currently there is
no sufficient evidence from large-scale multicenter random
prospective clinical trials to clearly indicate that HRT is safe
and that the prognosis and tumor recurrence rate may not be
affected by HRT. This study analyzed the effect of post-operative
HRT on the prognosis and relevant clinical factors in patients with
ovarian cancer.
Patients and methods
Patients
The medical cases included in this study comprised
inpatients/outpatients with a pathologically confirmed diagnosis of
ovarian cancer who were registered at the Department of
Gynecological Oncology of the Cancer Hospital of Guangxi Medical
University, China, between August 1999 and June 2003. The study was
endorsed by the Ethics Committee of the Guangxi Medical University.
The patients received an explanation of the aims of the study,
provided signed informed consent and understood that they had the
option of withdrawing from the study at any time without affecting
their oncological or general medical treatment. The patients
received cytoreductive surgery (total hysterectomy plus bilateral
appendix resection), followed by 6–8 courses of platinum-based
combination chemotherapy.
At 20 days following cytoreductive surgery, 90
patients were randomly divided into a HRT group and a non-HRT group
(n=45 each), using the envelope method. A total of 15 patients were
lost at follow-up or were non-compliant within 6 months. Of the
remaining 75 patients, 31 patients, with an average age of 40.3
years (range 20–45), were in the HRT group. These patients included
21 cases of serous cystadenocarcinoma and 10 cases of mucinous
cystadenocarcinoma, 11 of which were International Federation of
Gynecology and Obstetrics (FIGO) stage Ib-II, and 20 of which were
stage III. In the non-HRT group, the 44 patients, with an average
age of 42.9 years (range 20–45), presented with 26 serous
cystadenocarcinoma cases and 18 mucinous cystadenocarcinoma cases,
10 of which were FIGO stage Ib-II and 34 of which were stage III.
No significant difference was found with regard to age,
pathological type, differentiation level, clinical stage or
treatment the between HRT and non-HRT groups.
The patients were regularly reviewed every 6 months
by general examination, pelvic examination, CA125 assay, liver and
kidney function tests, pelvic ultrasound scan and breast check.
Follow-up lasted from 10 to 43 months (average 31.4 months).
Another 77 female individuals who were visiting the
hospital for a routine health examination were selected as controls
for the questionnaire study on quality of life. These individuals
were all post-menopausal, 53–67 years of age, and had never been
treated with HRT.
HRT
The 31 patients in the HRT group were randomly
divided into two treatment groups of similar age, clinical stage
and pathological type. One treatment group (n=14) received Premarin
0.625 mg/d + medroxyprogesterone 4 mg/d; the other (n=17) received
nylestriol 2.5 mg/15 d + medroxyprogesterone 4 mg/d. HRT continued
for 6–43 months (average 28.7), with no significant difference in
the treatment period between the two groups (Chi-square test,
p>0.05). To encourage compliance, certain oncologists,
independent of this study, were assigned to guide the patients
throughout the treatment period. Patients lost to follow-up and
those who were non-compliant within 6 months were termed as
withdrawn and were not included in the results and analysis.
Specimen collection and processing
The first blood sample (fasting) was obtained 1 week
following the end of menses, in the morning prior to surgery. The
second blood sample (fasting) was drawn 20 days following the
operation. At that point, HRT was commenced. The third blood sample
(fasting) was obtained 6 months or 1 year after HRT had begun. The
serum was separated by centrifugation and stored at −20˚C until
analyzed for serum calcitonin (CT) and transforming growth factor
(TGF)-α.
Tissue samples obtained during surgery were
paraffin-embedded and sectioned using a microtome. One slide was
stained with hematoxylin and eosin for histopathological
examination, and three additional slides were prepared for
immunohistochemical staining of estrogen receptor (ER)-α, ERβ and
progesterone receptor (PR), respectively.
Determination of CT and TGFα
Serum CT was determined using a CT RIA kit (Beijing
Meidike Biotechnology, Beijing, China), and TGFα was determined
using an ELISA kit (Promega, Madison, WI, USA), according to the
respective manufacturers’ instructions.
Detection of ERα, ERβ and PR
expression
Immunohistochemical staining of tissue slides for
the expression of ERα, ERβ and PR was performed using a
streptavidin-peroxidase staining kit (Fuzhou Maixin Biotechnology
Development, Fuzhou, China) according to the manufacturer’s
instructions. Anti-PR antibody was purchased from Fuzhou Maixin
Biotechnology Development. Anti-ERα and -ERβ antibodies were
provided by Professor Lihui Wei (People’s Hospital, Beijing
University, Beijing, China).
The immunohistochemical staining intensity of tumor
and endothelial cells of the tissues was scored in a blinded manner
by two experienced pathologists, using the following scale: 3,
intracytoplasmic granules strongly stained brown; 2,
intracytoplasmic granules stained a medium brown; 1,
intracytoplasmic granules weakly stained light brown; and 0,
intracytoplasmic granules not stained distinctly compared with
cytoplasm. The cells were counted under a high-power field, and the
rate of positive cells was calculated as the number of positive
cells divided by the total number of cells. The positive rates were
assigned points as follows: <5%, 0 points; 6–25%, 1 point;
26–50%, 2 points; 51–75%, 3 points; and >75%, 4 points. The
staining intensity score was multiplied by the positive rate points
to yield the total score for each field. For each slide, five
high-power fields were examined, and the average of the five total
scores was taken as the final score. Slides with a final score of
0–2 were considered to be negative, and slides with a final score
>2 were considered to be positive.
Assessment of quality of life
Patient quality of life was assessed using two
questionnaires: the EORTC-C30, developed by the European
Organization for Research and Treatment of Cancer (17), and the GMU-Gynae Index (18), a specific life quality questionnaire
developed by the Department of Gynecology, Guangxi Medical
University, China. The EORTC-C30 consists of five functional
sub-categories (five body function categories, two role categories,
two cognition categories, four emotion categories and two social
function categories), three symptom subscales (fatigue, pain,
nausea and vomiting, shortness of breath, insomnia, loss of
appetite, constipation and diarrhea), and a scale of general health
status. The GMU-Gynae Index assesses sexual life quality (sexual
difficulties, emotional exchange between the couple, regression of
sexual life and sexual desire), symptoms of lower urinary tract
infection (urethral burning and frequent urination), autonomic
dysfunction (itchy skin, dry skin and formication).
Following 6–12 months of HRT, the patients in the
HRT and non-HRT groups were asked by their assigned oncologists to
personally respond to the questions. Spouses were allowed to
substitute only when the patient was incapacitated.
Statistical data analysis
Survival data were analyzed using Kaplan-Meier
survival curves and the log-rank test. The Student’s t-test was
used to analyze averaged data, and the Chi-square test was used for
ratio comparisons. All analyses were performed with SPSS.10
software (Statsoft, USA). P<0.05 was considered to be
statistically significant.
Results
Effect of HRT on the prognosis of
malignant ovarian cancer
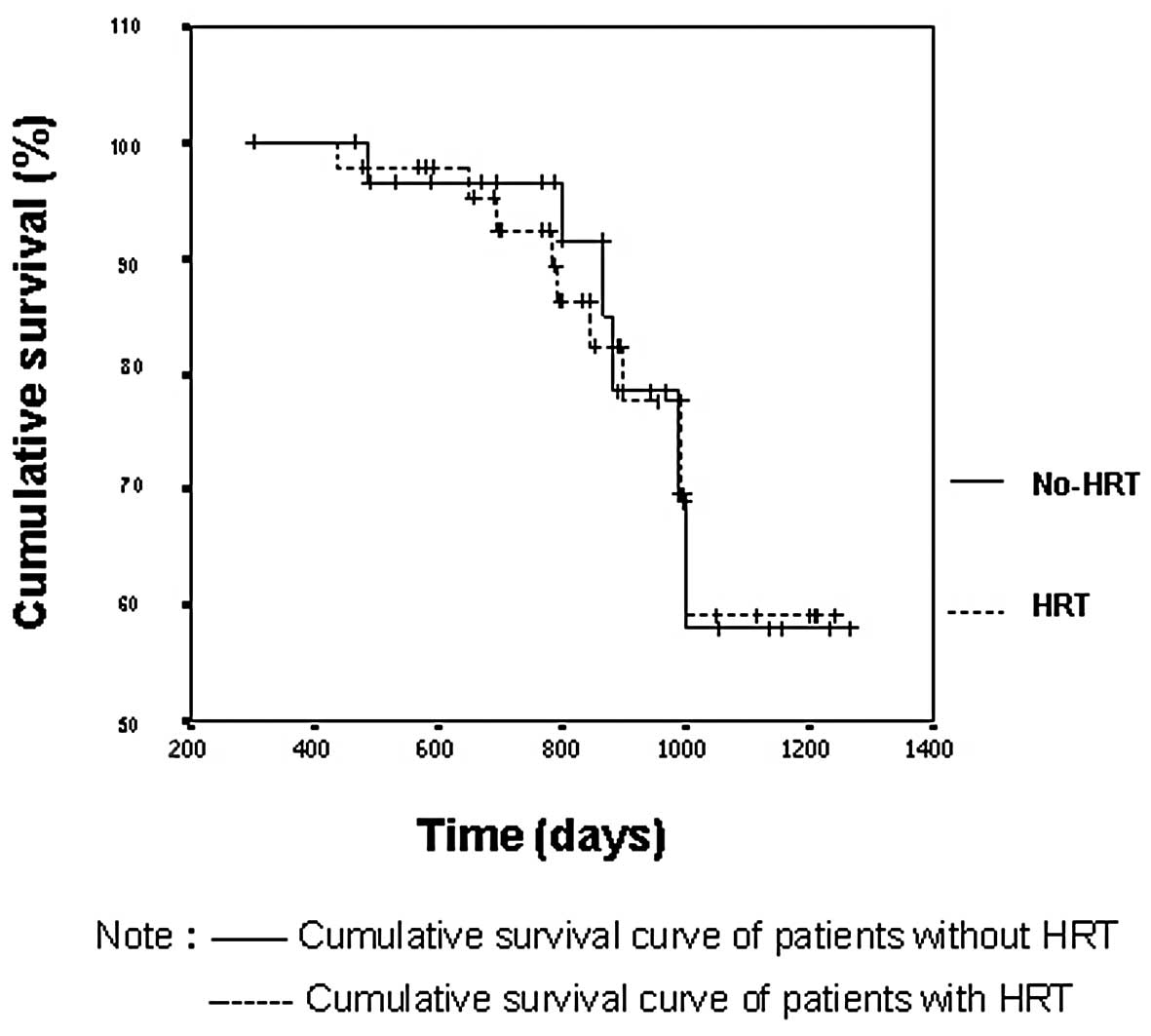
The Kaplan-Meier curves showed that the cumulative
survival was similar between the HRT and non-HRT groups. The
average survival period was 1108±52 days in the HRT group and
1086±43 days in the non-HRT group (Fig.
1), with no statistically significant difference between the
two groups (log-rank test, p=0.9399). No significant difference was
found in clinical stage or pathological type between the two groups
(log-rank test, p>0.05; Table
I).
 | Table IEffect of hormone replacement therapy
(HRT) on prognosis, according to pathology. |
Table I
Effect of hormone replacement therapy
(HRT) on prognosis, according to pathology.
| Pathology | Cases (n) | Average survival time
(days) | Mid-survival time
(days) | P-value |
|---|
| |
| |
|---|
| | HRT | Non-HRT | HRT | Non-HRT | |
|---|
| Type |
| Serous | 47 | 1134±34 | 1165±35 | 1097±62 | 1142±55 | 0.702 |
| Mucous | 28 | 1016±97 | 1031±73 | 986±152 | 994±67 | 0.678 |
| Stage |
| I–II | 21 | 1108±59 | 1084±46 | 1048±66 | 996±88 | 0.988 |
| III | 54 | 1062±62 | 1018±54 | 998±71 | 1000±61 | 0.767 |
Factors with a possible effect on the prognosis of
malignant ovarian tumors, including age, clinical stage,
pathological type, greater omental metastasis, retroperitoneal
lymph node metastasis, pleural effusion, ascites, HRT and
postoperative residual lesion size, were analyzed according to a
Cox model. At a level of a=0.05, only post-operative residual size
was identified as an influential factor in the Cox model.
No breast disease or breast cancer was observed in
either the HRT or non-HRT group at the end of follow-up.
Relationship between ERα, ERβ, and PR
expression and survival during HRT
The log-rank analysis revealed no significant
difference (p>0.05) in cumulative survival time among patients
with different ERα, ERβ and PR expression status (Table II).
 | Table IISurvival time in patients with and
without hormone replacement therapy (HRT), according to the
expression of estrogen receptor (ER)-α, ERβ and progesterone
receptor (PR). |
Table II
Survival time in patients with and
without hormone replacement therapy (HRT), according to the
expression of estrogen receptor (ER)-α, ERβ and progesterone
receptor (PR).
| Expression | Cases (n) | Average survival time
(days) | P-value |
|---|
| |
| |
|---|
| | HRT | Non-HRT | |
|---|
| ERα (+) | 60 | 1008±75 | 944±122 | 0.559 |
| ERα (−) | 15 | 966±99 | 961±89 | 0.493 |
| ERβ (+) | 44 | 1040±70 | 1102±86 | 0.856 |
| ERβ (−) | 31 | 980±100 | 957±114 | 0.852 |
| PR (+) | 53 | 1220±42 | 1101±54 | 0.351 |
| PR (−) | 22 | 955±93 | 936±87 | 0.912 |
Effect of HRT on serum TGFα and serum CT
levels
Although serum TGFα levels declined following
surgery in the HRT and non-HRT groups (p<0.05), these levels did
not differ significantly (p>0.05) between the two groups prior
to surgery, following surgery, or 6–12 months following surgery
(Table III).
 | Table IIIEffect of hormone replacement therapy
(HRT) on the serum transforming growth factor (TGF)-α levels. |
Table III
Effect of hormone replacement therapy
(HRT) on the serum transforming growth factor (TGF)-α levels.
| Treatment group | Cases (n) | Serum TGFα
(ng/ml) |
|---|
| |
|
|---|
| | Prior to surgery | Following
surgery | 6–12 months following
surgery |
|---|
| HRT | 31 | 30.0±22.6 | 12.7±7.3 | 12.6±9.8 |
| Non-HRT | 44 | 27.0±19.9 | 12.6±7.7 | 11.5±8.7a |
As shown in Table
IV, no significant difference was found in the serum CT levels
between the HRT and non-HRT groups prior to or following surgery
(p>0.05). At 6–12 months following surgery, the serum CT levels
in the HRT group was higher than those in the non-HRT group
(p<0.05). However, the serum CT levels in the HRT group did not
differ among the three time periods (p>0.05).
 | Table IVChanges in serum calcitonin (CT)
levels according to hormone replacement therapy (HRT). |
Table IV
Changes in serum calcitonin (CT)
levels according to hormone replacement therapy (HRT).
| Treatment
group | Cases (n) | Serum CT
(μg/l) |
|---|
| |
|
|---|
| | Prior to
surgery | Following
surgery | 6–12 months
following surgery |
|---|
| HRT | 31 | 93.43±14.38 | 91.91±10.52 | 90.09±18.46 |
| Premarin | 14 | 95.88±15.19 | 97.65±12.36 | 98.14±10.63 |
| Nylestriol | 17 | 92.40±16.57 | 93.18±10.01 | 91.60±14.77 |
| Non-HRT | 44 | 94.71±11.27 | 92.18±14.90 | 141.26±13.42 |
Effect of HRT on quality of life
As shown in Table V,
no significant differences were observed in the EORTC-C30
cognition, role and social function scores among the HRT, non-HRT
and normal menopause groups (p>0.05), in contrast to the
physical function and emotional function scores (p<0.05). The
EORTC-C30 symptom sub-scale score differed significantly between
the HRT and non-HRT groups (p<0.05), but not between the HRT and
normal menopause groups (p>0.05). The urethral symptom score was
not significantly different among the three groups (p>0.05). A
difference was found between the HRT and non-HRT groups with
respect to general health status (p<0.05). However, no
difference was noted between the non-HRT and normal menopausal
groups (p>0.05).
 | Table VEORTC-C30 and GMU-Gynae index scores
in the hormone replacement therapy (HRT), non-HRT and normal
menopause groups. |
Table V
EORTC-C30 and GMU-Gynae index scores
in the hormone replacement therapy (HRT), non-HRT and normal
menopause groups.
| Parameter | Normal menopause
(n=77) | Ovarian
malignancy |
|---|
| |
|
|---|
| | HRT (n=31) | Non-HRT (n=44) |
|---|
| Functional
subscale |
| Body function | 1.03±0.39 | 1.84±1.50 | 12.69±10.20 |
| Role function | 1.30±0.92 | 3.23±1.81 | 13.54±3.91 |
| Emotion
function | 9.94±7.03 | 1.45±0.82 | 12.90±11.61 |
| Cognition
function | 2.81±0.82 | 3.03±0.84 | 4.93±1.61 |
| Social
function | 1.24±0.22 | 2.42±1.95 | 4.44±2.03 |
| Symptom
subscale | 2.59±2.02 | 6.82±2.61 | 21.82±10.85 |
| General
condition | 28.21±9.64 | 27.51±11.3 | 13.84±6.42 |
| Sexual
behavior | 0.95±0.56 | 1.05±0.74 | 10.10±3.21 |
| Urinary
symptoms | 2.40±1.21 | 2.35±1.73 | 3.55±1.58 |
| Autonomic
dysfunction | 1.82±4.87 | 1.77±1.08 | 13.09±4.30 |
The GMU-Gynae index sexual behavior score of the HRT
group was significantly different from that of the non-HRT group
(p<0.05), but was not different from that of the normal
menopause group (p>0.05). The GMU-Gynae index autonomic
dysfunction score of the HRT group differed significantly from the
scores of the non-HRT and normal menopause groups (p<0.05), with
no difference between the scores of the two control groups
(p>0.05).
Discussion
Patients with ovarian cancer who receive
cytoreductive surgery suffer from post-menopausal symptoms due to
the sudden reduction in estrogen levels and loss of ovarian
function. In theory, HRT may improve this condition, but there
remains controversy in the clinical arena. A number of in
vitro experiments have suggested that estrogen is capable of
stimulating the proliferation of ovarian cancer cells, and certain
epidemiological studies have reported that HRT increases the risk
of ovarian and breast cancer, creating concern about the effect of
HRT on the prognosis of patients. Hopkins et al (6), after a systematic evaluation,
concluded that HRT has no significant impact on recurrence,
deterioration or mortality of ovarian cancer. These authors
suggested that HRT improved the quality of life and should be
useful for patients with menopausal syndrome.
As with other studies (19), we found no significant difference in
the cumulative survival period between HRT and non-HRT groups
(p>0.05), indicating that post-surgery HRT has no negative
impact on tumor-free survival time, overall survival time or
overall survival rate in patients with ovarian cancer.
Even after applying a Cox model risk analysis to
account for other factors that may affect survival, HRT was not a
significant factor in determining prognosis. Due to the limited
number of cases in our study and the relatively short follow-up
duration, more case observations with a longer follow-up period
would be beneficial for confirming our findings.
Our previous in vitro study (20) has shown that exogenous estrogen
stimulated the proliferation of ER-positive, but not ER-negative,
tumor cells. The response of hormone receptors on cancer cells to
exogenous estrogen may eventually cause a biological behavior
change (21), but no concrete
conclusion has been reached regarding receptor and cell responses
to the clinical application of HRT. In the present study, the
cumulative survival period did not differ significantly between HRT
and non-HRT patients, regardless of the expression of ERα, ERβ or
PR in the tumor tissues, indicating no direct correlation between
ERα, ERβ or PR expression and HRT effects. This lack of correlation
may be explained by the fact that the hormone concentrations used
in HRT are within normal ranges, resulting in failure to inhibit or
stimulate tumor cell proliferation in in vitro
experiments.
Findings of our previous in vitro study
(20) have shown that the estrogen
stimulation of cancer cell proliferation may occur via a TGFα
autocrine pathway in tumor cells. However, the present study failed
to correlate HRT with any change in serum TGFα concentration or
prognosis. This failure may be attributed to the fact that TGF is
only a minor player in the highly complicated system of
intracellular signal crosstalk in vivo. Alternatively, as
the estrogen and progesterone levels used in HRT are within the
normal concentration ranges, they may be insufficient to evoke the
TGF pathway in the few cancer cells remaining following adequate
cytoreductive surgery and multi-course chemotherapy, as was the
case in our study.
Bodurka-Bevers (22)
reported that approximately 21% of patients with a malignant
ovarian tumor suffered from depression and approximately 29% from
anxiety. The ability of HRT to improve the quality of life for
these patients remains controversial (23). In the present study, the EORTC and
GMU-Gynae index results showed improved physical functions and
emotional symptoms after HRT, and the general life quality in the
HRT group was better than that in the non-HRT group. HRT also
markedly improved sexual performance and automatic dysfunction, as
in other reports. The primary concern about life quality for
ovarian cancer patients was reported to be sexual dissatisfaction,
followed by body state changes induced by surgery, chemotherapy and
radiotherapy (24). By improving
physical function, emotional symptoms, sexual quality and autonomic
dysfunction, HRT may greatly enhance the quality of life for
patients with ovarian cancer.
Epidemiological investigations (25,26)
have shown that long-term oral estrogen may increase the risk for
breast cancer. We did not confirm this in our study, perhaps due to
the relatively short follow-up period and simultaneous progesterone
usage.
The current literature (27,28)
does not support the view that HRT facilitates the development and
recurrence of ovarian cancer. Thus, ovarian malignancy after
clinical management of cytoreduction and adequate chemotherapy is
not a contra-indication for HRT. HRT may be a good option for
patients with serious symptoms of menopause and osteoporosis.
Nevertheless, the use of HRT still lacks the support of large-scale
multi-center prospective double-blind randomized studies,
particularly regarding its effect on tumor growth in patients with
gross residual tumor. Therefore, care should be taken to limit the
use of HRT as much as possible to patients with satisfactorily
controlled ovarian malignancy. The suitable duration of HRT is
currently under debate, with no definite conclusions based on
large-scale studies. Consideration should be given to an
individual’s specific clinical circumstances as well as the
severity of menopause symptoms. Due to the indefinite conclusions
regarding its impact on ovarian cancer and its association with the
long-term risk for breast cancer, HRT should be recommended only
when the patient has been adequately informed as to whether their
individual cancer status allows them to use HRT.
Acknowledgements
This study was supported by a grant from the
Provincial Research Project Funding of Guangxi, China (No. GSR
9817101).
References
|
1
|
Williams T, Toups KL, Saggese D, et al:
Epithelial ovarian cancer: disease etiology, treatment, detection,
and investigational gene, metabolite, and protein biomarkers. J
Proteome Res. 6:2936–2962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobs IJ and Menon UM: Progress and
challenges in screening for early detection of ovarian cancer. Cell
Proteomics. 3:355–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok SC, Kwonga J, Welch WR, et al:
Etiology and pathogenesis of epithelial ovarian cancer. Dis
Markers. 23:367–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lawrenson K and Gayther SA: Ovarian
cancer: a clinical challenge that needs some basic answers. Plos
Med. 6:126–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deraco M, Baratti D, Laterza B, et al:
Advanced cytoreduction as surgical standard of care and
hyperthermic intraperitoneal chemotherapy as promising treatment in
epithelial ovarian cancer. Eur J Surg Oncol. 37:4–9. 2011.
View Article : Google Scholar
|
|
6
|
Hopkins ML, Fung MF, Le T, et al: Ovarian
cancer patients and hormone replacement therapy: a systematic
review. Gynecol Oncol. 92:827–832. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh P and Oehler MK: Hormone replacement
after gynaecological cancer. Maturitas. 65:190–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chikman B, Lavy R, Davidson T, Wassermann
I, Sandbank J, Siegelmann-Danieli N and Halevy A: Factors affecting
rise in the incidence of infiltrating lobular carcinoma of the
breast. Med Assoc J. 12:697–700. 2010.PubMed/NCBI
|
|
9
|
Braem MG, Onland-Moret NC, van den Brandt
PA, et al: Reproductive and hormonal factors in association with
ovarian cancer in the Netherlands cohort study. Am J Epidemiol.
172:1181–1189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou B, Sun Q, Cong R, Gu H, Tang N, Yang
L and Wang B: Hormone replacement therapy and ovarian cancer risk:
a meta-analysis. Gynecol Oncol. 108:641–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinds L and Price J: Menopause, hormone
replacement and gynaecological cancers. Menopause Int. 16:89–93.
2010.PubMed/NCBI
|
|
12
|
Taube M, Hockenstrom T and Isakasson M:
Low sex steroid environment affects survival and steroid secretion
of ovarian tumor cell in primary cultures. Int J Oncol. 20:589–594.
2002.PubMed/NCBI
|
|
13
|
Mabuchi S, Ohmichi M, Kimura A, et al:
Estrogen inhibits paclitaxel-induced apoptosis via the
phosphorylation of apoptosis signal-regulating kinase 1 in human
ovarian cancer cell lines. Endocrinology. 145:49–58. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seeger H and Mueck AO: The effect of
estradiol metabolites and progestogens on the proliferation of
human ovarian cancer cells. Panminerva Med. 48:13–17.
2006.PubMed/NCBI
|
|
15
|
Zheng H, Kavanagh JJ and Hu W: Hormonal
therapy in ovarian cancer. Int J Gynecol Cancer. 17:325–330. 2007.
View Article : Google Scholar
|
|
16
|
Levgur M: Estrogen and combined hormone
therapy for women after genital malignancies. J Reprod Med.
49:837–848. 2004.PubMed/NCBI
|
|
17
|
Aaronson NF, Ahmedzai S, Bergman B, et al:
The European Organization for Research and Treatment of Cancer
QLQ-C30: a quality of life instrument for use in international
clinical trials in oncology. J Natl Cancer Inst. 85:365–376. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong-Mian P, Li L, Xinqiu C, et al:
Effect of HRT for the quality of life in patients with gynecologic
malignancies of reproductive age. Guangxi Yikedaxue Xuebao.
23:921–923. 2006.
|
|
19
|
Eeles RA, Tan S, Wiltshaw E, et al:
Hormone replacement therapy and survival after surgery for ovarian
cancer. BMJ. 302:259–262. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li L, Gao K, Zhang W, et al: Effects of
estradiol and progesterone on ovarian cancer cell line. Zhonghua
Zhongliu Zazhi. 25:553–554. 2003.
|
|
21
|
Landon SP, Crew AJ, Ritchie AA, et al:
Growth inhibition of oestrogen receptor-positive human ovarian
carcinoma by antioestrogens in vitro and in a xenograft model. Eur
J Cancer. 30A:682–686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bodurka-Bevers D, Basen-Engquist K,
Carmack CL, et al: Depression, anxiety, and quality of life in
patients with epithelial ovarian cancer. Gynecol Oncol. 78:302–308.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biglia N, Mariani L, Marenco D, et al:
Hormonal replacement therapy after gynaecological cancer. Gynakol
Geburtshilfliche Rundsch. 46:191–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biglia N, Gadducci A, Ponzone R, et al:
Hormone replacement therapy in cancer survivors. Maturitas.
48:333–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wenzel LB, Huang HQ, Armstrong DK, et al:
Health-related quality of life during and after intraperitoneal
versus intravenous chemotherapy for optimally debulked ovarian
cancer: a Gynecologic Oncology Group Study. J Clin Oncol.
25:437–443. 2007. View Article : Google Scholar
|
|
26
|
Ponzone R, Biglia N, Jacomuzzi ME, et al:
Vaginal oestrogen therapy after breast cancer: is it safe? Eur J
Cancer. 41:2673–2681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bebar S and Ursic-Vrscaj M: Hormone
replacement therapy after epithelial ovarian cancer treatment. Eur
J Gynaecol Oncol. 21:192–196. 2000.PubMed/NCBI
|
|
28
|
Ursic-Vrscaj M, Bebar S and Zakelj MP:
Hormone replacement therapy after invasive ovarian serous
cystadenocarcinoma treatment: the effect on survival. Menopause.
8:70–75. 2001. View Article : Google Scholar : PubMed/NCBI
|