Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer mortality worldwide (1). It contributes to 14.8% of all cancer
mortality in Egypt, with a higher incidence in males (17.3%) than
in females (11.5%). It is the second most frequent cancer type in
Egyptian males after bladder cancer and the eighth most frequent in
Egyptian females (2). The high
incidence of HCC in Egypt is attributed to the high prevalence of
hepatitis C virus (HCV). HCV is currently the most significant
public health problem in Egypt with an overall prevalence of 17.4%
in males and 12.2% in females, and it increases with age to a
prevalence of 39.4% in 55–59-year olds (3). Chronic hepatitis usually leads to the
sequential occurrence of liver fibrosis and cirrhosis with a high
risk of development of HCC. It has been estimated that 20% of
HCV-infected patients develop liver cirrhosis and approximately 40%
of these develop HCC within 10–15 years (4).
The insulin-like growth factor (IGF) signalling
system is an essential regulator of growth and development. IGF-1
has a strong effect on cell proliferation and differentiation and
is a potent inhibitor of apoptosis (5). We previously showed that during
experimental hepatocarcinogenesis the IGF-1 receptor (IGF-1R),
which mediates IGF-1 and IGF-2 signals, and its downstream proteins
are initially overexpressed in preneoplastic foci, which may
reflect that hepatocytes in their early stage of transformation are
more sensitive to stimulation by circulatory IGFs. This
overexpression is gradually reduced in later stages, and it is
almost completely lost in poorly differentiated HCC (6,7), which
may indicate an IGF-independent state. The liver is the major site
of IGF-1 production (8). Serum
IGF-1 levels are influenced by many factors including age and
nutrition, but growth hormone (GH) is the principal regulator of
IGF-1 production in the liver and secretion into the blood stream.
IGF-2 is produced in various tissues throughout life. Serum
concentration of IGF-2 remains stable following puberty, and is not
regulated by GH (9). The
bioavailability of IGFs is modulated by high-affinity binding
proteins known as insulin-like growth factor binding proteins
(IGFBPs) (from 1 to 7), of which the liver is a significant source.
Most of the IGF-1 in circulation is bound by IGFBP-3, whose
circulating levels are more than 10-fold higher than any of the
other binding proteins (10).
Several studies have reported the reduction of serum IGF-1 levels
associated with the development of human HCC from cirrhosis
(11,12), the increase of IGF-2 levels in HCC
(13), and the reduction of IGFBP-3
levels in liver cirrhosis in comparison to healthy subjects
(14,15).
Patients with liver cirrhosis should be carefully
monitored for the early detection of HCC. Currently, surveillance
for HCC development is based on a six-month α-fetoprotein (AFP)
determination and ultrasound examination. However, AFP levels do
not discriminate between benign liver disease and HCC.
Additionally, they have poor sensitivity and specificity (16) and vary with the etiology of liver
disease, treatment and tumor stage (17). Therefore, it is of utmost importance
to identify sensitive biomarkers that allow the prediction of HCC
development at an early stage, and predict the severity of
cirrhosis stage, and at the same time are easily measurable and
minimally invasive (18).
The present study was carried out to i) investigate
whether serum levels of IGF-1, IGF-2 and IGFBP-3 may be used to
discriminate between the stages of hepatic dysfunction in Egyptian
patients with liver cirrhosis assessed by Child-Pugh (CP) score,
and subsequently ii) whether they may be used individually or in
combination as biomarkers for the development of HCC in patients
with liver cirrhosis and to correlate the levels with the HCC stage
and other standard HCC biomarkers such as AFP.
Materials and methods
Patients
A total of 241 subjects were recruited into the
present study between March 2010 and April 2011 at the Faculty of
Medicine, Alexandria University Hospital. The subjects were divided
into three groups: group I included 79 patients with chronic HCV
and liver cirrhosis. This group was further subdivided into three
subgroups according to the CP score system (11 patients with CP A,
male/female = 5/6, 29 patients with CP B, male/female = 14/15 and
39 patients with CP C, male/female = 19/20). Group II included 62
patients with HCC in addition to HCV-induced liver cirrhosis
(male/female = 48/14). Group III included 100 healthy volunteers as
controls (male/female = 40/60) (Table
I). For all subjects, height and weight were measured and the
body mass index (BMI) was calculated as body weight in kilograms
divided by the square of height in meters (kg/m2).
Subjects with BMI >30, with a history of alcohol abuse, heart
disease, kidney disease, diabetes mellitus, endocrine-related
diseases, tobacco-related cancers and hepatitis B virus infection
were excluded from the study. At the time of enrolment, the
patients were evaluated for complete medical history and physical
examination. Ultrasound of the liver, liver functions and complete
blood picture (CBC), serum albumin, serum bilirubin and prothrombin
time were performed for all patients. Screening for HCV Ab was
performed routinely using an ELISA assay, and confirmed by PCR
according to Kato et al (19). In the present study, the HCC staging
followed the Barcelona Clinic Liver Cancer (BCLC) staging
system.
 | Table IClinical characteristics of the
subjects enrolled in the present study. |
Table I
Clinical characteristics of the
subjects enrolled in the present study.
| Group I | Group II | Group III | |
|---|
|
|
|
| |
|---|
| Clinical
parameters | Patients with
HCV-cirrhosis (n=79) | Patients with HCC
on HCV-cirrhosis | Healthy
subjects | P-valuea |
|---|
|
| | | |
|---|
| CP A | CP B | CP C | | | |
|---|
| No. of subjects (%
from total) | 11 (13.9) | 29 (36.7) | 39 (49.4) | 62 | 100 | |
| Age (years) (Mean ±
SD) | 47.5 ±4.9 | 46.7 ±6.3 | 46.8 ±6.8 | 48.7±4.6 | 46.8±6.7 | 0.1 |
| BMI (Mean ±
SD) | 24.3 ±0.6 | 24.7 ±0.8 | 24.8 ±0.6 | 25.1±2.3 | 24.7±2.3 | 0.3 |
| Blood glucose
(mg/dl) (Mean ± SD) | 90.5 ±14.6 | 91.3 ±18.9 | 85.8 ±19.5 | 91.7±17.5 | 90.6±10.5 | 0.4 |
Ethical approval
All patients and healthy controls who participated
in the present study signed an informed consent form. The study
protocol was approved by the Ethics Committee of the Faculty of
Medicine, Alexandria University, Egypt and is in accordance with
the Helsinki Declaration of 1975.
Sample collection, determination of
glucose and AFP
Venous blood (5 ml) was withdrawn from the subjects
following 12 h overnight fasting. Serum fasting blood glucose was
measured immediately for all subjects with a Cobas Integra 400
analyzer (Roche Diagnostics, USA). AFP was measured using a Siemens
ADVIA Centaur analyzer (Siemens Healthcare Diagnostics, Germany).
Serum samples were then stored at −80°C until use.
Quantitative detection of serum IGF-1,
IGF-2, and IGFBP-3
Serum IGF-I, IGF-2 and IGFBP-3 were measured using
the following ELISA kits according to the manufacturer's
instructions: IGF-1 ELISA kit (DRG International, USA), IGF-2
active non-extraction ELISA kit (Diagnostic Systems Laboratories,
USA) and IGFBP-3 Quantikine ELISA Kit (R&D Systems, USA).
Absorbance was read at 450 nm for the three kits in a microplate
reader.
Statistical analysis
Statistical analysis was performed using the SPSS
statistical package version 16.00 (SPSS Inc, Chicago, IL, USA).
Data were expressed as the mean ± standard deviation. A receiver
operating curve (ROC) was used to establish the cut-off values that
provided the maximal diagnostic accuracy. Positive and negative
predictive values were also determined for each of the following
parameters; IGF-1, IGF-2, IGFBP-3, IGF-1 and IGF-2, IGF-1 and
IGFBP-3, IGF-2 and IGFBP-3 in predicting HCC. P<0.05 was
considered statistically significant.
Results
Subjects
The present study was conducted on 241 subjects
divided into three groups: group I included patients with chronic
HCV and liver cirrhosis (n=79), group II included patients with HCC
developed from HCV-induced cirrhosis (n=62), and group III included
healthy subjects (n=100). Clinical characteristics of the subjects
are shown in Table I. Group I was
classified into 11 patients with CP A (mean score 5.66±0.49), 29
patients with CP B (mean score 8.22±0.84) and 39 patients with CP C
(mean score 11.62±1.11). No significant difference was found
between the three subgroups in terms of age, BMI and glucose levels
(Table I). Similarly, there were no
significant differences in these parameters between patients with
cirrhosis and healthy subjects (group III), and HCC patients (group
II), and between groups II (HCC) and III (controls), respectively
(p>0.05).
AFP levels in the study groups
The mean AFP levels were 3.5±1.9, 110±227.1 and
207±257.4 ng/ml in the healthy subjects, patients with cirrhosis
and HCC patients, respectively (Table
II). These values were significant between patients with
cirrhosis and control subjects, HCC and control subjects, as well
as between cirrhosis and HCC patients. Furthermore, we measured AFP
levels in each of the three cirrhosis subgroups of group I, which
were 51.73±108.8, 127.6±248.8 and 114±236.2 ng/ml in CP A, CP B and
CP C, respectively (Table III).
No significant difference was observed in the AFP values in
patients with cirrhosis classified according to CP scores
(p>0.05).
 | Table IISerum levels of IGF-1, IGF-2, IGFBP-3
and α-fetoprotein in patients with cirrhosis, HCC and healthy
subjects. |
Table II
Serum levels of IGF-1, IGF-2, IGFBP-3
and α-fetoprotein in patients with cirrhosis, HCC and healthy
subjects.
| Group I | Group II | Group III | F test | Significance
between groupsa |
|---|
|
|
|
|---|
| Cirrhosis patients
(n=79) | HCC patients
(n=62) | Healthy subjects
(n=100) |
|---|
| IGF-1 (ng/dl) | 249±131 | 166.7±121.4 | 394.7±60.2 | 97.39a | I, III
II, III
I, II |
| IGF-2 (ng/dl) | 649.1±473.4 | 549±405 | 2197.5±499.9 | 334.7a | I, III
II, III |
| IGFBP-3
(ng/dl) | 1474.3±1042.2 | 456.2±268 | 3131.4±1159.7 | 100.6a | I, III
II, III
I, II |
| AFP (ng/dl) | 110±227.1 | 207±257.4 | 3.5±1.9 | 11.5a | I, III
II, III
I, II |
 | Table IIISerum levels of IGF-1, IGF-2 and
IGFBP-3 and α-fetoprotein in patients with cirrhosis classified
according to Child-Pugh scores into A, B and C. |
Table III
Serum levels of IGF-1, IGF-2 and
IGFBP-3 and α-fetoprotein in patients with cirrhosis classified
according to Child-Pugh scores into A, B and C.
| Group I | | |
|---|
|
| | |
|---|
| Child-Pugh A
(n=11) | Child-Pugh B
(n=29) | Child-Pugh C
(n=39) | F test | Significance
between groupsa |
|---|
| IGF-1 (ng/ml) | 377.3±71.7 | 268.5±100.2 | 198.5±138.3 | 11.5a | CP A, B
CP A, C
CP B, C |
| IGF-2 (ng/ml) | 1179.5±575.8 | 736.9±413.6 | 435.2±329.9 | 17.1a | CP A, B
CP A, C
CP B, C |
| IGFBP-3
(ng/ml) | 2638.6±919.2 | 1891.5±1148.4 | 853.4±396.9 | 14.6a | CP A, C
CP B, C |
| AFP (ng/ml) | 51.73±108.8 | 127.6±248.8 | 114±236.2 | 0.5 | |
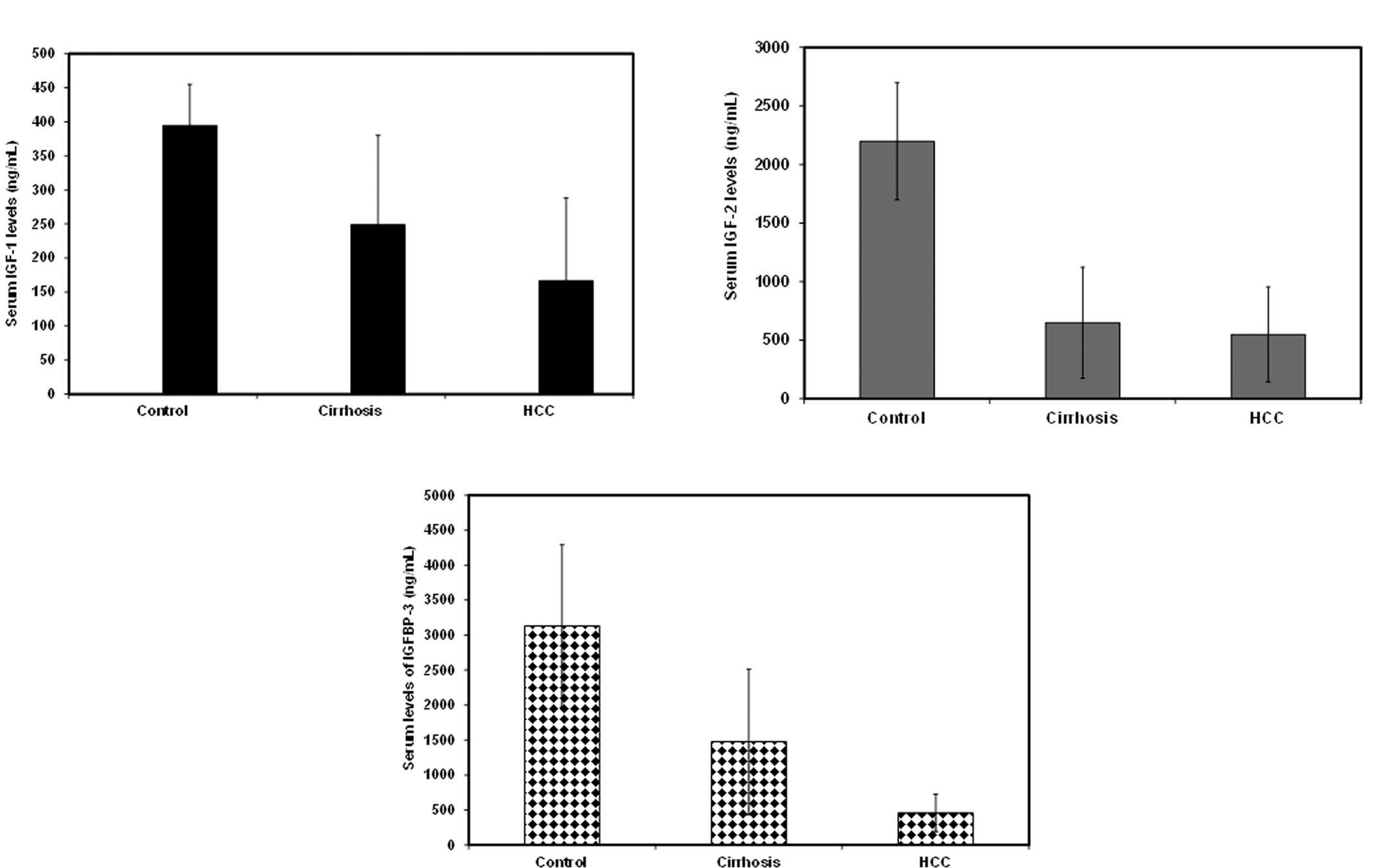
Serum IGF-1, IGF-2 and IGFBP-3 levels are
reduced in cirrhosis and HCC patients
In the present study, serum levels of IGF-1, IGF-2
and IGFBP-3 were measured in each of the three groups (patients
with cirrhosis, with HCC and healthy subjects) (Table II, Fig.
1). The levels in the normal subjects were 394.7±60.2,
2197.5±499.9 and 3131.4±1159.7 ng/ml for IGF-1, IGF-2 and IGFBP-3,
respectively. IGF-1 levels were found to be significantly reduced
in cirrhosis (249±131 ng/ml) and in HCC patients (166.7±121.4
ng/ml) in comparison to healthy subjects. The reduction of IGF-1
levels was also statistically significant between cirrhosis and HCC
patients. Similarly, serum IGF-2 levels decreased significantly in
cirrhosis (649.1±473.4 ng/ml) and in HCC patients (549±405 ng/ml)
in comparison to healthy subjects; however, there was no
significant difference between IGF-2 levels in patients with
cirrhosis and those with HCC. Serum IGFBP-3 levels followed the
same pattern with a significant reduction in cirrhosis
(1474.3±1042.2 ng/ml) and in HCC patients (456.2±268 ng/ml) in
comparison to healthy subjects. The reduction in serum IGFBP-3
levels was significant between control and cirrhosis patients,
control and HCC patients, as well as between cirrhosis and HCC
patients (p≤0.05) (Table II,
Fig. 1). In the present study, the
majority of the HCC patients enrolled were either in the
intermediate stage B (44%) or advanced stage C (39%), fewer were in
the early stage A (5%) or in the terminal stage D (12%). IGFBP-3
levels showed a negative correlation with tumor stage, which was
not statistically significant (p>0.05).
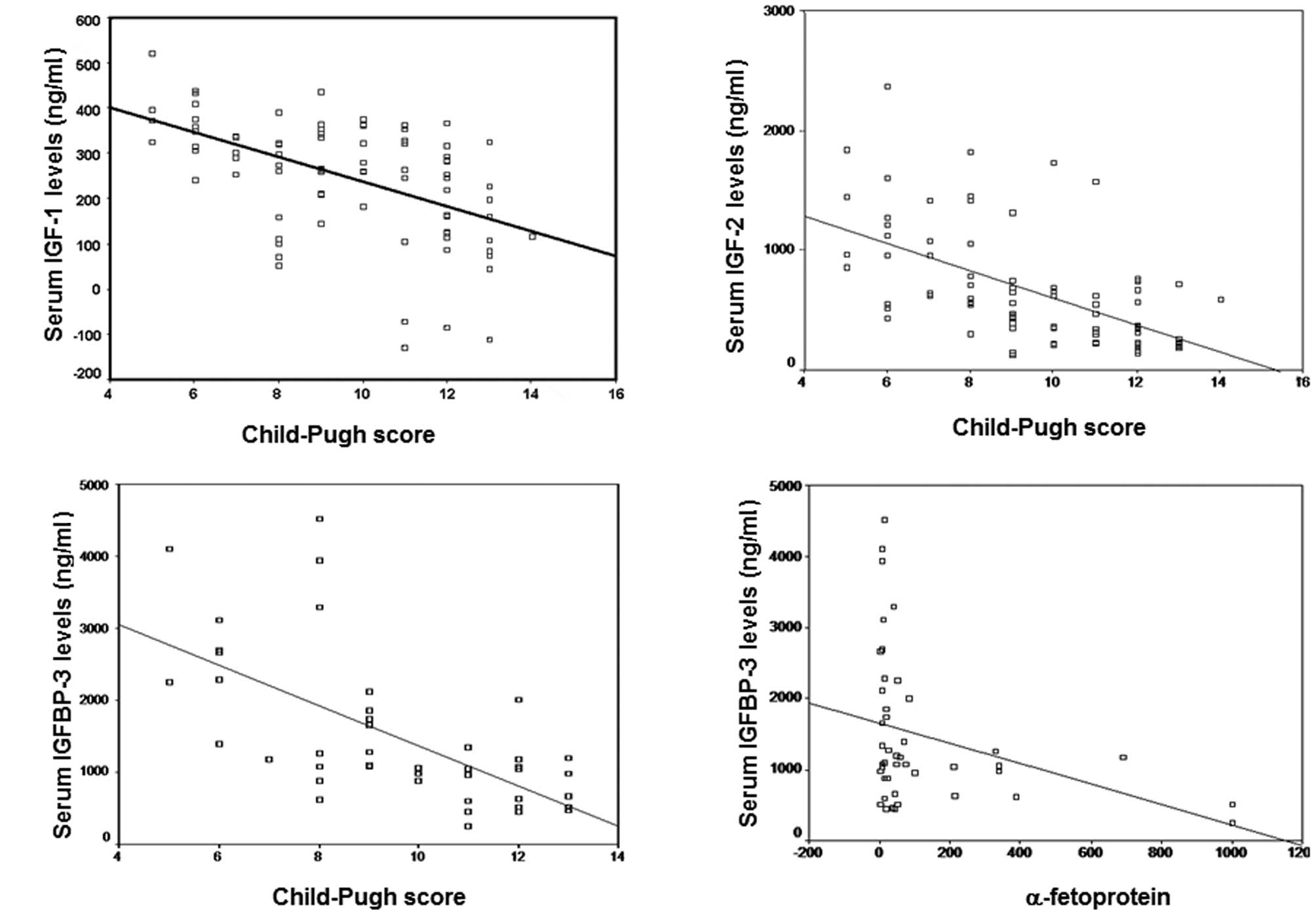
Serum IGF-1, IGF-2 and IGFBP-3 levels
negatively correlated with CP score
The mean serum levels of IGF-1, IGF-2 and IGFBP-3 in
the three CP stages of HCV-induced cirrhosis in Egyptian patients
are shown in Table III. For IGF-1
and IGF-2 there was a significant reduction in serum levels between
CP A and B, CP A and C, as well as between CP B and C,
respectively. However, IGFBP-3 levels were reduced significantly
between CP A and C, and between B and C, but not between A and B.
The negative correlation between the CP score and IGF-1 (r=−0.51),
IGF-2 (r=0.58) and IGFBP-3 levels (r=0.63), respectively, is shown
in Fig. 2A-C (p≤0.05). Furthermore,
the serum levels of IGF-1 and IGF-2 were found to be significantly
lower in HCC patients than those with CP A and B liver cirrhosis
(p≤0.05). However, no significant difference was observed between
IGF-1 and IGF-2 levels in HCC cases and those with CP C stage of
liver cirrhosis (p=0.16). By contrast, the mean levels of IGFBP-3
were significantly lower in HCC patients than the mean levels of CP
A, B and C patients, respectively (p≤0.05).
IGFBP-3 levels negatively correlate with
AFP in liver cirrhosis, but not in HCC patients
As we propose the serum levels of IGF-1, IGF-2 and
IGFBP-3 to be markers for progression of hepatic dysfunction and
development of HCC in patients with cirrhosis, we studied whether
there is a correlation between their levels and those of the
well-established HCC marker AFP. A significant negative correlation
was found only between IGFBP-3 and AFP in patients with liver
cirrhosis (r=−0.32, p=0.03) (Fig.
2D). By contrast, no significant difference was found between
the levels of IGF-1 and IGF-2 and AFP in the same group of
patients. Furthermore, no correlation was found between AFP levels
and the levels of IGF-1, 2 and IGFBP-3 in HCC patients.
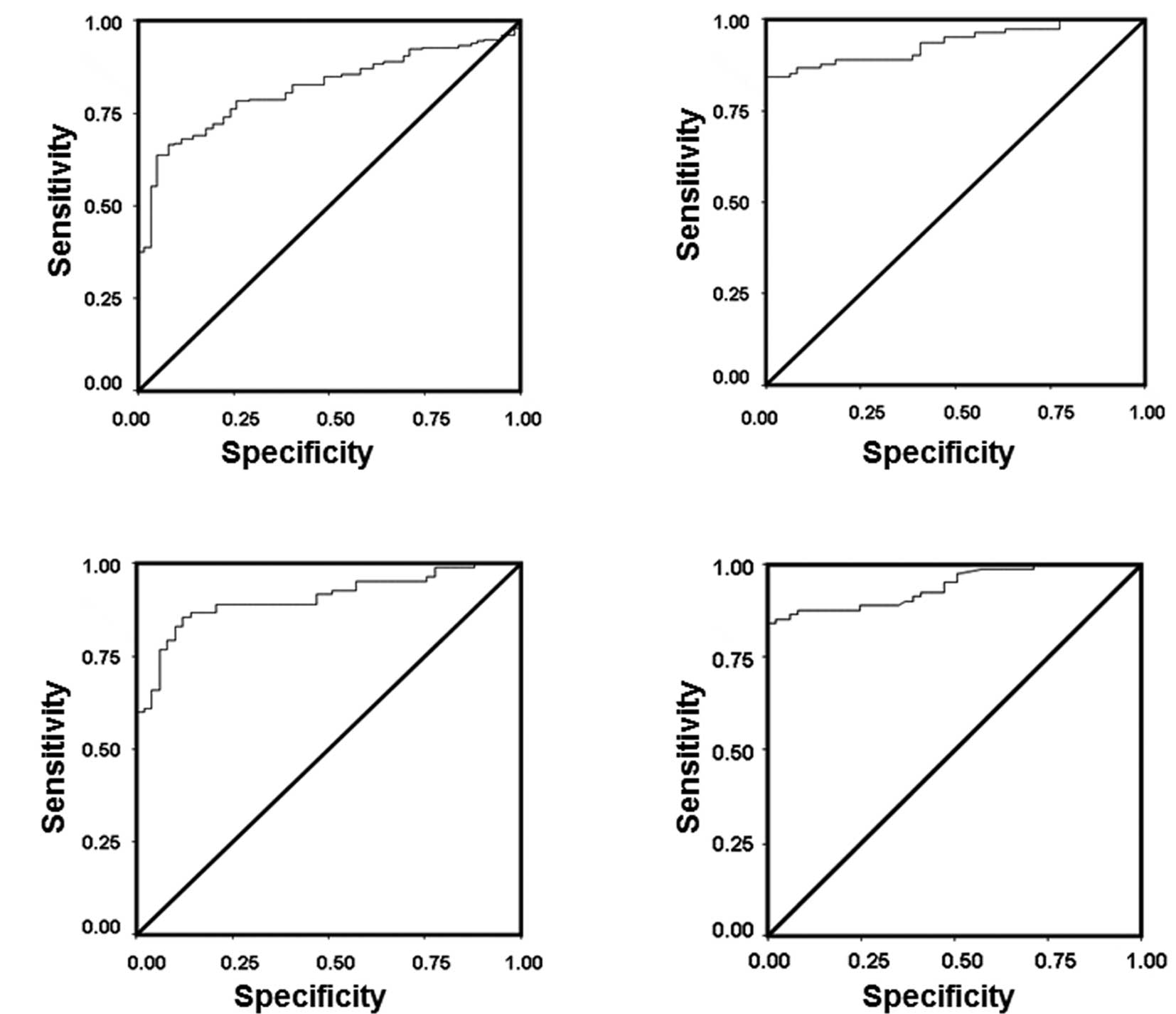
Serum IGFBP-3 levels are more effective
predictors than IGF-1 and IGF-2 for the development of HCC in
Egyptian patients with cirrhosis
Receiver operating curves (ROC) were used to
demonstrate the diagnostic accuracy of IGF-1, IGF-2 and IGFBP-3
individually and in combination in the discrimination between
cirrhosis and HCC; and to determine the cut-off values for IGF-1,
IGF-2 and IGFBP-3 serum levels for prediction of the development of
HCC in Egyptian patients with liver cirrhosis (Fig. 3). The sensitivity, specificity,
cut-off value, positive and negative predictive values (PPV, NPV)
and diagnostic accuracy for each parameter are shown in Table IV. The areas under the ROC curves
(AUC) were 0.78 for IGF1, 0.74 for IGF-2, 0.93 for IGFBP-3
(Fig. 3A), 0.78 for combined IGF-1
and IGF-2 (Fig. 3B), 0.93 for
combined IGF-1 and IGFBP-3 (Fig.
3C) and 0.9 for combined IGF-2 and IGFBP-3 (Fig. 3D) (Table IV). These data indicate that
IGFBP-3 levels, whether alone or in combination with IGF-1 or
IGF-2, had the highest AUC value (0.9–0.93), indicating a higher
power to discriminate between HCC and cirrhosis, and, hence a high
clinical value. In our study, IGFBP-3 is proposed as a marker for
predicting the development of HCC at an optimal cut-off value of
<682.6 at 87% sensitivity and 80% specificity. The PPV and NPV
of IGFBP-3 at the selected cut-off point were 78 and 88%,
respectively (Table IV).
 | Table IVSensitivity, specificity, diagnostic
accuracy, positive and negative predictive values and area under
the ROC curves for IGF-1, IGF-2, IGFBP-3 and combinations of two
parameters together at the optimal cut-off values. |
Table IV
Sensitivity, specificity, diagnostic
accuracy, positive and negative predictive values and area under
the ROC curves for IGF-1, IGF-2, IGFBP-3 and combinations of two
parameters together at the optimal cut-off values.
| Sensitivity
(%) | Specificity
(%) | Cut-off value | PPV (%) | NPV (%) | Diagnostic accuracy
(%) | AUC |
|---|
| IGF-1 | 81 | 62 | <207.4 | 60.6 | 88.2 | 80.8 | 0.78 |
| IGF-2 | 75 | 60 | <414.5 | 49.2 | 83.8 | 74.3 | 0.74 |
| IGFBP-3 | 87 | 80 | <682.6 | 78 | 88 | 84.3 | 0.93 |
| IGF-1, IGF-2 | 73 | 84 | <772 | 52.4 | 68.3 | 59.1 | 0.78 |
| IGF-1, IGFBP-3 | 89 | 82 | <885 | 81.6 | 82 | 81.9 | 0.93 |
| IGF-2, IGFBP-3 | 86 | 84 | <1246 | 80.3 | 79.5 | 80 | 0.90 |
Discussion
In the present study, we investigated the serum
levels of IGF-1, IGF-2 and IGFBP-3 in early, intermediate and late
stages of cirrhosis, assessed by the CP score, as well as in HCC
patients. We also studied their correlation with the HCC stage, and
with AFP serum levels. In this study, we focused on HCV-induced
cirrhosis since the majority of HCC cases in Egypt develop from
chronic hepatitis C infection. We found that serum levels of IGF-1,
IGF-2, IGFBP-3 were reduced significantly in cirrhosis and in HCC
patients in comparison to the controls. Moreover, the reduction in
IGF-1, IGFBP-3, but not IGF-2 levels was significant in HCC in
comparison to patients with cirrhosis. The reduction in IGF-1,
IGF-2 and IGFBP-3 levels negatively correlated with the CP scores
(A, B and C) and only IGFBP-3 levels showed a significant negative
correlation with AFP levels and a negative correlation with the HCC
stage, which was not statistically significant.
In agreement with these results, Aishima et
al (20) showed an
insignificant negative correlation between HCC staging and serum
IGFBP-3 and IGF-1 levels. Moreover, in the present study, IGFBP-3
levels significantly discriminated between cirrhosis and HCC at a
sensitivity of 87%, specificity of 80% and a cut-off value of
<682.6 ng/ml. The PPV and NPV at the optimal IGFBP-3 cut-off
value were 78 and 88%, respectively. Experts in risk prediction
encourage the use of predictive values to assess the clinical
relevance of biomarkers (21).
IGF-1 and IGF-2 had an AUC of 0.78 and 0.74, respectively, and
their combined detection did not increase their AUC, sensitivity or
specificity. By contrast, when IGFBP-3 values were combined with
those of either IGF-1 or IGF-2, their AUCs were increased to 0.93
and 0.9, respectively, with a concomitant increase in both the
sensitivity and specificity. Therefore, concomitant to AFP, IGFBP-3
is a promising marker for the prediction of HCC developing from
HCV-cirrhosis in Egyptian patients.
Our results are consistent with several published
reports on the serum levels of IGFBP-3 in cirrhosis and in HCC
(15,22–24)
and in chronic hepatitis prior to developing cirrhosis (25). Furthermore, in HCC patients with or
without cirrhosis, IGFBP-3 mRNA levels (13,26,27)
and protein levels (28) were lower
than those in non-tumor tissues. The decrease in IGFBP-3 mRNA
correlated with a tumor-specific IGFBP-3 promoter hypermethylation
(26). Findings of other studies
have shown that IGFBP-3 protein and IGF-1R were lost or
underexpressed in poorly differentiated HCC, but overexpressed in
well-differentiated HCC in comparison to normal liver tissue and
that IGFBP-3 levels significantly correlated with tumor size,
histological differentiation, capsular and portal invasion
(20), establishing a role for
IGFBP-3 in negatively regulated cell proliferation. The same
authors reported that the addition of exogenous IGFBP-3 markedly
blocked IGF-1- and IGF-2-stimulated proliferation of KYN-2 and
HepG2 cells, and also suppressed IGF-1-induced invasion in KYN-2
cells. The results of Aishima et al have shown that the
serum levels of IGFBP-3 correlate with the tissue levels of IGFBP-3
protein (20). Thus, in the present
study, IGFBP-3 serum levels serve not only as a biomarker for the
prediction of hepatic dysfunction and progression towards
malignancy, but may have a pathophysiological significance. The
molecular mechanism underlying the reduction in serum IGFBP-3
levels in HCV-induced HCC remains to be elucidated. Previous
reports demonstrated the association between the hepatitis B virus
oncogenic protein (HBx) modulation of DNA methylation and the
downregulation of IGFBP-3 expression in cell lines, animal models
and human tumor samples (29).
Furthermore, it has been shown that HBx recruits histone
deacetylase 1 to repress IGFBP-3 transcription (30). Moreover, IGFBP-3 has been shown to
trigger intracellular signalling: Stimulation of phosphotyrosine
phosphatase and phosphatidylinositol-3 kinase activities and
increase in intracellular calcium (31–33)
potentially through its own ‘putative’ receptor. However, no
underlying genetic polymorphisms have been detected in IGFBP-3 to
contribute to its downregulation in HCC (34).
In agreement with our results, Wu et al
(15) showed a significant
reduction of serum IGF-1, IGF-2 and IGFBP-3 levels in patients with
cirrhosis compared to controls. Progressive reduction in IGF-2
levels observed in the present study in cirrhosis and in HCC
patients are consistent with El-Houseini et al (35). In partial disagreement with our
results, a recent study investigated the same parameters in similar
patient groups and reported that IGF-2, despite being lower in HCC
cases than in healthy controls, was significantly higher compared
to patients with liver cirrhosis and, therefore, high serum IGF-2
levels can be used as a tumor marker to discriminate HCC from
cirrhosis (36). We did not find a
significant difference in the serum levels of IGF-2 in patients
with cirrhosis in comparison to those with HCC, and both exhibited
lower serum levels than in healthy controls. The differences
between those findings and ours may be due to the sample size, as
well as the methods used to measure IGF-2. Rehem and El-Shikh
(36) conducted their studies on a
smaller sample size (60 patients with liver cirrhosis, 20 HCC
patients and 20 controls) compared to our sample size (72 patients
with liver cirrhosis, 62 HCC patients and 100 healthy controls). It
is an established fact that a larger sample size results in better
assessment with lower bias. Furthermore, we have used ELISA to
measure serum IGF-2 levels, while in the other study
radioimmunoassay (RIA) was used (36). Moreover, Rehem and El-Shikh
(36) did not report any data
concerning the diagnostic accuracy of IGF-1 and IGFBP-3, nor did
they demonstrate any significant correlation between IGF-2 and AFP
in these cases. However, the authors concluded that IGF-2 and AFP
may be used as complementary tumor markers to discriminate HCC from
cirrhosis. In contrast, our analysis of the sensitivity,
specificity and diagnostic accuracy of the three IGF members
studied revealed that IGFBP-3 was a more effective predictor for
the development of HCC than IGF-1 and IGF-2.
Other studies have demonstrated a significant
increase in IGF-2 mRNA expression in human cirrhotic liver, in
liver cancers and in the peripheral blood of HCC, in human hepatoma
cell lines, in comparison to that of normal adult liver (37–40).
This discrepancy may be attributed to the fact that in the present
study we measured only the free IGF-2 levels in serum but not mRNA.
IGF-2 levels may be lower than the mRNA since the bulk of the
former is bound to IGFBPs. We believe that serum IGF-2 levels may
not be used as a marker for HCC progression, as in our setting
IGF-2 had an AUC of 0.74, whereas it has been previously reported
that ROC curves with an AUC≤0.75 are not clinically useful
(41). A comprehensive study by
Tovar et al (13)
demonstrated overexpression of IGF-2 mRNA resulting from
reactivation of the fetal promoters P3 and P4, downregulation of
IGFBP-3, allelic loss of IGF-2R and activation of IGF-1R in a
specific subclass of human HCC; the ‘proliferation’ subclass. It is
expected that a reduction in the serum levels of IGF-1 and to a
lesser extent IGF-2 would occur in cirrhotic liver, since the main
pool of circulating IGF-1 is synthesized in liver parenchyma
(42), whose mass is greatly
reduced in cirrhotic liver, whereas IGFBP-3 is produced by Küpffer,
endothelial and hepatic stellate cells, which also synthesize IGF-2
(43–46). Liver damage is apparently not the
only cause for reduced IGF-1 levels in HCC, as reduced IGF-1 levels
were reported in males with HCC without a history of liver
cirrhosis and in virus-free metastatic liver cancer (47,48),
and did not correlate with the CP score in patients with liver
cirrhosis followed up until the development of HCC (11). In the present study, the gradual
decrease in circulating IGF-1 levels from HCC precursors to
malignancy is consistent with our previous observation, which
indicated the initial overexpression of IGF-1R in preneoplastic
stages is followed by a gradual decrease and complete loss in HCC
in rats (7). Moreover, our findings
are in concordance with reports by Huynh et al (28) and Aishima et al (20), who showed lower levels of IGF-1R
expression in a significant number of human HCC by
immunohistochemistry. Low serum levels of IGF-1 do not prove a
specific function of IGF-1 in hepatocarcinogenesis per se,
but from this and previous reports from human and experimental
hepatocarcinogenesis the following may be speculated: i) the low
levels of IGF-1 in cirrhosis and in HCC may be caused by separate
events since chronic liver disease is known to be an IGF-1
deficiency state, as reviewed by Bonefeld and Moller (49); however, the cause of low IGF-1 serum
levels in HCC patients with no history of cirrhosis remains to be
elucidated. ii) IGF-1 may only be required in initiating events of
hepatocarcinogenesis; iii) the autocrine and paracrine effects of
IGF-1 may be more relevant to cell transformation than its
endocrine effect, and iv) IGFBP-3 may have other roles in
modulating IGF-1 effects than its binding capacity.
In conclusion, although our results have showm that
serum IGF-1, IGF-2 and IGFBP-3 are reduced with the progression of
hepatic dysfunction, only IGFBP-3 may be considered as the most
promising serological marker for the prediction of the development
of HCC in Egyptian chronic HCV patients with liver cirrhosis.
Future studies should be directed towards understanding the
association between serum and tissue growth factor levels.
Acknowledgements
The authors are grateful to Professor Abbas Omar,
Professor of Clinical Oncology and Nuclear Medicine, Faculty of
Medicine, and Dr Hanan Mostafa, lecturer of Internal Medicine,
Medical Research Institute, Alexandria University for providing
blood samples from HCC patients, and to Ms. Hoda Mabrouk, Faculty
of Medicine, Alexandria University for technical assistance. This
study was supported by a research grant from the Science and
Technology Development Fund (STDF), Egypt (Grant No. 445) to Dr
Eiman Aleem.
Abbreviations:
|
IGF-1
|
insulin-like growth factor-1
|
|
IGF-2
|
insulin-like growth factor-2
|
|
IGFBP-3
|
insulin-like growth factor binding
protein-3
|
|
IGF-1R
|
insulin-like growth factor-1
receptor
|
|
GH
|
growth hormone
|
|
HCV
|
hepatitis C virus
|
|
HCC
|
hepatocellular carcinoma
|
|
CP
|
Child- Pugh score
|
|
AFP
|
α-fetoprotein
|
|
ROC
|
receiver operating curve
|
|
AUC
|
area under the ROC curve
|
References
|
1
|
Hsieh PM, Hung KC and Chen YS: Tumor lysis
syndrome after transarterial chemoembolization of hepatocellular
carcinoma: case reports and literature review. World J
Gastroenterol. 15:4726–4728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anwar WA, Khaled HM, Amra HA, El-Nezami H
and Loffredo CA: Changing pattern of hepatocellular carcinoma (HCC)
and its risk factors in Egypt: possibilities for prevention. Mutat
Res. 659:176–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sievert W, Altraif I, Razavi HA, et al: A
systematic review of hepatitis C virus epidemiology in Asia,
Australia and Egypt. Liver Int. 31(Suppl 2): 61–80. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furstenberger G and Senn HJ: Insulin-like
growth factors and cancer. Lancet Oncol. 3:298–302. 2002.
View Article : Google Scholar
|
|
6
|
Nehrbass D, Klimek F and Bannasch P:
Overexpression of insulin receptor substrate-1 emerges early in
hepatocarcinogenesis and elicits preneoplastic hepatic
glycogenosis. Am J Pathol. 152:341–345. 1998.PubMed/NCBI
|
|
7
|
Aleem E, Nehrbass D, Klimek F, Mayer D and
Bannasch P: Upregulation of the insulin receptor and type I
insulin-like growth factor receptor are early events in
hepatocarcinogenesis. Toxicol Pathol. 39:524–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen CJ and Pollak M: Circulating IGF-I:
new perspectives for a new century. Trends Endocrinol Metab.
10:136–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenfeld RG, Kofoed E, Little B, et al:
Growth hormone insensitivity resulting from post-GH receptor
defects. Growth Horm IGF Res. 14(Suppl A): S35–S38. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones JI and Clemmons DR: Insulin-like
growth factors and their binding proteins: biological actions.
Endocr Rev. 16:3–34. 1995.PubMed/NCBI
|
|
11
|
Mazziotti G, Sorvillo F, Morisco F, et al:
Serum insulin-like growth factor I evaluation as a useful tool for
predicting the risk of developing hepatocellular carcinoma in
patients with hepatitis C virus-related cirrhosis: a prospective
study. Cancer. 95:2539–2545. 2002. View Article : Google Scholar
|
|
12
|
Su WW, Lee KT, Yeh YT, et al: Association
of circulating insulin-like growth factor 1 with hepatocellular
carcinoma: one cross-sectional correlation study. J Clin Lab Anal.
24:195–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tovar V, Alsinet C, Villanueva A, et al:
IGF activation in a molecular subclass of hepatocellular carcinoma
and pre-clinical efficacy of IGF-1R blockage. J Hepatol.
52:550–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sidlova K, Pechova M, Kotaska K and Prusa
R: Insulin-like growth factor binding protein-3 in patients with
liver cirrhosis. Physiol Res. 51:587–590. 2002.PubMed/NCBI
|
|
15
|
Wu YL, Ye J, Zhang S, Zhong J and Xi RP:
Clinical significance of serum IGF-I, IGF-II and IGFBP-3 in liver
cirrhosis. World J Gastroenterol. 10:2740–2743. 2004.PubMed/NCBI
|
|
16
|
Bruix J and Llovet JM: Hepatocellular
carcinoma: is surveillance cost effective? Gut. 48:149–150. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spangenberg HC, Thimme R and Blum HE:
Serum markers of hepatocellular carcinoma. Semin Liver Dis.
26:385–390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Srinivas PR, Kramer BS and Srivastava S:
Trends in biomarker research for cancer detection. Lancet Oncol.
2:698–704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kato N, Yokosuka O, Omata M, Hosoda K and
Ohto M: Detection of hepatitis C virus ribonucleic acid in the
serum by amplification with polymerase chain reaction. J Clin
Invest. 86:1764–1767. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aishima S, Basaki Y, Oda Y, et al: High
expression of insulin-like growth factor binding protein-3 is
correlated with lower portal invasion and better prognosis in human
hepatocellular carcinoma. Cancer Sci. 97:1182–1190. 2006.
View Article : Google Scholar
|
|
21
|
McShane LM, Altman DG and Sauerbrei W:
Identification of clinically useful cancer prognostic factors: what
are we missing? J Natl Cancer Inst. 97:1023–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shaarawy M, Fikry MA, Massoud BA and Lotfy
S: Insulin-like growth factor binding protein-3: a novel biomarker
for the assessment of the synthetic capacity of hepatocytes in
liver cirrhosis. J Clin Endocrinol Metab. 83:3316–3319. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mattera D, Capuano G, Colao A, et al:
Increased IGF-I: IGFBP-3 ratio in patients with hepatocellular
carcinoma. Clin Endocrinol (Oxf). 59:699–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elsammak MY, Amin GM, Khalil GM, Ragab WS
and Abaza MM: Possible contribution of serum activin A and IGF-1 in
the development of hepatocellular carcinoma in Egyptian patients
suffering from combined hepatitis C virus infection and hepatic
schistosomiasis. Clin Biochem. 39:623–629. 2006. View Article : Google Scholar
|
|
25
|
Okan A, Comlekci A, Akpinar H, et al:
Serum concentrations of insulin-like growth factor-I and
insulin-like growth factor binding protein-3 in patients with
chronic hepatitis. Scand J Gastroenterol. 35:1212–1215. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanafusa T, Yumoto Y, Nouso K, et al:
Reduced expression of insulin-like growth factor binding protein-3
and its promoter hypermethylation in human hepatocellular
carcinoma. Cancer Lett. 176:149–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo SM, Tan WM, Deng WX, Zhuang SM and Luo
JW: Expression of albumin, IGF-1, IGFBP-3 in tumor tissues and
adjacent non-tumor tissues of hepatocellular carcinoma patients
with cirrhosis. World J Gastroenterol. 11:4272–4276.
2005.PubMed/NCBI
|
|
28
|
Huynh H, Chow PK, Ooi LL and Soo KC: A
possible role for insulin-like growth factor-binding protein-3
autocrine/paracrine loops in controlling hepatocellular carcinoma
cell proliferation. Cell Growth Differ. 13:115–122. 2002.
|
|
29
|
Park IY, Sohn BH, Yu E, et al: Aberrant
epigenetic modifications in hepatocarcinogenesis induced by
hepatitis B virus X protein. Gastroenterology. 132:1476–1494. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shon JK, Shon BH, Park IY, et al:
Hepatitis B virus-X protein recruits histone deacetylase 1 to
repress insulin-like growth factor binding protein 3 transcription.
Virus Res. 139:14–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ricort JM, Lombet A, Lassarre C and Binoux
M: Insulin-like growth factor binding protein-3 increases
intracellular calcium concentrations in MCF-7 breast carcinoma
cells. FEBS Lett. 527:293–297. 2002. View Article : Google Scholar
|
|
32
|
Ricort JM and Binoux M: Insulin-like
growth factor-binding protein-3 activates a phosphotyrosine
phosphatase. Effects on the insulin-like growth factor signaling
pathway. J Biol Chem. 277:19448–19454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ricort JM and Binoux M: Insulin-like
growth factor binding protein-3 stimulates phosphatidylinositol
3-kinase in MCF-7 breast carcinoma cells. Biochem Biophys Res
Commun. 314:1044–1049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weng CJ, Hsieh YH, Tsai CM, et al:
Relationship of insulin-like growth factors system gene
polymorphisms with the susceptibility and pathological development
of hepatocellular carcinoma. Ann Surg Oncol. 17:1808–1815. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
El-Houseini ME, Mohammed MS, Elshemey WM,
Hussein TD, Desouky OS and Elsayed AA: Enhanced detection of
hepatocellular carcinoma. Cancer Control. 12:248–253.
2005.PubMed/NCBI
|
|
36
|
Rehem RN and El-Shikh WM: Serum IGF-1,
IGF-2 and IGFBP-3 as parameters in the assessment of liver
dysfunction in patients with hepatic cirrhosis and in the diagnosis
of hepatocellular carcinoma. Hepato-gastroenterology. 58:949–954.
2011.PubMed/NCBI
|
|
37
|
Scharf JG, Ramadori G and Dombrowski F:
Analysis of the IGF axis in preneoplastic hepatic foci and
hepatocellular neoplasms developing after low-number pancreatic
islet transplantation into the livers of streptozotocin diabetic
rats. Lab Invest. 80:1399–1411. 2000. View Article : Google Scholar
|
|
38
|
Price JA, Kovach SJ, Johnson T, et al:
Insulin-like growth factor I is a comitogen for hepatocyte growth
factor in a rat model of hepatocellular carcinoma. Hepatology.
36:1089–1097. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qian J, Yao D, Dong Z, et al:
Characteristics of hepatic IGF-ii expression and monitored levels
of circulating IGF-ii mRNA in metastasis of hepatocellular
carcinoma. Am J Clin Pathol. 134:799–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
El Tayebi HM, Salah W, El Sayed IH, et al:
Expression of insulin-like growth factor-II, matrix
metalloproteinases, and their tissue inhibitors as predictive
markers in the peripheral blood of HCC patients. Biomarkers.
16:346–354. 2011.PubMed/NCBI
|
|
41
|
Fan J, Upadhye S and Worster A:
Understanding receiver operating characteristic (ROC) curves. CJEM.
8:19–20. 2006.PubMed/NCBI
|
|
42
|
Scott CD, Martin JL and Baxter RC:
Production of insulin-like growth factor I and its binding protein
by adult rat hepatocytes in primary culture. Endocrinology.
116:1094–1101. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chin E, Zhou J, Dai J, Baxter RC and Bondy
CA: Cellular localization and regulation of gene expression for
components of the insulin-like growth factor ternary binding
protein complex. Endocrinology. 134:2498–2504. 1994.PubMed/NCBI
|
|
44
|
Villafuerte BC, Koop BL, Pao CI, Gu L,
Birdsong GG and Phillips LS: Coculture of primary rat hepatocytes
and nonparenchymal cells permits expression of insulin-like growth
factor binding protein-3 in vitro. Endocrinology. 134:2044–2050.
1994.PubMed/NCBI
|
|
45
|
Scharf J, Ramadori G, Braulke T and
Hartmann H: Synthesis of insulinlike growth factor binding proteins
and of the acid-labile subunit in primary cultures of rat
hepatocytes, of Kupffer cells, and in cocultures: regulation by
insulin, insulinlike growth factor, and growth hormone. Hepatology.
23:818–827. 1996. View Article : Google Scholar
|
|
46
|
Scharf JG, Schmidt-Sandte W, Pahernik SA,
Ramadori G, Braulke T and Hartmann H: Characterization of the
insulin-like growth factor axis in a human hepatoma cell line
(PLC). Carcinogenesis. 19:2121–2128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stuver SO, Kuper H, Tzonou A, et al:
Insulin-like growth factor 1 in hepatocellular carcinoma and
metastatic liver cancer in men. Int J Cancer. 87:118–121. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Major JM, Stolzenberg-Solomon RZ, Pollak
MN, Snyder K, Virtamo J and Albanes D: Insulin-like growth factors
and liver cancer risk in male smokers. Br J Cancer. 103:1089–1092.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bonefeld K and Moller S: Insulin-like
growth factor-I and the liver. Liver Int. 31:911–919. 2011.
View Article : Google Scholar : PubMed/NCBI
|