Introduction
Gastrointestinal stromal tumours (GISTs) are rare,
however they are the most common mesenchymal tumours of the
digestive tract. he annual GIST incidence in the western world is
estimated at 11–15 cases/million individuals (1) and the disease predominantly affects
middle-aged or elderly individuals; however, the disease has been
identified in a wide range of ages. GISTs are highly resistant to
chemotherapy and surgical resection is the current mainstay
treatment (2). Risk of recurrence
or metastasis is high. Factors of malignancy prediction include
tumour size and mitotic index, as well as the tumour site (1,3).
The pathogenesis of GISTs has been linked to
activating mutations occurring in two proto-oncogenes, v-kit
Hardy/Zuckerman 4 feline sarcoma viral oncogene homologue KIT
(KIT) and the platelet derived growth factorα
(PDGFRα) (4,5). The genes encode homologous type III
tyrosine kinases (6). Activating
mutations render the receptors constitutively activated, resulting
in the dysregulation of several signalling pathways and
uncontrolled cellular proliferation (7). Mutations in the juxtamembrane domain
of KIT are most frequently identified and this region is
encoded by exons 9 and 11, while mutations in the tyrosine kinase
domain (exons 13 and 17) are rare. In PDGFRα, juxtamembrane
(exon 12) mutations are rare, however, the kinase domain, encoded
by exon 14 and 18, is frequently mutated (8–10).
An increased understanding of GIST pathogenesis has
led to targeted treatment with tyrosine kinase inhibitors (TKIs),
including imatinib and sunitinib. These drugs block the protein
tyrosine kinase domain, preventing downstream signalling (1). The majority of activating mutations
render the tumour more susceptible to TKIs and therefore TKI
treatment is recommended as an adjuvant to surgery in patients at
high risk for relapse, as well as for metastatic GIST (1,7,11).
Overall, GISTs with KIT exon 11 mutations exhibit the best
response to TKI therapy when compared with wild-type tumours and
other mutations (12). It was
previously hypothesised that the conformational changes caused by
the mutations enable improved access of the drug to the tyrosine
kinase domain of the protein (13).
However, some primary and secondary mutations are associated with
imatinib resistance. Determination of mutations in the KIT
and PDGFRα genes by direct sequencing is therefore
essential for individual targeted treatment of GIST patients
(2).
A number of previous studies have characterised GIST
samples at the molecular level, generating population-specific data
for USA, Europe and Asia. Information on KIT and
PDGFRα mutations associated with GIST in African individuals
remains extremely scarce (14). The
present study is the first to report on the molecular
characterisation of 46 African GIST samples. We have developed
initial data with the aim to determine the GIST mutation pattern in
the South African population.
Materials and methods
Patient selection and samples
Ethics approval was granted from the Human Research
Ethics Committee of the University of the Witwatersrand. Archival
material was used in the present study. A total of 46
formalin-fixed paraffin-embedded (FFPE) GIST classified samples
were received from all regions in South Africa. A haematoxylin and
eosin slide from each block was reviewed by experienced
pathologists and the tumour area selected for DNA extraction.
DNA extraction
Each FFPE tissue block was sectioned using a clean
microtome blade and five 4-μm sections were cut and baked at
48.2°C for 2 h or overnight. Sections were deparaffinised in
methylcyclohexane followed by rehydration in a series of 100%
ethanol, 95% ethanol and distilled water washes. DNA was extracted
using the Qiagen DNeasy kit (Whitehead Scientific, Brackenfell,
South Africa) following the manufacturer’s instructions.
Polymerase chain reaction (PCR)
amplification and DNA cycle sequencing
PCR conditions were optimised for each exon, as
previously described (9,15). Primers used are listed in Table I. PCR was prioritised into two
rounds, according to the most prevalent mutations. The first round
was performed on all samples and amplified KIT exons 9, 11,
13 and 17 along with PDGFRα exons 12, 14 and 18. The second
round PCR was only performed on the sample if the first round of
sequenced results were all wild-type. The second round consisted of
amplification of KIT exons 10, 12, 14, 16, 18 and 19 and
PDGFRα exons 10, 13, 15, 16, 17, 19, 20 and 21. KIT
exon 9 was not sequenced but resolved in a 4% MetaPhor agarose gel
(Whithead Scientific). PCR fragment length analysis was used to
determine the presence of a 6-bp duplication (codons 502–503)
(10,16). All other amplified exons were
screened on a 2% agarose gel and subsequently sequenced in both
directions using the BigDye terminator kit v3.1 (Applied
Biosystems, Bedford, MA, USA). Reactions were loaded on either an
ABI 3170 or 3500 genetic analyser (Applied Biosystems). Sequences
were analysed using Sequence Scanner v1.0 (Applied Biosystems).
 | Table IPCR primers for KIT and
PDGFRα exons. |
Table I
PCR primers for KIT and
PDGFRα exons.
| Gene | Exon | Forward
(5′–3′) | Reverse
(5′–3′) |
|---|
| KIT | 9 |
GATGTGGGCAAGACTTCTG |
TGGTAGACAGAGCCTAAACATCC |
| 10 |
GAGTGGCTGTGGTAGAGATC |
CAGTCATTAGAGCACTCTGG |
| 11 |
CCAGAGTGCTCTAATGACTG |
ACTGTTATGTGTACCCAAAAAG |
| 12 |
GCACAAATGGTCCTTCAATTC |
AAAGCACAACTGGCAAACTGA |
| 13 |
GCTTGACATCAGTTTGCCAGT |
GGCAGCTTGGACACGGCTTTA |
| 14 |
TTATGGGAGGCAGAATTAATCTAT |
CCCTTATGACCCCATGAACT |
| 16 |
ACTGTCTTTTCCTTTCCTGACC |
ACACAAAACTCTTTAGAGAATCACTC |
| 17 |
GGTTTTCTTTTCTCCTCCAACC |
TGCAGGACTGTCAAGCAGAG |
| 18 |
AATTTTGTTGAGCTTCTGAATTAAC |
ACTTCAAGAAGATGCTCTGAGTCT |
| 19 |
GGTGTCCTGCTTCCTTGTGAT |
CCTCAACATCTGGGTTTCTGTC |
|
PDGFRα | 10 |
TGTTCCCGTGGCTCCACTCATTG |
TTCCGCCTGGGGCAGATGC |
| 12 |
TCCAGTCACTGTGCTGCTTC |
GCAAGGGAAAAGGGAGTCTT |
| 13 |
GCTGGCTACGGTGCAGAAAG |
TATCCCCATGCTCAAAAAATCC |
| 14 |
TGAGAACAGGAAGTTGGTAGCTCA |
GATGGAGAGTGGAGGATTTAAGCCT |
| 15 |
GGACAATTCATGGCTTTTCTGTT |
TTCCATTTGTGATGCCTGTAAGA |
| 16 |
CCCAGTTAGCTCCCATGCCTACCT |
CATCCCTATACACTTCCCTCTAAAT |
| 17 |
TGCCTCTGCAACCTGATGATT |
CTCCGTCCACACTCCACTCAC |
| 18 |
CAGCTACAGATGGCTTGATC |
GGATGAGCCTGACCAGTGAG |
| 19 |
CCCTTTTCTATTTCCACTGCTGTGG |
CATCCTGGGGCTTGAAAGAAC |
| 20 |
TCATGCCAAGTGTTTCAGCAAT |
GCCCCTCCCTCCCCCTAGAC |
| 21 |
CCGGGGGCCTGTGTTCACAGT |
TGCGGAACAGCACAGCTCAC |
Results
Patient demographics
In the present study, 46 GIST samples were obtained
from 45 patients. The majority (80%) of the tumours were localised.
The most common site of the tumour was the stomach (48%), followed
by the small bowel (20%) and large bowel or colon (9%). The rest of
the tumours (24%) occurred along various parts of the GI tract,
including the liver, pancreas, retroperitoneal and intraperitoneal
cavity. Patient age ranged between 17 and 86 years, with an average
age of 56 years. Ethnicity was known for 35 patients and of these,
23 were African and 12 were Caucasian. There was a higher male to
female ratio of 1.25:1. In addition, one patient had
neurofibromatosis 1 (NF1). By assessing the tumour size, mitotic
index and tumour site, the risk for malignancy was evaluated for 43
of the samples. The majority of the samples (23 GISTs) were high
risk, with a mitotic count >10/50 high-powered fields and/or a
tumour diameter >10 cm. Eight and 12 samples were classified as
either intermediate or low risk, respectively. Follow-up data were
not available for the majority of the cases.
Mutational analysis
Overall, 36 (78.3%) samples had a mutation in one of
the two genes while the remaining 10 samples were considered
wild-type (21.7%). Of the samples with mutations, 32 (88.9%)
occurred in KIT exon 11, three (8.3%) in PDGFRα exon
18 and one (2.8%) in KIT exon 9. The wild-type samples
included tumours from a juvenile boy (aged 17 years) and the known
NF1 patient.
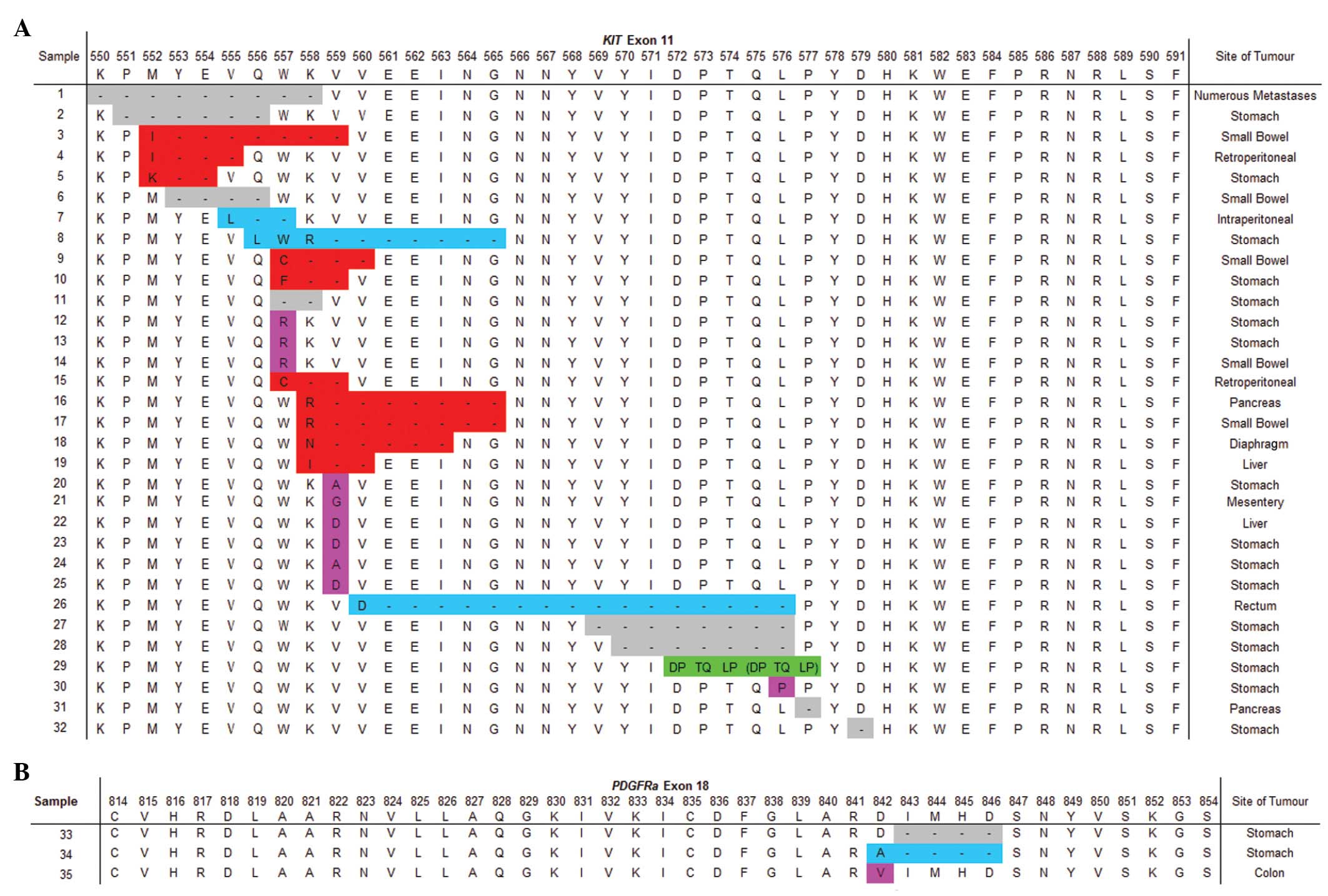
Mutations occurring in KIT exon 11 included
substitutions, deletions, deletions with a missense mutation,
deletions and nucleotide insertions resulting in missense mutations
and a duplication (Fig. 1A) in 10,
8, 10, 3 and 1 case(s), respectively. KIT exon 11 mutations
occurred in 73% of GISTs arising in the stomach, 56% in the small
bowel and accounted for 85% of tumours detected in other areas of
the GI tract. The most commonly (46.9%) affected KIT exon 11
codon was 559, but mutations were also identified in codons
550–579. Two of the three PDGFRα exon 18 mutations occurred
in GIST arising in the stomach and the other was in the colon.
PDGFRα exon 18 mutations included a substitution, deletion
and deletion with nucleotide insertion resulting in a missense
mutation. These affected PDGFRα exon 18 codons 842 to 846
(Fig. 1B). A single KIT exon
9 mutation was identified in a GIST arising from the liver. This
patient had an additional primary GIST of the small bowel with a
KIT exon 11 mutation (absent in the liver GIST).
Single nucleotide polymorphisms
(SNPs)
Seven different SNPs were detected in 17 patients
with one or more SNPs (Table II).
Seven of these patients had wild-type tumours. Thirteen were of
African ethnicity, two were Caucasian and two were of unknown
ethnicity. Of the seven SNPs, one in KIT exon 17 (K779K;
AAA→AAG) has not been previously identified.
 | Table IISNPs detected in KIT and
PDGFRα in 46 GIST samples from South Africa. |
Table II
SNPs detected in KIT and
PDGFRα in 46 GIST samples from South Africa.
| Gene | Exon | Description | Site of
tumoura | Mutation
statusa | Ethnicitya |
|---|
| KIT | 11 | P585P (CCC>TCC)
rs121913515 | Small bowel
(1) | Wild-type (1) | African (1) |
| KIT | 18 | L862L (CTG>CTC)
rs3733542 | Stomach (1) | Wild-type (2) | African (2) |
| Small bowel
(1) |
| PDGFRα | 12 | P567P (CCG>CCA)
rs1873778 | Stomach (3) | K11 mutation
(3) | African (4) |
| Small bowel
(1) | Wild-type (1) |
| PDGFRα | 13 | Intronic
(AAA>A3AG) rs41279523 | Large bowel
(1) | Wild-type (1) | African (1) |
| PDGFRα | 17 | K779K (AAA>AAG)
Unknown | Rectum (1) | Wild-type (1) | African (1) |
| PDGFRα | 18 | V824V (GTC-GTT)
rs2228230 | Stomach (9) | Wild-type (5) | African (10) |
| Small bowel
(2) | K11 mutation
(7) | Caucasian (2) |
| Large bowel
(1) | P18 mutation
(1) | Unknown (2) |
| Other (2) | | |
|
PDGFRα | 20 | R914R (CGG>CGA)
rs56384252 | Rectum (1) | Wild-type (1) | African (1) |
Discussion
Molecular profiling of GIST is recommended for the
determination of the best course of treatment for a patient with
unresectable or metastatic disease (2). Previously, our laboratory reported
KIT and PDGFRα mutational incidence in 17 samples
from South African GIST patients (14). The present study expands this
previous report and aimed to determine the molecular profile of 46
GIST samples. In addition, the present findings were compared with
those from developing and first world countries. Overall, a
mutation was identified in 78.3% of South African GISTs. The
majority of these mutations occured in KIT exon 11 (88.9%)
followed by PDGFRα exon 18 (8.3%). The KIT
exon 9 mutation was rare and was identified in only one sample.
GIST pathology studies have been previously
performed in a number of countries worldwide, including Africa and
South Africa (17,18). Previously, Tran et
al(19) revealed that GIST
incidence was higher in African-Americans compared with Caucasians.
However, information on GIST incidence and mutational profiles is
scarce in Africa. The genetic profile of South African GIST
patients remains poorly understood and must be determined.
The frequency of specific mutations varies
worldwide, however, current results demonstrate that KIT
mutations are the most common while those in PDGFRα
have only been detected in KIT wild-type GISTs (Table III) (1). Variance in reported mutation rates for
various populations is thought to be due to a number of factors,
including ethnicity, study design and selection criteria for each
study (7,20).
 | Table IIIKIT and PDGFRα
mutational rates in various populations. |
Table III
KIT and PDGFRα
mutational rates in various populations.
| Country | Gene | Number of
studies/total sample number | Mutated exons (%)
| Reference |
|---|
KIT
| PDGFRα
|
|---|
| 11 | 9 | 13 | 17 | 18 | 12 |
|---|
| Taiwan | KIT | 2/188 | 66.1 | 14.2 | 0.0 | 0.0 | - | - | 20, 28 |
| PDGFRα | 1/134 | - | - | - | - | 0.0 | 0.8 | |
| Japan | KIT | 2/118 | 58.7 | 7.0 | 0.0 | NP | - | - | 30–32 |
| PDGFRα | 1/70 | - | - | - | - | 4.3 | 2.9 | |
| China | KIT | 1/165 | 63.0 | 6.6 | 1.3 | 0.6 | - | - | 29 |
| PDGFRα | 1/165 | - | - | - | - | 3.0 | 0.0 | |
| Brazil | KIT | 1/55 | 69.0 | 3.6 | 0.0 | 0.0 | - | - | 33 |
| PDGFRα | 1/55 | - | - | - | - | 1.8 | 5.5 | |
| Spain | KIT | 1/166 | 47.5 | 2.4 | 0.6 | 1.6 | - | - | 27 |
| PDGFRα | 1/166 | - | - | - | - | 4.0 | 4.0 | |
| USA | KIT | 4/818 | 56.5 | 8.9 | 0.4 | 0.6 | - | - | 5,21–23 |
| PDGFRα | 3/693 | - | - | - | - | 8.1 | 0.7 | |
| Europe | KIT | 3/591 | 62.6 | 9.9 | 1.4 | 0.45 | - | - | 24–26 |
| PDGFRα | 3/579 | - | - | - | - | 3.9 | 0.8 | |
| South Africa | KIT | 1/46 | 69.6 | 2.8 | 0.0 | 0.0 | - | - | Present study |
| PDGFRα | 1/46 | - | - | - | - | 6.5 | 0.0 |
Previous studies performed in European and North
American populations (western populations) revealed that 85–90% of
GISTs exhibited an activating mutation in KIT or
PDGFRα(1). The most
frequently observed mutation is KIT exon 11, followed by
KIT exon 9 and PDGFRα exon 18 (21–25).
Mutations have been detected at much lower frequencies in
KIT exons 13 and 17 and PDGFRα exons 12 and 14
(21–25). These trends are comparable in other
populations as well as in the present findings, despite small
sample numbers. In the present study, none of the mutations
identified resulted in frame shift or nonsense mutations. Point
mutations in KIT exon 11 were detected in codons 557, 559
and 576 only, with codon 559 being the most frequently affected.
Deletions in KIT exon 11 affected codons 558 followed by
557. Three of the five deletions, in more distal regions of this
exon, involved codons 568 and/or 570. The duplication occurred
following codon 570. The above findings are consistent with
previous literature (7,8,26,27).
Additional mutations in the remaining exons of KIT and
PDGFRα that were sequenced were not identified.
Previous Taiwanese studies demonstrated that
PDGFRα mutations are rare in Taiwan. Only 1/134 patients
revealed a PDGFRα mutation in exon 12 and no mutations in
the more prevalent exon 18 were identified (20,28).
By contrast, a Chinese study was consistent with western trends
described above (29). Japanese
studies revealed the same trend but also demonstrated a higher
prevalence of PDGFRα exon 12 mutations (30–32).
In addition, this was observed in Brazilian and Hispanic
populations, revealing higher mutation rates in PDGFRα exon
12 than exon 18 (27,33). In the present study, a more common
pattern of PDGFRα exon 18 mutations was observed, occurring
in 6.5% of GIST samples, while no exon 12 mutations were
identified.
KIT exon 9 mutations are the second most
prevalent mutations detected in western and Asian populations.
However, the present study revealed similar findings to those of
previous Brazilian and Hispanic studies, demonstrating a reduced
KIT exon 9 mutation frequency than PDGFRα
exons (27,33). A single sample revealed a KIT
exon 9 mutation. Therefore, we assume that KIT exon 9
mutations are rare in South African GIST patients. However, a
larger study is required to yield more concrete findings. The
KIT exon 9 mutation was identified in a metastatic tumour of
a Caucasian patient with a primary tumour in the small bowel with a
KIT exon 11 deletion. However, the metastatic tumour did not
possess the KIT exon 11 mutation, indicating that the
metastatic tumour was an independent GIST to the primary tumour.
Duplication of codons 502 and 503 of KIT exon 9 was not
detected in any of the African patients.
Molecular characterisation is vital for the
prediction of response, choice and dose of TKIs. Imatinib is the
first choice TKI, while sunitinib is the second line of therapy in
cases of resistance (12). As a
general rule, mutations in KIT exon 11 are sensitive to
imatinib (11). GIST cases with a
KIT exon 9 mutation tend to be more aggressive and should
recieve a higher daily dose of imatinib (10–12).
The effect of mutations in PDGFRα have variable
effects on imatinib sensitivity. However, the most common mutation
identified, the D842V substitution, is resistant to imatinib
treatment (10,15). In the present study, a single known
resistant mutation was identified, a D842V mutant.
Genetic diversity is a common trait in African
populations (34). As the majority
of the patients were of African ethnicity, it was predicted that
silent variations would also be detected and it was identified that
77.8% of the patients containing an SNP were of African ethnicity.
A novel SNP was detected in KIT exon 17 that has not
previously been described. An SNP was observed in 17/45 patients,
of which 7 were identified to be wild-type for KIT and
PDGFRα. A previous Brazilian study (33) analysed SNP rates and identified
polymorphisms in 3/11 wild-type GIST cases. SNPs do not lead to
phenotypic alterations, however, their impact on disease
progression or treatment is currently unknown.
The mutation status of GISTs affects the efficacy of
TKIs in treatment. Therefore, it is vital to determine the genetic
profile of a GIST to provide the best possible care for patients.
The genetic profile of African GIST patients, including South
Africans, is currently unknown and further investigation is
required in this area of research. The present study is likely to
contribute to the understanding of the molecular profile of GIST in
South African patients.
Acknowledgements
The authors thank Novartis Oncology
and University of the Witwatersrand for their contribution towards
publishing this article.
References
|
1
|
Bachet JP and Emile JF: Diagnostic
criteria, specific mutations and genetic predisposition in
gastrointestinal stromal tumors. Appl Clin Genet. 3:85–101.
2010.PubMed/NCBI
|
|
2
|
Blay JY and Reichardt P: Advanced
gastrointestinal stomal tumour in Europe: a review of updated
treatment recommendations. Expert Rev Anticancer Ther. 9:1–8.
2009.PubMed/NCBI
|
|
3
|
Gupta P, Tewari M and Shukla HS:
Gastrointestinal stromal tumor. Surg Oncol. 17:129–138. 2008.
View Article : Google Scholar
|
|
4
|
Hirota S, Isozaki K, Moriyama Y, et al:
Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heinrich MC, Corless CL, Duensing A, et
al: PDGFRA activating mutations in gastrointestinal stromal tumors.
Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stenman G, Eriksson A and Claesson-Welsh
L: Human PDGFA receptor gene maps to the same region on chromosome
4 as the KIT oncogene. Genes Chromosomes Cancer. 1:155–158. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lasota J and Miettinen M: Clinical
significance of oncogenic KIT and PDGFRA mutations in
gastrointestinal stromal tumours. Histopathology. 53:245–266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corless CL, Fletcher JA and Heinrich MC:
Biology of gastrointestinal tumors. J Clin Oncol. 22:3813–3825.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Corless C, Schroeder A, Griffith D, et al:
PDGFRA mutations in gastrointestinal stromal tumors: frequency,
spectrum and in vitro sensitivity to imatinib. J Clin Oncol.
23:5357–5364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cassier PA and Blay JY: Molecular response
prediction in gastrointestinal stromal tumors. Target Oncol.
5:29–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blay JY: A decade of tyrosine kinase
inhibitor therapy: Historical and current perspectives on targeted
therapy for GIST. Cancer Treat Rev. 37:373–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demetri GD, von Mehren M, Antonescu CR, et
al: NCCN Task force report: Update on the management of patients
with gastrointestinal stromal tumours. J Natl Compr Canc Netw.
8:S1–41. 2010.PubMed/NCBI
|
|
13
|
Mol CD, Dougan DR, Schneider TR, et al:
Structural basis for the autoinhibition and STI-571 inhibition of
c-Kit tyrosine kinase. J Biol Chem. 279:31655–31663. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Babb C, Schnugh D, Louw M, et al: Getting
the gist: what is the importance of molecular genetics in
gastro-intestinal stromal tumours (GIST). S Afr Gastroenterol Rev.
8:4–5. 2010.
|
|
15
|
Heinrich MC, Owzar K, Corless CL, et al:
Correlation of kinase genotype and clinical outcome in the North
American Intergroup Phase III Trial of imatinib mesylate for
treatment of advanced gastrointestinal stromal tumor: CALGB 150105
Study by Cancer and Leukemia Group B and Southwest Oncology Group.
J Clin Oncol. 26:5360–5367. 2008.
|
|
16
|
Yuzawa S, Opatowsky Y, Zhang Z, Mandiyan
V, Lax I and Schlessinger J: Structural basis for activation of the
receptor tyrosine kinase KIT by stem cell factor. Cell.
130:323–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdulkareem FB, Rotimi O, Elesha SO and
Banjo AA: Immunophenotyping of gastrointestinal mesenchymal tumours
in Lagos, Nigeria. West Afr J Med. 28:358–362. 2009.PubMed/NCBI
|
|
18
|
Hartley RJ, Becker JHR, Van Der Walt H and
Luyhengo T: Gastro-intestinal stromal tumours (GISTs) - the
Pretoria experience and a literature review. S Afr J Surg.
49:128–131. 2011.PubMed/NCBI
|
|
19
|
Tran T, Davila JA and El-Serag HB: The
epidemiology of malignant gastrointestinal stromal tumors: an
analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol.
100:162–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tzen CY, Wang MN and Mau BL: Spectrum and
prognostication of KIT and PDGFRA mutation in gastrointestinal
stromal tumors. Eur J Surg Oncol. 34:563–568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agaram NP, Baren A, Arkun K, Dematteo NP,
Besmer P and Antonescu CR: Comparative ultrastructural analysis and
KIT/PDGFRA genotype in 125 gastrointestinal stromal tumors.
Ultrastruct Pathol. 30:443–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miettinen M, Sobin LH and Lasota J:
Gastrointestinal stromal tumors of the stomach: a
clinicopathologic, immunohistochemical and molecular genetic study
of 1765 cases with long-term follow-up. Am J Surg Pathol. 29:52–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miettinen M, Makhlouf H, Sobin LH and
Lasota J: Gastrointestinal stromal tumors of the jejunum and ileum:
a clinicopathologic, immunohistochemical and molecular genetic
study of 906 cases before imatinib with long-term follow-up. Am J
Surg Pathol. 30:477–489. 2006. View Article : Google Scholar
|
|
24
|
Debiec-Rychter M, Dumez H, Judson I, et
al: Use of c-KIT/PDGFRA mutational analysis to predict the clinical
response to imatinib in patients with advanced gastrointestinal
stromal tumours entered on phase I and II studies of the EORTC Soft
Tissue and Bone Sarcoma Group. Eur J Cancer. 40:689. 2004.
View Article : Google Scholar
|
|
25
|
Debiec-Rychter M, Sciot R, Le Cesne A, et
al: KIT mutations and dose selection for imatinib in patients with
advanced gastrointestinal stromal tumours. Eur J Cancer.
42:1093–1103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andersson J, Bumming P, Meis-Kindblom JM,
et al: Gastrointestinal stromal tumors with KIT exon 11 deletions
are associated with poor prognosis. Gastroenterology.
130:1573–1581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martín J, Poveda A, Llombart-Bosch A, et
al: Deletions affecting codons 557–558 of the c-KIT gene indicate a
poor prognosis in patients with completely resected
gastrointestinal stromal tumors: a study by the Spanish group for
Sarcoma Research (GEIS). J Clin Oncol. 23:6190–6198. 2005.
|
|
28
|
Yeh CN, Chen TW, Lee HL, et al: Kinase
mutations and imatinib mesylate response for 64 Taiwanese with
advanced GIST: preliminary experience from Chang Gung Memorial
Hospital. Ann Surg Oncol. 14:1123–1128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He HY, Fang WG, Zhong HH, et al: Status
and clinical implication of c-KIT and PDGFRA mutations in 165 cases
of gastrointestinal stromal tumor (GIST). Zhonghua Bing Li Xue Za
Zhi. 35:262–266. 2006.(In Chinese).
|
|
30
|
Sakurai S, Fukasawa T, Chong JM, Tonaka A
and Fukayama M: C-kit gene abnormalities in gastrointestinal
stromal tumors (tumors of interstitial cells of Cajal). Jpn J
Cancer Res. 90:1321–1328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakurai S, Oguni O, Hironaka M, Fukayama
M, Morinaga S and Saito K: Mutations in c-kit gene exons 9 and 13
in gastrointestinal stromal tumors among Japanese. Jpn J Cancer
Res. 92:494–498. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirota S, Ohashi A, Nishida T, Isozaki K,
Kinoshita K, Shinomura Y and Kitamura O: Gain-of-function mutations
of platelet-derived growth factor receptor α gene in
gastrointestinal stromal tumors. Gastroenterology. 125:660–667.
2003.
|
|
33
|
Braggio DA, Braggio E, Small IA, et al:
The profile of platelet-derived growth factor receptor alpha
(PDGFRA) gene alterations in GIST patients (pts) from Brazil. J
Clin Oncol. 26:105612008.
|
|
34
|
Campbell MC and Tishkoff SA: African
genetic diversity: implications for human demographic history,
modern human origins and complex disease mapping. Annu Rev Genomics
Hum Genet. 9:303–333. 2008. View Article : Google Scholar
|