Introduction
Cytotoxic drugs are still widely used to treat
malignant tumors via tumor cell death by apoptosis (1). One of the most representative
cytotoxins is cisplatin, which reacts with target genomic DNA to
form DNA adducts (2). Cytotoxins
are typically non-selective and this lack of selectivity
occasionally results in significant toxicity to normal cells.
Moreover, treatment of cancer patients with chemotherapeutic agents
frequently allows tumors to acquire a multidrug-resistant (MDR)
phenotype (3,4). Overexpression of MDR1, other
ATP-dependent transporters, amplification of drug-inactivating
enzymes, mutations or modifications of drug targets, alterations in
DNA repair machinery and increased resistance to apoptosis cause
this resistance (3–5). Toxicities of chemotherapy, along with
drug resistance, are major therapeutic limitations that result in
poor clinical outcomes in cancer patients.
Targeting of low extracellular pH, elevated enzymes
in tumor tissues, the hypoxic environment inside the tumor and
tumor-specific antigens expressed on tumor cell surfaces were
previously investigated as possible strategies for improved
therapeutic outcomes (6–9). Singh et al highlighted recent
trends in pro-drug and conjugate rationale and a design for cancer
treatment, by analyzing comparative accounts of the advantages and
disadvantages associated with each approach (10). A variety of receptors related to
cellular growth factors or cytokines on tumor cells have been shown
to be overexpressed (11), and we
believe that targeting these receptors is a promising strategy. For
example, the transfection of the tumor necrosis factor (TNF)
receptor gene in cancer cells, or the exposure of cancer cells to
certain reagents, may increase the expression of TNF receptors,
resulting in the enhancement of the cytotoxic effect of TNF
(12). Identifying new receptors on
tumor cells, in addition to further investigation of therapeutic
strategies, is still crucial for cancer treatment.
In the process of finding potential candidate
receptors in cancer cells, the orexin 2 receptor (OX2R, also known
as hypocretin receptor 2) was found to be a noteworthy target. The
orexin family comprises orexins-A and -B, and their corresponding
receptors are OX1R and OX2R, respectively. These two receptors
belong to the seven-transmembraned G-coupled receptor superfamily
(13). It has become clear that
orexin receptors regulate narcolepsy (14,15).
Immunohistochemistry (IHC) analyses have demonstrated that certain
peptides that bind to orexin receptors were selectively expressed
in the hypothalamus, particularly in the lateral and medial
hypothalamic regions (16).
At present, the presence of orexin receptors
reported in human cancer cells is limited. The expression of OX1R
has been found in cell lines from human colon cancer (17). However, the study pertaining to the
expression of OX2R is limited to clinical samples of
cortisol-secreting adrenocortical adenomas (18). Moreover, the role of orexin
receptors in cancer cells is as yet unknown. Thus, identifying the
location of orexin receptors remains a challenge.
OX2R was selected as a possible candidate since the
expression levels of OX2R in normal cells are limited (16) and, thus, OX2R may be a cancer
cell-specific target. Although a high expression of OX2R has been
identified in hypothalamic samples, systemic administration of
antitumor drugs targeting OX2R may not interact with the
hypothalamus due to the presence of the blood-brain-barrier. In
addition, orexin receptors are a well known target in the field of
neuroscience, since they closely correlate with narcolepsy and
other diseases (14,15). Investigating other functions of OX2R
would be helpful in understanding the roles of orexins.
In the present study, following the screening of
OX2R expression in a variety of cancer cell lines, we investigated
cancer tissue array samples to further examine OX2R expression. In
addition, we examined the possibility of OX2R as a target for
immunotoxin or antibody-drug conjugate (ADC) cancer therapy.
Materials and methods
Materials
Hepatocellular carcinoma tissue array, ARY- HH0075
was purchased from Folio Biosciences (Columbus, OH, USA). Digestive
system disease tissue array, DID381 was purchased from US Biomax
(Rockville, MD, USA). Human cancer tissue array, VA2 was purchased
from SuperBioChips (Seoul, Korea). All other reagents were of
reagent grade quality. All tissue samples were collected under the
ethical standards with the donor’s complete informed consent under
the control of manufacturers.
Polyclonal antibody against OX2R
Two New Zealand white rabbits were immunized with
three specific peptides of OX2R to obtain a polyclonal antibody for
OX2R. Three peptides (CRNWSSASELNETQE, FAHTEDRETVYAWF and
C-AVAAEIKQIRARRK) were selected; GenBank reference: CAI19665.1. The
immunization period was 64 days and immunization was performed four
times during this period. Rabbit serum (10 ml) were purified with a
peptide affinity column. The antibody titer of ELISA for
CRNWSSASELNETQE was estimated to be 1 in 4,000 and for
C-AVAAEIKQIRARRK to be 1 in 16,000. All procedures were performed
by Japan Bio Services (Saitama, Japan).
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted according to the
manufacturer’s instructions (TRIzol, Invitrogen, Carlsbad, CA,
USA). Following quantification of the extracted RNA, first strand
complementary DNA (cDNA) of each cancer cell line was
synthesized.
OX2R expression was measured by RT-PCR, using 1 μg
RNA and oligo (dT) as reverse transcription primers. A control
reaction, which omitted reverse transcriptase was included to check
for the presence of genomic DNA. OX2R was amplified using a Thermal
Cycler (Applied Biosystems, Foster City, CA, USA) in a 20-μg
reaction medium containing 0.1 μg of AmpliTaq Gold® DNA
Polymerase (Applied Biosystems), using the following cycling
conditions: 95°C for 10 min, followed by 35 cycles of 95°C for 30
sec, 57°C for 30 sec and 72°C for 60 sec, followed by a 10 min
extension at 72°C. The sequences for the sense and anti-sense
primers of OX2R and β-actin used were: 5′-caccgtgttcccaggcttag-3′,
5′-ttctggctcggatctgcttt-3′, 5′-cactgtgttggcgtacaggt-3′ and
5′-tcatcaccattggcaatgag-3′. Amplicons were separated by
electrophoresis in 2% agarose gel, stained with ethidium bromide
and viewed under UV illumination. Gene sequence of OX2R was
performed by Invitrogen Japan (Tokyo, Japan).
Western blotting
Proteins (30 μg) were evaluated by the bicinchoninic
acid (BCA) protein assay (Thermo Fisher Scientific, Waltham, MA,
USA), loaded onto a NuPAGE 10% Bis-Tris gel (Invitrogen) and
blotted onto a Polyscreen polyvinylidene fluoride (PVDF) transfer
membrane (ParkinElmer, Waltham, MA, USA) at 100 V for 1 h in a
transfer buffer. The PVDF membranes were incubated with primary
monoclonal antibody at a 1:2,000 dilution and with α-tubulin
antibody (ab24246, Abcam, Cambridge, MA, USA) at a 1:20,000 in
phosphate-buffered saline (PBS)-0.1% Tween (PBS-T) and 1% bovine
serum albumin (BSA) overnight at 4°C. The membranes were washed,
incubated with a secondary anti-rabbit horseradish
peroxidase-conjugated antibody (GE Healthcare, Fairfield, CT, USA)
at a 1:20,000 dilution for 90 min at a room temperature and washed
for 60 min with PBS-T. Antibody complexes were visualized using
enhanced chemiluminenscense (ECL) plus western blotting reagent (GE
Healthcare). The images were scanned by LAS-3000 UVmini (Fuji Film,
Tokyo, Japan).
IHC
IHC was performed using tissue arrays. Tissue arrays
were deparaffinized by xylene treatment and washed with an alcohol
gradient (from 99 to 50%) and PBS. Arrays were incubated with
polyclonal antibody against OX2R at a 1:1,000 dilution overnight at
4°C. Slides were developed with alkaline phosphatase (AP)-goat
anti-rabbit IgG (Invitrogen) and stained by BM purple AP substrate
(Roche Diagnostics, Rotkreuz, Switzerland). Slides were also
lightly counterstained with hematoxylin and eosin. Negative
controls in this study were prepared by omitting the primary
antibody step.
Immunohistochemical scoring
Samples were scored independently by an experienced
histopathologist. Immunostaining was scored as ++/+++ (strong), +
(moderate) or - (negative).
Results
OX2R mRNA expression in cancer cells
assessed by RT-PCR
One of the main objectives of this study was to
confirm the expression levels of OX2R in cancer cells. We
investigated 36 cancer cell lines originating from 10 different
organs, ranging from glioblastoma to uterine cervix, and screened
all 36 cell lines to assess this objective (Table I). Total RNA isolated from cancer
cell lines was transcribed to cDNA, and then assessed by PCR as
described in Materials and methods. Primer sequences and RT-PCR
conditions were optimized by referring to other investigations
analyzing the expression of OX2R (19,20)
and, through several trials, to obtain a clear single band.
 | Table ICell lines used for RT-PCR and western
blotting screening. |
Table I
Cell lines used for RT-PCR and western
blotting screening.
| Organ | Cell line |
|---|
| SCCHN | YCUT231, YCUT891,
YCUM 862, YCUM911, YCUMs861, YCUL891, Wmm-scc |
| Glioblastoma
multiforme | SN19, U251tg, A172,
U373, SF295 |
| Breast | BT-20, MDA-MB-231,
ZR-75-1, T47D |
| Liver | Hep3B, HepG2,
PLC/PFR-5, HuH-7 |
| Gallbladder | TGBC1, TGBC2, TGBC14,
TGBC44, NOZ, OCUG |
|
Cholangiocarcinoma | MzChA1, MzChA2, OZ,
HuCCT-1 |
| Pancreas | PANC1, SU.86.86 |
| Uterine cervix | Hela, HT-29 |
| Oesophageal
carcinoma | OE19 |
| Colon | DLD-1 |
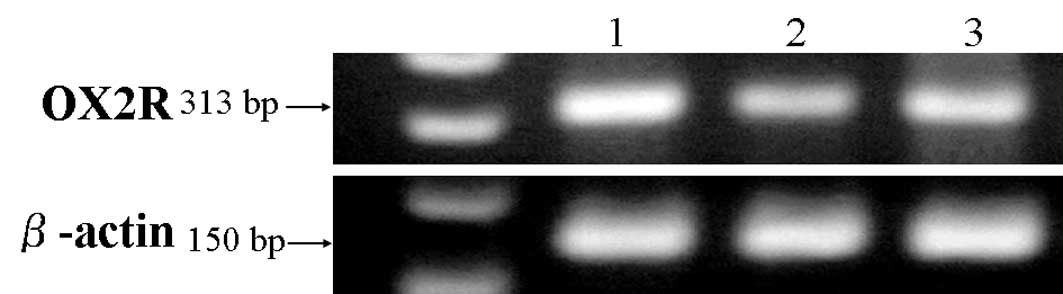
Of the 36 cell lines, three cell lines, originating
from the gallbladder (TGBC2), glioblastoma (SF295) and squamous
cell carcinoma of the head and neck (YCUM862), expressed OX2R mRNA
(Fig. 1). In addition, we performed
gene sequences of PCR products to confirm its homology to OX2R. The
homology between PCR products and OX2R was 98%. We also screened
for the expression of OX1R mRNA, but this was not identified in any
of the cell lines tested in this study (data not shown).
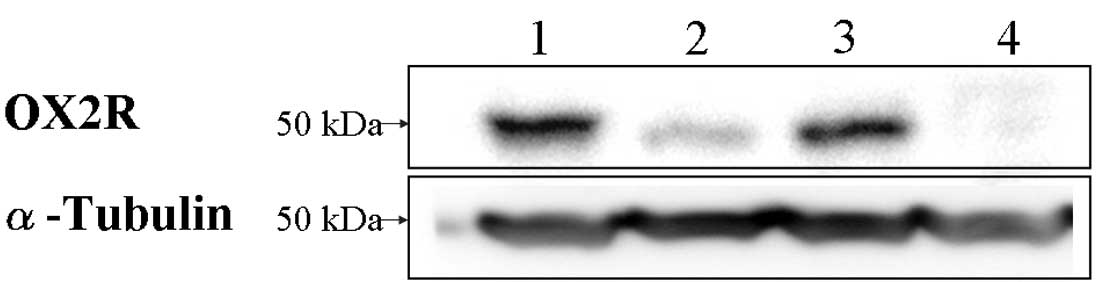
OX2R protein expression in cancer cells
assessed by western blotting
To investigate the expression of OX2R protein on
cancer cells, we performed western blot analyses on the same cancer
cell lines. Since antibodies against OX2R available for both
western blotting and IHC were limited, we produced a polyclonal
antibody against OX2R. We immunized two rabbits with three peptides
specific to OX2R and purified serum as described in Materials and
methods. Fig. 2 shows that all 3
cancer cell lines originated from the gallbladder (TGBC2),
glioblastoma (SF295) and squamous cell carcinoma of the head and
neck (YCUM862), which were positive for OX2R mRNA expressed the
protein of OXR2. Conversely, OX2R was not detected in the OE19 cell
line, which did not express the mRNA of OX2R in RT-PCR analysis and
was used as a negative control (Fig.
2). This result indicates that the three cell lines express
OX2R.
OX2R expression in clinical tissue array
samples
Based on the results of the mRNA and protein
expression analysis, we performed an IHC analysis to analyze
localization of OX2R on multiple organs and its frequency of
expression among clinical cancer using the polyclonal antibody
against OX2R shown at the western blot analysis stage. We prepared
221 clinical samples from the three tissue arrays purchased, which
enabled us to obtain insights on the objectives described
previously. Details of tissue arrays and protocols are described in
Materials and methods.
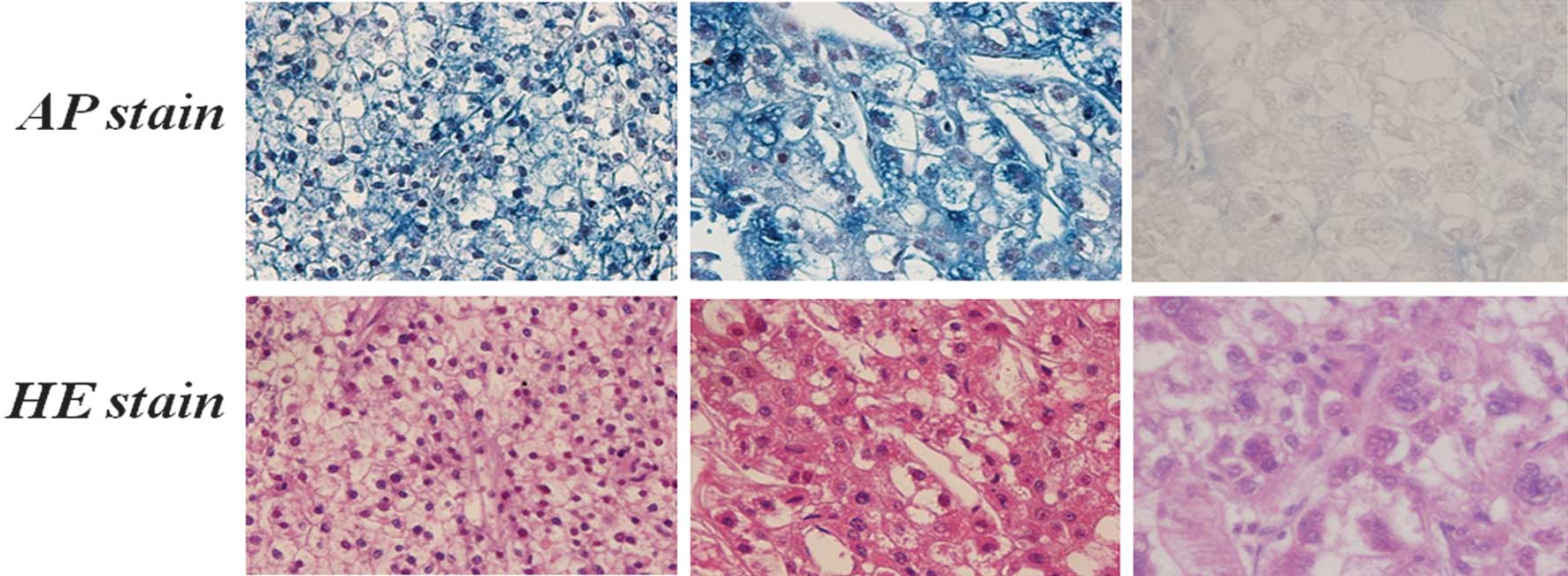
As shown in Table
II, 69 of the 221 samples expressed OX2R, including 8 samples
with strong staining and 61 samples with moderate staining. The
remaining 152 samples did not react with the OX2R antibody. We
observed that the expression of OX2R was located on the cell
membrane, where receptors should be located, as shown in Fig. 3. These results suggest that 31.2% of
clinical cancer samples express OX2R on their cell membrane. It is
also assumed that cancer organs such as the stomach, salivary gland
and larynx expressed OX2R more frequently than other organs.
 | Table IISummary of OX2R immunohistochemistry
analyses in a variety of cancers using three tissue arrays. |
Table II
Summary of OX2R immunohistochemistry
analyses in a variety of cancers using three tissue arrays.
| | Staining
intensity | |
|---|
| |
| |
|---|
| Organ | No. of patients | − | + | ++/+++ | Positive samples
(%) |
|---|
| Liver | 80 | 71 | 7 | 2 | 11 |
| Stomach | 34 | 16 | 17 | 1 | 53 |
| Colon | 20 | 13 | 7 | 0 | 35 |
| Salivary gland | 15 | 8 | 6 | 1 | 47 |
| Larynx | 15 | 7 | 7 | 1 | 53 |
| Rectum | 9 | 6 | 2 | 1 | 33 |
| Lung | 9 | 8 | 1 | 0 | 11 |
| Esophagus | 6 | 6 | 0 | 0 | 0 |
| Small intestine | 6 | 0 | 5 | 1 | 100 |
| Nasopharynx | 6 | 4 | 2 | 0 | 33 |
| Pancreas | 6 | 3 | 3 | 0 | 50 |
| Gallbladder | 5 | 2 | 3 | 0 | 60 |
| Tongue | 2 | 2 | 0 | 0 | 0 |
| Maxilla | 2 | 0 | 1 | 1 | 100 |
| Bile duct | 2 | 2 | 0 | 0 | 0 |
| Mandible | 1 | 0 | 1 | 0 | 100 |
| Lower lip | 1 | 1 | 0 | 0 | 0 |
| Tonsil | 1 | 1 | 0 | 0 | 0 |
| Anus | 1 | 1 | 0 | 0 | 0 |
| Total | 221 | 151 | 62 | 8 | |
Discussion
This study showed the presence of OX2R on several
clinical cancer samples including salivary gland, stomach and small
intestine cancer. We demonstrated the existence of OX2R on cell
lines as well as on clinical samples using tissue arrays. These
results suggest that OX2R is overexpressed in certain types of
cancer and may have biological roles in cancer cells. To reveal the
functions of OX2R in cancer cells, we conducted additional
experiments to confirm the reaction of OX2R-positive cells with a
ligand, orexin B. We observed minimal growth promotion of
OX2R-positive cancer cells despite increasing ligand doses (data
not shown). Thus, it is suggested that orexin B does not affect the
growth of OX2R-expressing cancer cells and that traditional
molecular-targeted approaches, such as anti-OX2R antibodies, may
not work effectively. Reacting specifically to OX2R and exhibiting
toxicity only inside the OX2R-expressing cells may be criteria for
drug candidates to target OX2R as a potential mode of action.
Based on insights revealed in this study, we believe
that antibodies or ligands conjugated to natural toxins or
chemicals, termed immunotoxins or ADCs (21–23),
are likely to be an ideal strategy to target receptors such as
OX2R. Immunotoxin or ADCs is likely to be an appropriate approach
to treat OX2R-positive cancer cells, since antibodies or ligands
that bind OX2R themselves are often non-cytotoxic, as previously
noted. Immunotoxin is a rationally designed anticancer agent with
potent toxins that target cell-surface antigens or receptors on
cancer cells. An immunotoxin strategy enables the OX2R antibody to
add specific cytotoxicity to cancer cells by fusing the OX2R
antibody to toxins. This strategy may also avoid toxicity to the
hypothalamus where OX2R is normally expressed with the function of
the blood-brain-barrier to maintain homeostasis (24,25).
In addition to the direct killing of cancer cells,
antibody-dependent cell-mediated cytotoxicity (ADCC) occurs if the
antibody to OX2R is conjugated with toxic moiety.
OX2R immunotoxin is an alternative option for
patients with resistance to current antibody therapies, since its
anti-tumor effect varies from current molecular-targeting drugs.
Moreover, dual-specific immunotoxin enables the enhancement of this
type of multi-anti-tumor effect. Conjugating the OX2R antibody and
kinase inhibitors may elicit both the ADCC and multi-kinase
inhibition effects.
In this study, the presence of OX2R on a variety of
types of cancer was suggested. However, further studies are
required to confirm the presence of OX2R, since the number of
clinical samples in the same organs are limited. At present,
additional IHC experiments are underway using a larger number of
clinical samples to confirm statistical significance of the
presence of OX2R and the correlation with stage and cancer type.
Therefore, further investigations on OX2R and new immunotoxin/ADC
approaches may offer new therapeutic options for cancer
patients.
Acknowledgements
We thank Nana Kawaguchi and Kumi Kodama (Department
of Pharmacoepidemiology, Kyoto University) for technical assistance
with cell culturing and Motomichi Matsuzaki (Department of
Biomedical Chemistry, The University of Tokyo) for advice on
immunohistochemistry.
References
|
1
|
Hickman JA: Apoptosis induced by
anticancer drugs. Cancer Metastasis Rev. 11:121–139. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersson A, Fagerberg J, Lewensohn R and
Ehrsson H: Pharmacokinetics of cisplatin and its monohydrated
complex in humans. J Pharm Sci. 85:824–827. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szakacs G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilson TR, Longley DB and Johnston PG:
Chemoresistance in solid tumours. Ann Oncol. 17:315–324. 2006.
View Article : Google Scholar
|
|
5
|
Sharom FJ: ABC multidrug transporters:
structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Izumi H, Torigoe T, Ishiguchi H, Uramoto
H, Yoshida Y, Tanabe M, et al: Cellular pH regulators: potentially
promising molecular targets for cancer chemotherapy. Cancer Treat
Rev. 29:541–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Husain I, Mohler JL, Seigler HF and
Besterman JM: Elevation of topoisomerase I messenger RNA, protein,
and catalytic activity in human tumors: demonstration of tumor-type
specificity and implications for cancer chemotherapy. Cancer Res.
54:539–546. 1994.PubMed/NCBI
|
|
8
|
Liu H, Savaraj N, Priebe W and Lampidis
TJ: Hypoxia increases tumor cell sensitivity to glycolytic
inhibitors: a strategy for solid tumor therapy (Model C). Biochem
Pharmacol. 64:1745–1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boon T and Old LJ: Cancer Tumor antigens.
Current Opinion in Immunology. 9:681–683. 1997. View Article : Google Scholar
|
|
10
|
Singh Y, Palombo M and Sinko PJ: Recent
trends in targeted anticancer prodrug and conjugate design. Curr
Med Chem. 15:1802–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koji K, Oumi N, Ryuichi M and Ryozo N:
Targeted anticancer immunotoxins and cytotoxic agents with direct
killing moieites. Sci World J. 6:781–790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohara H, Hasegawa Y, Kawabe T, Ichiyama S,
Hara T, Shimono Y, et al: Effect of gene transfer of tumor necrosis
factor receptors into human lung carcinoma cell line. Jpn J Cancer
Res. 89:589–595. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakurai T, Amemiya A, Ishii M, Matsuzaki
I, Chemelli RM, Tanaka H, et al: Orexins and orexin receptors: a
family of hypothalamic neuropeptides and G protein-coupled
receptors that regulate feeding behavior. Cell. 92:573–585. 1998.
View Article : Google Scholar
|
|
14
|
Peyron C, Faraco J, Rogers W, Ripley B,
Overeem S, Charnay Y, et al: A mutation in a case of early onset
narcolepsy and a generalized absence of hypocretin peptides in
human narcoleptic brains. Nat Med. 9:991–997. 2000.PubMed/NCBI
|
|
15
|
Thannickal TC, Moore R, Nienhuis R,
Ramanathan L, Gulyani S, Aldrich M, et al: Reduced number of
hypocretin neurons in human narcolepsy. Neuron. 27:469–474. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nambu T, Sakurai T, Mizukami K, Hosoya Y,
Yanagisawa M and Goto K: Distribution of orexin neurons in the
adult rat brain. Brain Res. 827:243–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rouet-Benzineb P, Rouyer-Fessard C, Avondo
V, Pouzet C, Yanagisawa M, et al: Orexin acting at native OX1
receptor in colon cancer and neuroblastoma cells or at recombinant
OX1 receptor suppress cell growth by inducing apoptosis. J
Biological Chem. 279:45875–45886. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spinazzi R, Rucinski M, Neri G,
Malendowicz LK and Nussdorfer GG: Preproorexin and orexin receptors
are expressed in cortisol-secreting adrenocortical adenomas, and
orexins stimulate in vitro cortisol secretion and growth of tumor
cells. J Clin Endocrinol Metab. 90:3544–3549. 2005. View Article : Google Scholar
|
|
19
|
Karteris E, Chen J and Randeva H:
Expression of human prepro-orexin and signaling characteristics of
orexin receptors in the male reproductive system. J Clin Endocrinol
Metab. 89:1957–1962. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Digby JE, Chen J, Tang JY, Lehnert H,
Matthews RN and Randeva HS: Orexin receptor expression in human
adipose tissue: effects of orexin-A and orexin-B. J Endocrinology.
191:129–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reiter Y: Recombinant immunotoxins in
targeted cancer cell therapy. Adv Cancer Res. 81:93–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pastan I, Chaudhary V and FitzGerald DJ:
Recombinant toxins as novel therapeutic agents. Annu Rev Biochem.
61:331–354. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson DA and Laguzza BC: Antitumor
xenograft activity with a conjugate of a Vinca derivative and the
squamous carcinoma-reactive monoclonal antibody PF1/D. Cancer Res.
47:3118–3122. 1987.PubMed/NCBI
|
|
24
|
Levin VA: Relationship of octanol/water
partition coefficient and molecular weight to rat brain capillary
permeability. J Med Chem. 23:682–684. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun H, Dai H, Shaik N and Elmquist WE:
Drug efflux transporters in the CNS. Adv Drug Delivery Rev.
55:83–105. 2003. View Article : Google Scholar
|