Introduction
It is well known that metastasis of tumor cells is
the main cause of mortality in patients with cancer, and this is a
hot topic for investigators. Metastasis is a multi-step and highly
regulated cascade process, in which tumor cells separate from a
primary foci, cross the wall of vessels, circulate through the
whole organism within the blood, and eventually form new colonies
at remote sites following adhesion to the endothelium (1). The majority of tumor cells are killed
by the immune system during circulation in the vessels (2,3), and
natural killer cells (NK cells) are one of the main cellular immune
factors. The anti-metastasis function of NK cells has been reported
in various models of experimental and spontaneous metastasis
(4,5). In these reports, it is well accepted
that direct contact between NK cells and tumor cells is crucial
(6). Therefore, the mechanism by
which the surviving tumor cells avoid lethal contact with NK cells
is a key topic for anti-cancer research. Various studies have
demonstrated that a number of blood coagulation factors facilitate
the hematogenous metastasis of tumor cells (7,8). These
factors contribute to tumor angiogenesis, stroma formation, growth
or metastasis (9–11). Of all the known effects of cancer
progression, the protective effect of fibrinogen to tumor cells has
been well probed. A mass of fibrinogen may adhere to tumor cells
tightly, and coat the tumor cells to form a protective shield,
which inhibits the effector-target interaction and subsequently
blocks NK cytotoxicity against tumor cells (12,13).
Ganoderma lucidum (G. lucidum) has
been widely used as a traditional herb for disease treatment and
health promotion, particularly by cancer patients. G.
lucidum polysaccharides have been reported that may
significantly promote the immune parameters of patients with
advanced cancer (14). In their
study, Sliva et al demonstrated that G. lucidum
inhibits the migration of various cancer cells (15,16).
The antitumor activities of the polysaccharides appear to be due to
different mechanisms, such as inhibition of adhesion of tumor cells
to type I collagen, hyaluronan, fibronectin and laminin, promotion
of the expression of cytokines, promotion of tumor cells to induce
lymphocyte proliferation and suppression of tumor-induced
angiogenesis (17–20). This study was designed to observe
the effect of G. lucidum polysaccharides on the adhesion of
fibrinogen to melanoma cells and NK cytotoxicity to tumor cells. We
found that fibrinogen is capable of adhering to the melanoma cells
B16 and A375, and that α5β1 and
αvβ3 integrin are essential for adhesion.
Coated fibrin may protect melanoma cells from NK cytotoxicity, and
G. lucidum polysaccharides are capable of eliminating the
adhesion of fibrinogen to tumor cells, and then eradicating the
blocking effect of fibrinogen on NK cytotoxicity against melanoma
cells and decreasing the lung metastasis of melanoma cells in
mice.
Materials and methods
Reagents
Fibrinogen conjugated with Alexa Fluor 488 and IMDM
culture medium was purchased from Invitrogen (OR, USA), and
fibrinogen was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Blocking antibodies of mouse β1 (Biolegend, CA, USA,
HMb1-1) and mouse β3 integrins (Santa Cruz
Biotechnology, Santa Cruz, CA, USA, 2C9.G2), human
α5β1 (Chemicon, Millipore, Billerica, MA,
USA, HA5) and human αvβ3 integrins (Santa
Cruz Biotechnology, HM2034) were prepared according to the
manufacturer's instructions. G. lucidum polysaccharides were
purchased from Johnsun Mushroom (Hangzhou, China), and dissolved in
phosphate-buffered saline (PBS).
Cells and animals
Melanoma cells B16 and A375 were obtained from the
Shanghai Cell Bank (Chinese Academy of Science, Shanghai, China).
Cells were cultured in IMDM medium and 10% fetal bovine serum (FBS)
was added in a humidified cell incubator (37˚C, 5% CO2).
These cells were harvested with trypsin and EDTA. Subsequently,
PBS-washed cells were suspended in culture medium for further use.
Six- to eight-week-old C57Bl/6J healthy male mice (specific
pathogen free) were obtained from the Jilin University Animal
Center (Changchun, China). All of the protocols applied in the
animal experiments were approved by the Animal Care and Use
Committee of Northeast Dianli University (Jilin, China).
Flow cytometric assay
Melanoma cells were harvested and resuspended in
IMDM. For the adhesion assay, Alexa Fluor 488 conjugated fibrinogen
(green fluorescence) was used. Each aliquot (0.1 ml) of tumor cells
was mixed with fibrinogen (1 mg/ml) and incubated at 37˚C for 30
min. After washing twice, cells were suspended in 0.5 ml of PBS for
further analysis by a flow cytometer (FACScan, Beckman-Coulter,
Miami, FL, USA).
Preparation of NK cells
Human NK cells were isolated from human peripheral
blood mononuclear cells (PBMCs) with a negative magnetic bead NK
cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
In brief, B cells, T cells, stem cells, monocytes, dendritic cells,
granulocytes and erythroid cells were labeled by a cocktail of
magnetically biotin-conjugated antibodies. Highly pure NK cells
were separated by depletion of magnetically labeled cells in the
MicroBead. Mouse NK cells were separated from spleen cells with a
similar kit (Miltenyi Biotec).
NK cytotoxic assay
An NK cytotoxic assay was performed in the presence
or absence of fibrinogen. In brief, Na51CrO4
pre-labeled tumor cells in 0.05 ml (1×104) culture
medium were pre-incubated with 0.05 ml of various concentrations of
fibrinogen in the presence (0.1 mg/ml) or absence of G.
lucidum polysaccharides for 30 min at 37˚C. Subsequently, 100:1
of NK cells (0.05 ml) were added. Following 4 h of co-incubation at
37˚C, cell culture plates were centrifuged at 1500 rpm for 10 min,
and supernatants (0.05 ml) were collected for the radioactivity
measurement. The cytotoxicity was measured using the formula % = (A
− B)/(C − B) × 100, where A is radioactivity in the test well, B is
spontaneous radioactivity from the well target cells without effect
cells, and C is the total target released radioactivity.
Statistical analysis
Data were presented as the mean ± SD. The
significance of differences between the means was calculated by the
analysis of variance. When the difference of the means was shown to
be significant, multiple comparisons by pairs were calculated by
the T-test. Probability values of P<0.001, P<0.01 or
P<0.05 were considered to indicate a statistically significant
difference.
Results
Fibrinogen binds to A375 and B16 cells in
a αvβ3 and α5β1
integrin-dependent manner
Fibrinogen is known to adhere to
αvβ3 and αvβ1 integrins
on the surface of tumor cells. To confirm the adhesion of
fibrinogen to the tumor cells we used, fibrinogen conjugated with
Alexa Fluor 488 was incubated with B16 or A375 cells at 37˚C for 30
min with or without sufficient blocking antibodies. The results
showed that fibrinogen strongly bound to the A375 (~94%) and B16
(~98%) cell surface. Both αvβ3 and
α5β1 integrins bind to fibrinogen. In A375
and B16 cells, total eradication of the adhesion appeared when the
functions of the two integrins were blocked (Fig. 1). Our results revealed that these
two types of integrins mediated the adhesion of melanoma cells to
fibrinogen.
Fibrinogen protects tumor cells from NK
cytotoxicity
Fibrinogen may be coated on tumor cells and may
protect these cells from NK cytotoxicity. Moreover, the adhesion of
fibrinogen to tumor cells is essential. Melanoma cells strongly
adhere to fibrinogen (Fig. 1).
Therefore, we examined the ability of fibrinogen to protect
melanoma cells from NK cytotoxicity. The results revealed that
fibrinogen was capable of protecting tumor cells from NK
cytotoxicity in a concentration-dependent manner (Fig. 2).
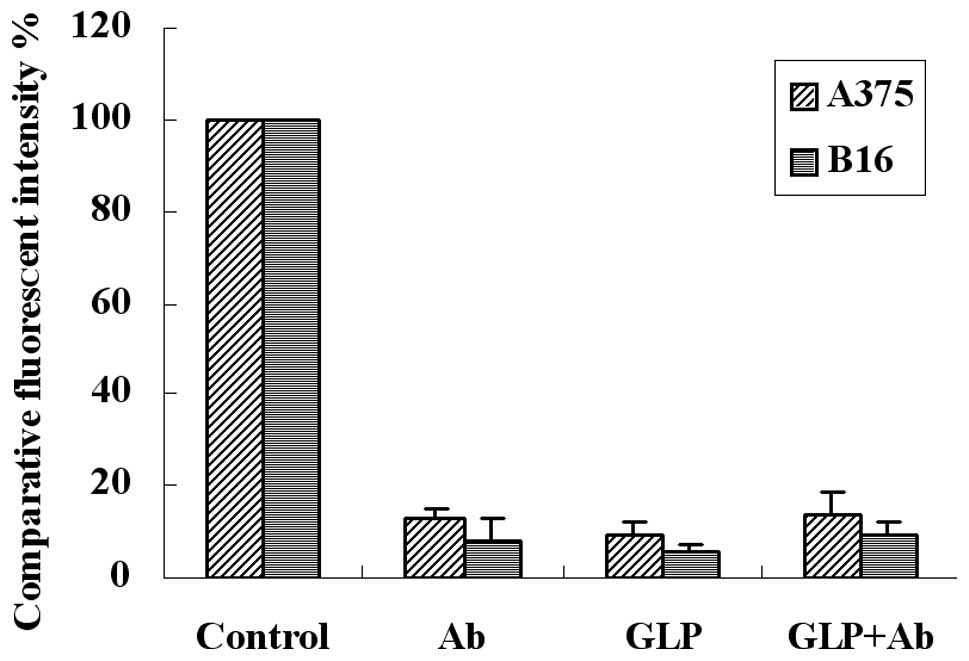
G. lucidum polysaccharides block the
adhesion of fibrinogen to melanoma cells
To observe the effects of G. lucidum
polysaccharides, we added them into the flow cytometric adhesion
system. The results revealed that G. lucidum polysaccharides
inhibited adhesion to the level of the blocking antibodies, and the
combination of G. lucidum polysaccharides and antibodies
(GLP+Ab) had no further blocking effect (Fig. 3). The results suggest that G.
lucidum polysaccharides eradicate melanoma cell-fibrinogen
adhesion by blocking αvβ3 and
α5β1 integrins.
G. lucidum polysaccharides eradicates the
blocking effect of fibrinogen on NK cytotoxicity against melanoma
cells
Fibrinogen coats are capable of blocking NK
cytotoxicity against melanoma cells, and fibrinogen adhesion
mediated by αvβ3 and
α5β1 integrins are eliminated by G.
lucidum polysaccharides. Therefore, we examined the effect of
G. lucidum polysaccharides on NK cytotoxicity affected by
fibrinogen. The results showed that G. lucidum
polysaccharides (FBG+GLP) reduced the blocking effect to almost the
level of the control (Fig. 4). The
results were also similar to the samples with antibodies added
(FBG+Ab), and a combination of G. lucidum polysaccharides
and antibodies (FBG+Ab+GLP) revealed no further improvement. Our
results suggested that G. lucidum polysaccharides are able
to eliminate the blocking effect of fibrinogen on NK cytotoxicity
to melanoma cells.
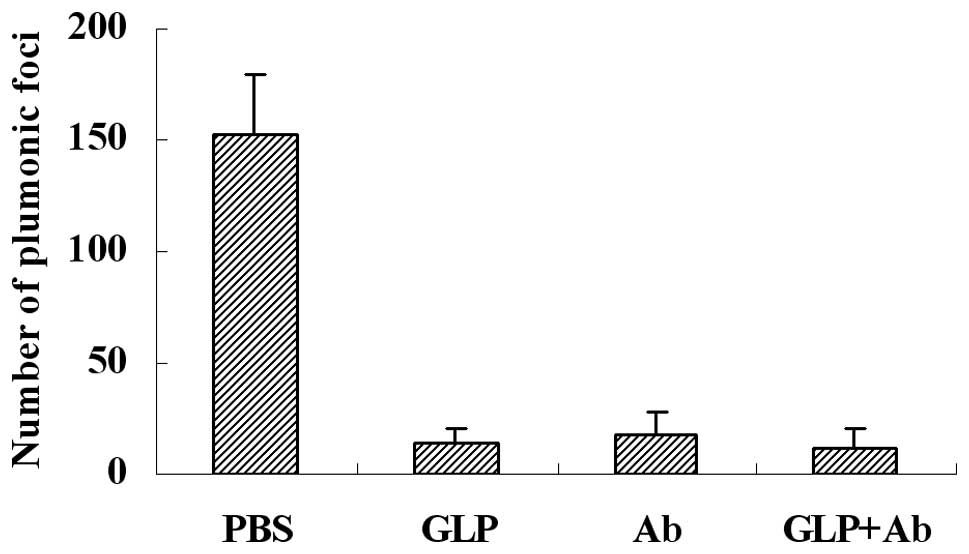
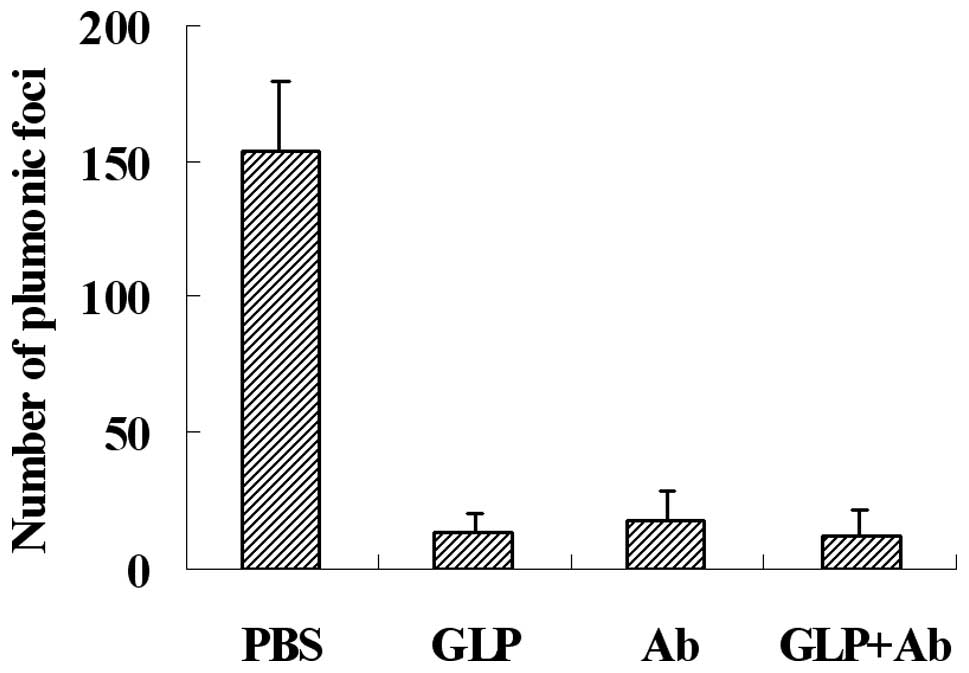
G. lucidum polysaccharides decrease the
lung metastasis of melanoma cells in mice
NK cytotoxicity is an important immune defense for
tumor metastasis, which may be blocked by fibrinogen, and G.
lucidum polysaccharides may eliminate the blocking effect of
fibrinogen on NK cytotoxicity against tumor cells. To detect the
effect of G. lucidum polysaccharides on the metastasis of
melanoma cells in a mouse model, we injected G. lucidum
polysaccharides or function-blocking antibodies 5 min prior to
injection of B16 tumor cells through the lateral tail vein. Our
results, as shown in Fig. 5, shown
that G. lucidum polysaccharides (GLP) and antibodies (Ab)
decreased metastasis efficiently. The inhibitive rate of G.
lucidum polysaccharides and antibodies was almost identical,
and the combination of G. lucidum polysaccharides and
antibodies (GLP+Ab) provided no further inhibition.
Discussion
It has been reported that G. lucidum
polysaccharides is capable of eliminating the adhesion of tumor
cells to various matrix proteins, including collagen, hyaluronan,
fibronectin and laminin, and subsequently affecting the
physiological phenomenon invoked by these proteins. Blood is rich
in fibrinogen, and fibrinogen is capable of strongly blocking NK
cytotoxicity. Although investigators have reported that G.
lucidum polysaccharides improve the NK cell profile or cytokine
secretion, the effects of G. lucidum polysaccharides on NK
cytotoxicity blocked by fibrinogen have not previously been
reported. In this study, we found that G. lucidum
polysaccharides eliminate melanoma cell-fibrinogen adhesion
mediated by αvβ3 and
α5β1 integrins, and eradicate the blocking
effect of fibrinogen on NK cytotoxicity against melanoma cells. We
suggest that it is a new area of study of anti-cancer activity of
G. lucidum polysaccharide.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (31101009), Doctoral Initiating
Project of Northeast Dianli University of China (BSJXM-200905), and
‘Twelfth Five’ scientific and technological research projects from
Jilin Provincial Department of Education (2011.74).
References
|
1
|
Liotta LA: Cancer cell invasion and
metastasis. Sci Am. 266:54–59. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fidler I: Metastasis: quantitative
analysis of distribution and fate of tumor emboli labeled with
125I-5 iodo-2-deoxyuridine. J Natl Cancer Inst. 45:773–779.
1970.PubMed/NCBI
|
|
3
|
Mehdi AB, Tozawa K, Fisher AB, Shientag L,
Lee A and Muschel RJ: Intravascular origin of metastasis from the
proliferation of endothelium-attached tumor cells: a new model for
metastasis. Nat Med. 6:100–102. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gorelik E, Wiltrout RH, Okumura K, Habu S
and Herberman RB: Role of NK cells in the control of metastatic
spread and growth of tumor cells in mice. Int J Cancer. 30:107–112.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanna N: The role of natural killer cells
in the control of tumor growth and metastasis. Biochim Biophys
Acta. 780:213–226. 1985.PubMed/NCBI
|
|
6
|
Arnon TI, Achdout H, Lieberman N, Gazit R,
Gonen-Gross T, Katz G, Bar-Ilan A, Bloushtain N, Lev M, Joseph A,
Kedar E, Porgador A and Mandelboim O: The mechanisms controlling
the recognition of tumor- and virus-infected cells by NKp46. Blood.
103:664–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rickles FR and Falanga A: Molecular basis
for the relationship between thrombosis and cancer. Thromb Res.
102:V215–V224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terraube V, Pendu R, Baruch D, Gebbink MF,
Meyer D, Lenting PJ and Denis CV: Increased metastatic potential of
tumor cells in von Willebrand factor-deficient mice. J Thromb
Haemost. 4:519–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dvorak HF, Nagy JA, Berse B, Brown LF, Yeo
KT, Yeo TK, Dvorak AM, van de Water L, Sioussat TM and Senger DR:
Vascular permeability factor, fibrin, and the pathogenesis of tumor
stroma formation. Ann N Y Acad Sci. 667:101–111. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nierodzik ML and Karpatkin S: Thrombin
induces tumor growth, metastasis, and angiogenesis: evidence for a
thrombin-regulated dormant tumor phenotype. Cancer Cell.
10:355–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langer F, Amirkhosravi A, Ingersoll SB,
Walker JM, Spath B, Eifrig B, Bokemeyer C and Francis JL:
Experimental metastasis and primary tumor growth in mice with
hemophilia A. J Thromb Haemost. 4:1056–1062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gunji Y, Lewis J and Gorelik E: Fibrin
formation inhibits the in vitro cytotoxic activity of human natural
and lymphokine-activated killer cells. Blood Coagul Fibrinolysis.
1:663–672. 1990.PubMed/NCBI
|
|
13
|
Atagi S, Sone S, Fukuta K and Ogura T:
Inhibition by fibrin coagulation of lung cancer cell destruction by
human interleukin-2-activated killer cells. Jpn J Cancer Res.
83:1088–1094. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Zhou S, Jiang W, Huang M and Dai X:
Effects of ganopoly (a Ganoderma lucidum polysaccharide
extract) on the immune functions in advanced-stage cancer patients.
Immunol Invest. 32:201–215. 2003.PubMed/NCBI
|
|
15
|
Sliva D, Labarrere C, Slivova V, Sedlak M,
Lloyd FP Jr, Ho NW, et al: Ganoderma lucidum suppresses
motility of highly invasive breast and prostate cancer cells.
Biochem Biophys Res Commun. 298:603–612. 2002. View Article : Google Scholar
|
|
16
|
Sliva D: Ganoderma lucidum (Reishi)
in cancer treatment. Integr Cancer Ther. 2:358–364. 2003.
View Article : Google Scholar
|
|
17
|
Zhang Q and Lin Z: Study on antitumour
activity and mechanism of Ganoderma polysaccharides B.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 19:544–547. 1999.PubMed/NCBI
|
|
18
|
Sun LX, Lin ZB, Li XJ, Li M, Lu J, Duan
XS, Ge ZH, Song YX, Xing EH and Li WD: Promoting effects of
Ganoderma lucidum polysaccharides on B16F10 cells to
activate lymphocytes. Basic Clin Pharmacol Toxicol. 108:149–154.
2011.
|
|
19
|
Wu QP, Xie YZ, Li SZ, La Pierre DP, Deng
ZQ, Chen Q, Li C, Zhang Z, Guo J, Wong CKA, Daniel Y, Yee A and
Burton BY: Tumour cell adhesion and integrin expression affected by
Ganoderma lucidum. Enzyme Microb Technol. 40:32–41. 2006.
View Article : Google Scholar
|
|
20
|
Kimura Y, Taniguchi M and Baba K:
Antitumour and antimetastatic effects on liver of triterpenoid
fractions of Ganoderma lucidum: mechanism of action and
isolation of an active substance. Anticancer Res. 22:3309–3318.
2002.PubMed/NCBI
|