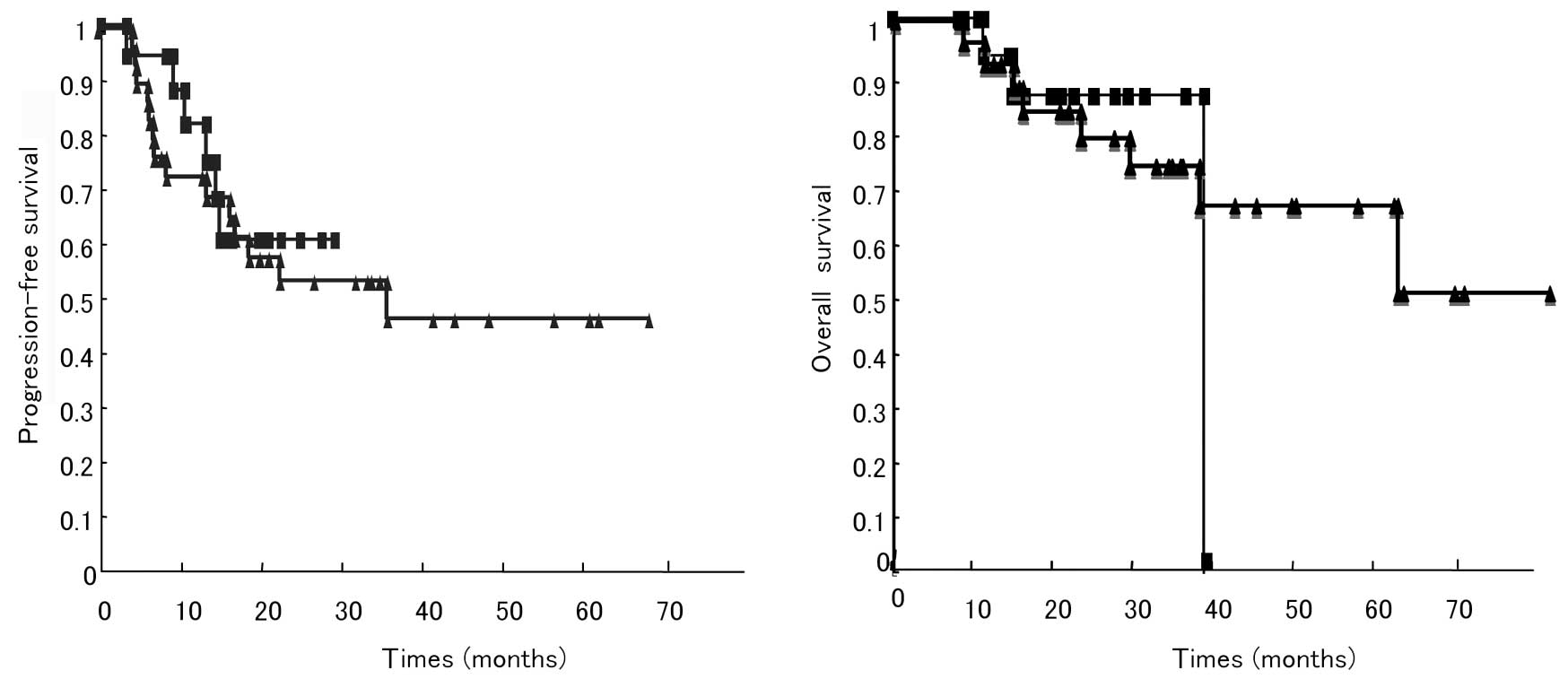
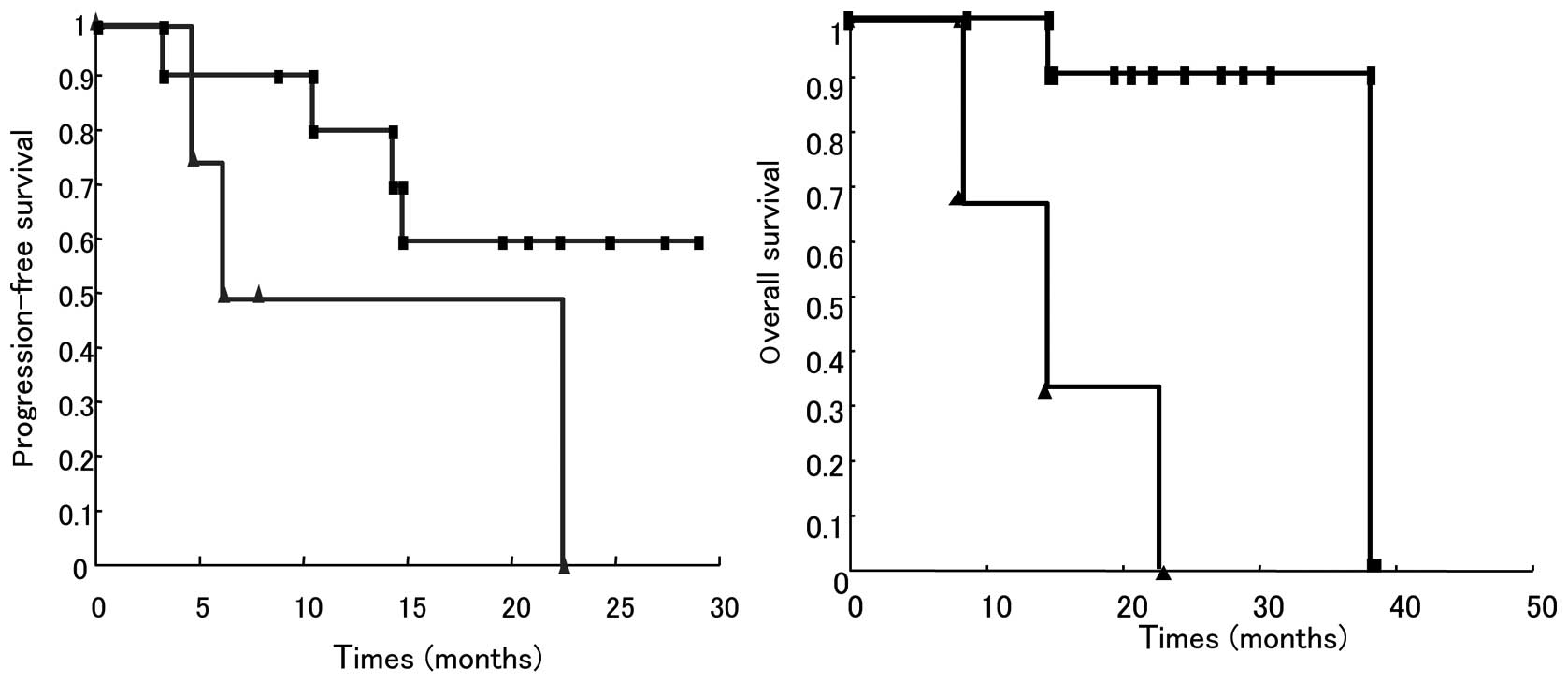
|
1
|
Center for Cancer Control and
Information Services, National Cancer Center, Japan, 2010
(ganjoho.jp).
|
|
2
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rose PG, Bundy BN, Watkins EB, Thigpen JT,
Deppe G, Maiman MA, Clarke-Pearson DL and Insalaco S: Concurrent
cisplatin-based radiotherapy and chemotherapy for locally advanced
cervical cancer. N Engl J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whitney CW, Sause W, Bundy BN, Malfetano
JH, Hannigan EV, Fowler WC Jr, Clarke-Pearson DL and Liao SY:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA
carcinoma of the cervix with negative para–aortic lymph nodes: a
Gynecologic Oncology Group and Southwest Oncology Group study. J
Clin Oncol. 17:1339–1348. 1999.PubMed/NCBI
|
|
5
|
Keys HM, Bundy BN, Stehman FB, Muderspach
LI, Chafe WE, Suggs CL III, Walker JL and Gersell D: Cisplatin,
radiation, and adjuvant hysterectomy compared with radiation and
adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl
J Med. 340:1154–1161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: an update of
radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar
|
|
7
|
Green JA, Kirwan JM, Tierney JF, Symonds
P, Fresco L, Colingwood M and Williams CJ: Survival and recurrence
after concomitant chemotherapy and radiotherapy for cancer of the
uterine cervix: a systematic review and meta-analysis. Lancet.
358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim YB, Cho JH, Keum KC, Lee CG, Seong J,
Suh CO and Kim GE: Concurrent chemoradiotherapy followed by
adjuvant chemotherapy in uterine cervical cancer patients with
high-risk factors. Gynecol Oncol. 104:58–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi CH, Lee JW, Kim WY, Nam HR, Kim BG,
Hum SJ, Lee JH and Bae DS: Phase II study of consolidation
chemotherapy after concurrent chemoradiation in cervical cancer:
preliminary results. Int J Rad Oncol Biol Phys. 68:817–822. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Japan Society of Gynecologic Oncology.
Cervical cancer treatment guidelines. 2007
|
|
11
|
Ikushima H, Osaki K, Furutani S, Yamashita
K, Kawanaka T, Kishida Y, Wamoto S, Takegawa Y, Kudoh T and
Nishitani H: Chemoradiation therapy for cervical cancer: toxicity
of concurrent weekly cisplatin. Rad Med. 24:115–121. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakuragi N: Up-to-date management of lymph
node metastasis and the role of tailred lymphadenenctomy in
cervical cancer. Int J Clin Oncol. 12:165–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berman ML, Keys H, Creasman W, Reasman W,
DiSaia P, Bundy B and Blessing J: Survival and patterns of
recurrence in cervical cancer metastatic to periaortic lymph nodes.
Gynecol Oncol. 19:8–16. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benedetti PP, Basile S and Angioli R:
Pelvic and aortic lymphadenectomy in cervical cancer: the
standardization of surgical procedure and its clinical impact.
Gynecol Oncol. 113:284–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vidaurreta J, Bermudez A, Di Paola G and
Sardi J: Laparoscopic staging in locally advanced cervical
carcinoma: a new possible philosophy? Gynecol Oncol. 75:366–371.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grigsby PW, Dehdashi F and Siegel BA:
FDG-PET evaluation of carcinoma of the cervix. Clin Pos Image.
2:105–109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rose PG and Bundy BN: Chemoradiation for
locally advanced cervical cancer: does it help? J Clin Oncol.
20:891–893. 2002.PubMed/NCBI
|
|
18
|
Grigsby PW, Perez CA, Chao KS, Herog T,
Mutch DG and Rader J: Radiation therapy for carcinoma of the cervix
with biopsy-proven positive para-aortic lymph nodes. Int J Rad
Oncol Biol Phys. 49:733–738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walker JL, Morrison A, DiSilvestro P and
von Gruenige VE: A phase I/II study of extended field radiation
therapy with concomitant paclitaxel and cisplatin chemotherapy in
patients with cervical carcinoma metastatic to the para-aortic
lymph nodes. Gynecol Oncol. 112:78–84. 2009. View Article : Google Scholar
|
|
20
|
Zhang MQ, Liu SP and Wang XE: Concurrent
chemotherapy with paclitaxel and nedaplatin followed by
consolidation chemotherapy in locally advanced squamous cell
carcinoma of the uterine cervix: preliminary results of phase II
study. Int Rad Oncol Biol Phys. 78:821–827. 2010. View Article : Google Scholar
|
|
21
|
Rose PG, Lessing JA, Gershenson DM and
McGehee R: Paclitaxel and cisplatin as first-line therapy in
recurrent or advanced squamous cell carcinoma of the cervix: a
gynecologic oncology group study. J Clin Oncol. 17:2676–2680.
1999.
|
|
22
|
Sit AS, Kelley JL, Gallion HH, Kunschner
AJ and Edwards RP: Paclitaxel and carboplatin for recurrence or
persistent cancer of the cervix. Cancer Invest. 22:368–373. 2004.
View Article : Google Scholar : PubMed/NCBI
|