Introduction
Traditionally, the biological behavior of solid
tumors and their responses to immune cells in vitro are
examined using stabilized tumor cell lines grown in a
two-dimensional (2-D) monolayer culture (1). Although this strategy has provided
valuable information with regard to the cytotoxicity of immune
cells to tumor cells, it is not sufficient to mimic the antitumor
activity of immune cells in vivo. Solid tumor cells grown in
three-dimensions (3-D) in vivo support each other and also
interact with the extracellular matrix (ECM). The behavior of a
single tumor cell is regulated by its interactions with its
immediate neighbors and the ECM (2). Similarly, the antitumor activity of
immune cells may be affected by the 3-D spatial array and ECM
(3,4). In this aspect, the in vitro 2-D
tumor cell culture model has limited value in the evaluation of the
clinical efficacy of the cytotoxicity of immune cells in
vivo. Therefore, it is necessary to establish an in
vitro tumor model that accurately reflects the in vivo
immune cell-tumor cell interactions. Such in vitro models
may be used for successful screening of tumor-reactive immune cells
prior to testing their in vivo antitumor immunity in animal
models, as well as for the treatment of patients.
Tissue engineering practices have suggested that the
3-D culture of cell models in vitro is better than the
planar cultures of cell lines, particularly in mimicking the in
vivo cell-cell and cell-matrix interactions (5). Furthermore, it has been reported that
the cellular microenvironment, cell-cell and cell-matrix
interactions within 3-D tissues greatly influence cellular
functions, including the adhesion, motility, invasiveness and
metastasis of cancers (6). Thus,
3-D culture may be an important strategy for the evaluation of the
behavior of tumor cells and the efficacy of immunotherapeutic
intervention. There are various methods available for the
establishment of 3-D tumor cultures in vitro, including the
spinner flask method (7), gyratory
rotation system (8) and
liquid-overlay technique (9). While
the established 3-D tumor models have been successfully used for
the evaluation of the effects of drugs (10–12),
they are not suitable for the screening of immune cells. Our
previous study used a tissue engineering approach and type I
collagen hydrogel as a matrix to successfully create hepatic tissue
in vitro(13). However,
whether the engineered 3-D tumor tissues cultured with a similar
approach may be used for evaluating the efficacy of antitumor
immune cells has not been explored. Given that immune and tumor
cell interactions in the engineered 3-D tumor model resemble those
in vivo, we hypothesized that the evaluation of the
antitumor activity of immune cells in an engineered 3-D tumor model
may be better than that in a 2-D tumor culture model.
In the present study, we first employed tissue
engineering technology to establish an in vitro 3-D gastric
tumor model. Furthermore, we compared the growth of tumor cells and
the cytotoxicity of cytokine-induced killer (CIK) cells in 2-D
culture and the engineered 3-D tumor culture models. In addition,
we compared the cytotoxicity of CIK cells, dendritic cell-activated
CIK (DC-CIK) cells and anti-CEA/CD3 bi-specific single-chain
antibody-activated CIK cells (CIK-CEA/CD3-bscAb) in the engineered
3-D model. Our results indicate that this engineered 3-D gastric
tumor model may be a simple and useful approach to screen antitumor
immune cells in vitro.
Materials and methods
Cell culture
The human undifferentiated stomach adenocarcinoma
cell line BGC823 was purchased from the Chinese Academy of Medical
Sciences (Beijing, China). Tumor cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Sigma, St. Louis, MO, USA)
containing 10% fetal calf serum (FCS; Hyclone, Logan, UT, USA) as
monolayer growth in 25-cm2 plastic tissue culture flasks
at 37°C in 5% CO2 and 95% humidity in an incubator
(HF212UV; Heal Force, Shanghai, China).
Establishment of different types of
gastric tumor cell culture models in vitro
BGC823 cells (2×105) were mixed with 500
μl collagen type I (2 mg/ml) prepared from Sprague Dawley
rat tails (14) in 2X DMEM (500
μl) and adjusted to pH 7.2 using 0.1 M NaOH on ice.
Subsequently, the mixture was added to 96-well plates
(1×104 cells/50 μl/well) and incubated at 37°C in
5% CO2 for 10–15 min, followed by adding 50 μl
complete culture media into each well for the establishment of the
3-D engineered model of gastric tumors in vitro.
Traditional gastric tumor culture models were
established and used as controls. Briefly, BGC823 cells
(1×104 cells/well) were cultured in 96-well plates and
used as a monolayer tumor culture model. The same numbers of BGC823
cells were cultured in 96-well plates that had been coated with
collagen type I (30 μl/well) and used as the liquid-overlay
tumor culture model.
Following culture for varying periods of time, the
cells in the monolayer and liquid-overlay models were stained with
hematoxylin and eosin (HE) and directly examined by the
Lab-Tell® II Chamber slide system (Nunc, Roskilde,
Denmark) on an inverted phase contrast microscope (TE2000-U, Nikon,
Tokyo, Japan). The cells in the engineered 3-D model were fixed and
paraffin-embedded, followed by staining with HE and examinination
under a microscope.
Immune cell isolation and culture
Heparinized peripheral blood samples (50 ml) were
obtained from healthy donors and peripheral blood mononuclear cells
(PBMCs) were isolated by density gradient Ficoll-Paque
centrifugation (TBD, Tianjin, China). PBMCs at 1×107
cells/ml were incubated in Cellix 901 serum-free medium (SIMA,
Beijing, China) in tissue culture flasks for 3 h and the
non-adherent cells were harvested, followed by gentle washing. The
adherent cells were exposed to fresh Cellix 901 medium containing
rh-IL-4 (1,000 U/ml) and GM-CSF (1,000 U/ml) every other day for
seven days. Then the DC cells were loaded with BGC823 tumor cell
lysate (20 μg/ml) in the presence of 3 μl/ml
Pseudomonas aeruginosa preparation (Weikexi, Haikou, China)
and cultured for 48 h. The cells were harvested, washed and stained
with fluorescent-conjugated antibodies against CD80, CD83, CD86 and
HLA-DR (Becton Dickinson, San Diego, CA, USA), followed flow
cytometry analysis on a FACScan (Becton Dickinson).
Generation of CIK and DC-CIK
cells
The harvested non-adherent cells were stimulated
with 1,000 U/ml IFN-γ (SIMA) in Cellix 601 serum-free medium (SIMA)
at 37°C for 24 h and activated by anti-CD3 (20 μg/ml) and 1
μg/ml anti-CD28 (Mabworks Biotech, Beijing, China) in the
presence of 1,000 U/ml IL-2 for 4–5 days to induce CIK cells.
Subsequently, one portion of the CIK cells was mixed with mature
DCs at a ratio of 100:1 and cultured for 1–2 days to generate
DC-CIK cells. The remaining CIK cells were continually cultured for
up to 13–14 days following stimulation. The phenotypes of CIK and
DC-CIK cells were characterized by flow cytometry analysis using
antibodies against CD3, CD4, CD8 and CD56 (Becton Dickinson).
Generation of CIK-CEA/CD3-bscAb
cells
The harvested CIK cells (1×107 cells)
were stimulated with 10 μg anti-CEA/anti-CD3 bi-specific Ab
(ABT, Beijing, China) at 4°C for 30 min and cultured in Cellix 601
serum-free medium.
Tumor cell transfection and CIK cell
labeling
BGC823 cells (1×106) were transfected
with 6 μg pEGFP-C1 plasmid (Clontech, Mountain View, CA,
USA) using the X-fect transfection reagent (Clontech) according to
the manufacturer’s instructions. The CIK cells (2×107
cells) were labeled with 8 μl PKH26 (Sigma), according to
the manufacturer’s instructions. The efficiency of cell
transfection and labeling was examined by flow cytometry
analysis.
Antitumor activity of CIK cells in
different tumor growth models
Morphology
Unmanipulated and pEGFP-C1-transfected BGC823 cells
were cultured in 96-well plates for 24 h for the generation of
different gastric tumor growth models. Subsequently, individual
wells of tumor cells were reacted in triplicate with CIK and
PKH26-labeled CIK cells (1×105 cells/well) at an
effector:target cell (E:T) ratio of 10:1. The morphology of tumor
cells was examined under an inverted phase contrast microscope, and
a confocal laser scanning microscope (ACAS-208, Olympus, Tokyo,
Japan) was used to observe the immune cell-tumor cell interactions
and the antitumor activity of CIK cells.
Cytotoxic effects
The cytotoxicity of CIK cells against individual
types of tumor cells was measured by lactate dehydrogenase (LDH)
assay using the CytoTox 96 nonradioactive cytotoxicity assay kit
(Promega, Madison, WI, USA), according to the manufacturer’s
instructions. Briefly, BGC823 cells (1×104/well) were
cultured for 24 h and then reacted in triplicate with
5×104 cells/well or 1×105 cells/well CIK
cells (E:T ratios of 5–10:1). The BGC823 cells were used as the
target cells and the CIK cells as the effector cells. The BGC823
and CIK cells alone were used as controls (Target spontaneous and
Effector spontaneous), and BGC823 cells treated with lysis solution
were used as maximum control (Target maximum). The supernatants of
the cultured cells were harvested, and the levels of LDH in the
supernatants were determined by reading at 492 nm on an ELISA
analyzer (DNM-9602G, Perlong, Beijing, China). The percentages of
specific cytotoxicity were calculated by the equation:
%Cytotoxicity = [A (Experimental) − A (Effector Spontaneous) − A
(Target Spontaneous)] × 100 / [A (Target maximum) − A (Target
spontaneous)].
Following co-culture of the engineered gastric tumor
cells with CIK cell for varying periods of time, these cells were
fixed and paraffin-embedded. Subsequently, the engineered gastric
tumor sections were stained with HE and examined under a light
microscope.
Cytotoxicity of different immune cells
against the engineered 3-D model of tumor cells
Following the establishment of the engineered 3-D
model in 96-well plates overnight, the tumor cells were reacted in
triplicate with 100 μl (1×105 cells) CIK, DC-CIK
and CIK-CEA/CD3-bscAb cells at an estimated E:T ratio of 10:1. The
engineered 3-D tumor cells or each type of CIK cells alone were
used as negative controls, while the 3-D tumor cells that had been
treated with lysis solution were used as maximum controls. The
supernatants were harvested and the cytotoxicity of different types
of CIK cells against the tumor cells was determined by LDH
assays.
Statistical analysis
Data are expressed as the mean ± SD. The difference
between groups was analyzed by ANOVA and Student’s t-test using the
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
result.
Results
Cancer cells form spheroids in
collagen matrix
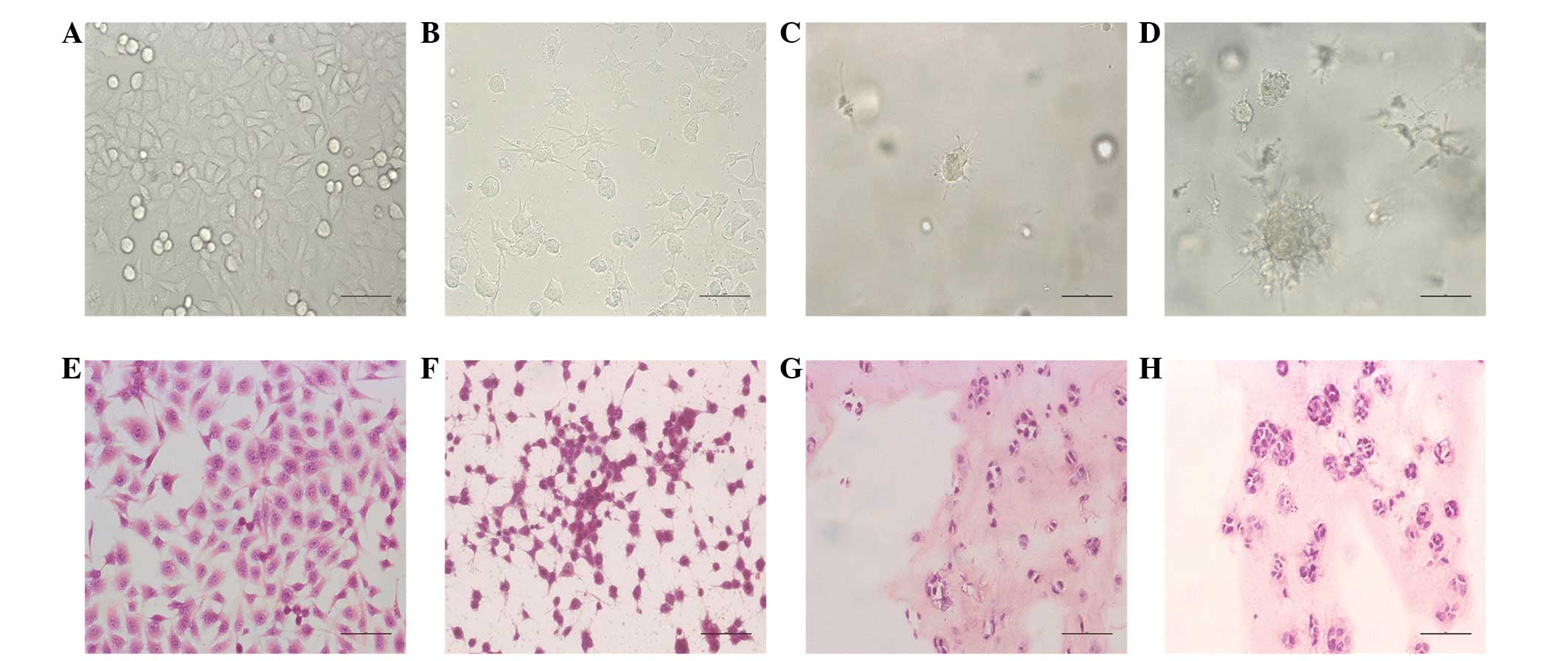
Compared with other tumor culture methods (Fig. 1A and B), human gastric cancer
(BGC823) cells grew in 3-D culture and formed tumor spheroids in
the engineered culture model (Fig.
1C). Phase contrast micrograph images showed that the numbers
and sizes of tumor cells spheroids increased with time (Fig. 1D). Histological characterization
revealed that the tumor cells formed numerous spheroids in the
collagen matrix in the engineered culture model (Fig. 1G and H). However, there were no
tumor cell spheroids in the monolayer culture (Fig. 1E and F).
Characterization of immune cells
To study the interaction of immune cells with tumor
cells in different culture models, we prepared human DCs and CIK
and DC-CIK cells in vitro. Flow cytometry analysis indicated
that the CIK cells were >95% CD3+ and 70%
CD8+, but only ∼30% CD3+CD56+
cells. Similarly, the purity of DC-CIK cells was >90% and the
majority of DC-CIK cells were CD3+, CD4+,
CD8+ and CD56+. The generated mature DCs had
90% purity and the majority of DCs had high levels of CD80, CD86,
CD83 and HLA-DR expression.
CIK cells migrate into the matrix and
kill cancer cells
To visualize the interaction of CIK cells with tumor
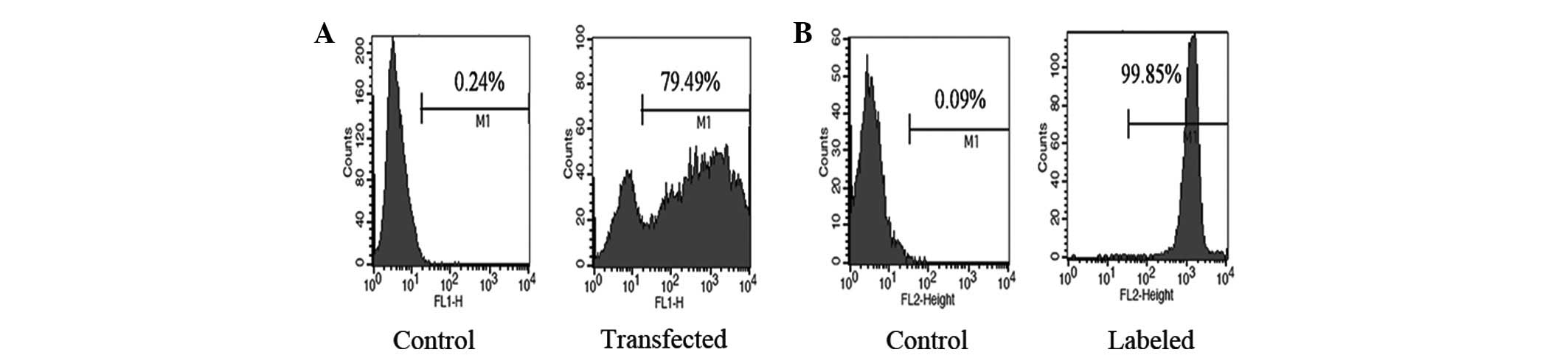
cells, we transfected tumor cells with pEGFP-C1 plasmids and found
that 79.49% of the tumor cells were GFP-positive (Fig. 2A). Similarly, we labeled the CIK
cells with PKH26 and found that 99.85% of CIK cells exhibited a red
color (Fig. 2B). After adding CIK
cells to the different tumor culture models, we found that the CIK
cells had direct contact with tumor cells in the control models
within a short time (Fig. 3A and
B). By contrast, the CIK cells first infiltrated into the
collagen matrix (Fig. 3C) and then
migrated towards and surrounded the tumor spheroids (Fig. 3D). Finally, the CIK cells destroyed
the spheroids (Fig. 3E) and killed
the tumor cells (Fig. 3F). Confocal
laser scanning and histological analyses indicated that the CIK
cells migrated into the collagen matrix and destroyed the spheroids
and tumor cells (Fig. 3G and
H).
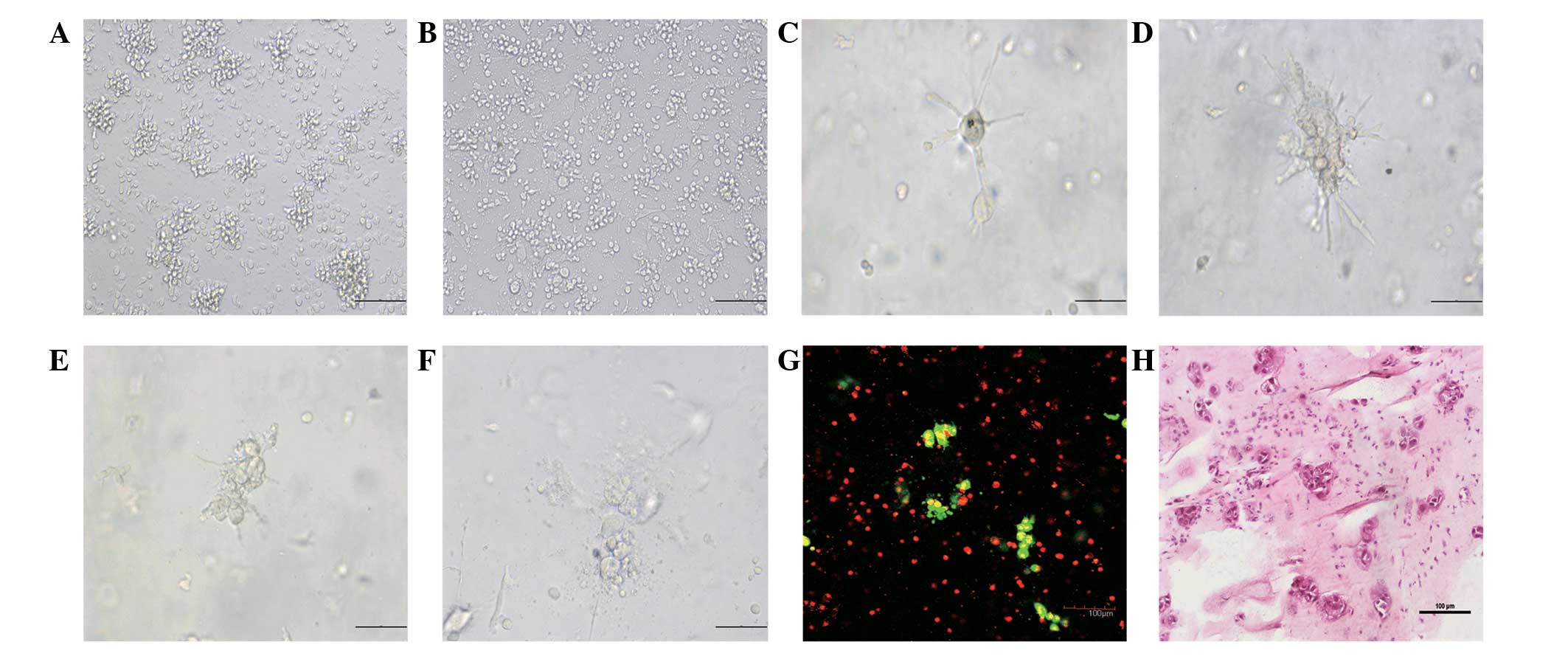 | Figure 3.Cytotoxic process of CIK cells
against tumor cells in different types of tumor culture models
in vitro. The same numbers of CIK cells were added to the
monolayer, liquid-overlay and 3-D gastric tumor cell culture models
and the cytotoxic process of CIK cells against the tumor cells was
characterized by longitudinal morphological observation,
histological examination and confocal analysis. Data shown are
representative images of different groups of cells from three
separate experiments. (A) The cytotoxicity of CIK cells against the
monolayer tumor cells at 12 h post-interaction; (B) the
cytotoxicity of CIK cells against the liquid-overlay tumor cells at
12 h post-interaction; (C) the structure of intact tumor spheroids
at 12 h post-CIK-tumor cell interaction; (D) the CIK cells migrated
and surrounded the tumor spheroids at 24 h post-CIK-tumor cell
interaction; (E) the CIK cells invaded the tumor spheroids at 36 h
post-CIK-tumor cell interaction; (F) the CIK cells destroyed the
tumor spheroids at 48 h post-CIK-tumor cell interaction; (G) the
confocal laser scanning microscopy of the interaction of CIK cells
with the tumor spheroids at 24 h post-CIK-tumor cell interaction;
and (H) histological examination of the interaction of CIK cells
with the tumor spheroids at 24 h post-CIK-tumor cell interaction.
(A, B, G and H) Magnification, ×200; scale bar equals 100
μm. (C, D, E and F) magnification, ×400; scale bar equals 50
μm. CIK, cytokine-induced killer; 3-D, 3-dimensional. |
Cytotoxicity of CIK cells in different
tumor culture models
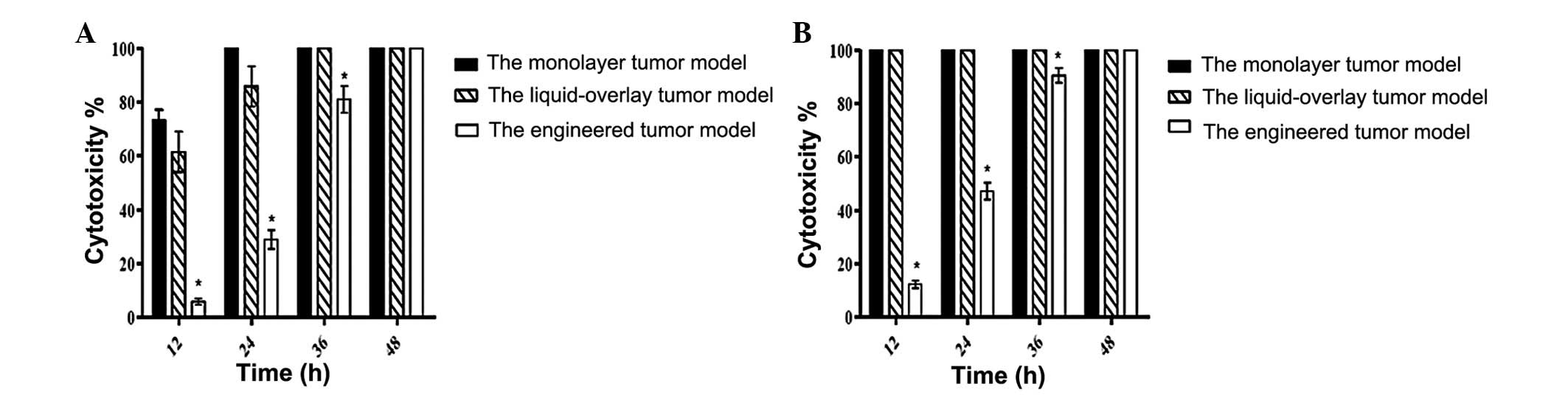
Quantitative analysis of the cytotoxicity of CIK
cells in different tumor culture models revealed that following
interaction of CIK cells with tumor cells for varying periods of
time, the cytotoxicity of CIK cells against tumor cells in the
engineered tumor culture model at 12 h post-interaction was low and
gradually increased over time. The cytotoxicity of CIK cells
against tumor cells in the engineered tumor culture models at 12,
24 and 36 h post-interaction was significantly lower than that in
the control models regardless of the ratios of E:T in our
experimental system (Fig. 4).
However, there was no significant difference in the cytotoxicity
against tumor cells among the tested models at 48 h
post-interaction.
Antitumor activity of different immune
cells in the 3-D model
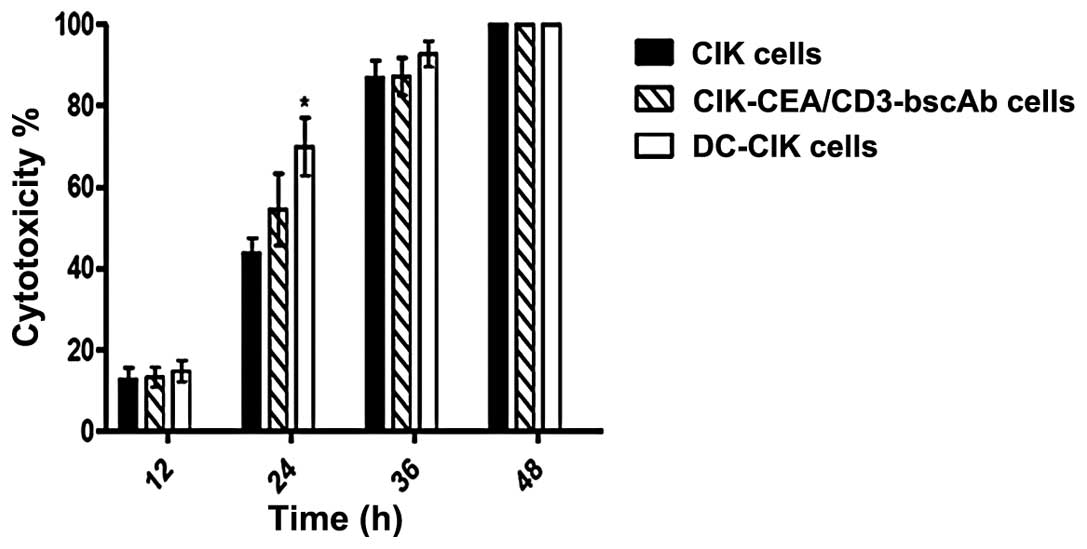
Finally, we employed the engineered tumor culture
model to examine the cytotoxicity of CIK cells, DC-CIK cells and
CIK-CEA/CD3-bscAb cells following interaction with tumor cells for
varying periods. As shown in Fig.
5, there was no significant difference in the cytotoxicity of
different types of CIK cells against tumor cells at 12 h
post-interaction. Notably, the cytotoxicity of DC-CIK cells against
tumor cells at 24 h was significantly stronger than that of CIK and
CIK-CEA/CD3-bscAb cells. Following interaction with tumor cells for
36 and 48 h, the cytotoxicity of the three types of CIK cells
against tumor cells in this model displayed similar levels.
Discussion
The 2-D cell culture models have been widely used
for studying the formation, function and pathology of tumors
(15). It has been suggested that
2-D cell cultures do not accurately reflect the cellular
environment in vivo due to the significant differences in
the spatial array and cellular surroundings (16). In the present study, we used the
tissue engineering approach to establish a 3-D gastric tumor
culture model in vitro. Our results showed that tumor cells
grew in the collagen matrix and formed spheroids in the matrix. The
engineered tumor 3-D culture model has a 3-D structure and should
be better than the 2-D cell culture models for mimicking the tumor
growth in vivo.
Engineering tissues have been traditionally
developed for the repair of damaged tissues and the reconstruction
of organs. The engineered scaffold materials to build the 3-D tumor
models have been used for evaluating the activity of antitumor
drugs in vitro(17–19). There are few studies using the
engineered tumor models to evaluate the antitumor activity of
immune cells in vitro. In the present study, we used
collagen as the scaffold material to establish a tumor 3-D culture
model. Collagen is a commonly used scaffold material in tissue
engineering and is also the primary structural protein of ECM that
maintains the stability of the basement membrane (20). Collagen has a porous structure that
supports tumor cell growth to form a 3-D tumor-like structure. The
porous structure of the scaffold provides a huge surface for cells
to adhere to and to interact with the scaffold. The porous
structure also allows the migration or entry of cultured cells into
the scaffold and nutrients to diffuse into the scaffold to support
the growth of the seeded cells (21). In addition, while collagen is liquid
at a low temperature, it is polymerized to form a collagen gel
after increasing the temperature and adjusting the pH to alkaline.
Moreover, the collagen gel is translucent and can be easily
paraffin-embedded, which allows the examination of the immune and
tumor cell interactions by microscopy. Therefore, collagen has a
number of advantages in establishing the 3-D tumor model for
evaluating the antitumor activity of immune cells in
vitro.
Immunotherapies have been widely used for the
treatment of cancer patients in the clinic (22–25).
DCs are important antigen-presenting cells and T cells are critical
for antitumor immunity. Currently, the antitumor activity of immune
cells in vitro is primarily evaluated by the 2-D cell
culture (26,27). In this experimental system, the
immune cells are able to contact the tumor cells directly, leading
to cytotoxicity against tumor cells. However, this model poorly
reflects the cytotoxic process of immune cells against tumor cells
in vivo, as tumor cells are surrounded by ECM in
vivo. Indeed, immune cells have to migrate through the ECM
barriers, such as the basement membrane (28). Collagen is the primary structural
protein of ECM and may be considered as an artificial ECM (29). We found that tumor cells formed
spheroids in the engineered 3-D tumor culture model and that the
cytotoxic process of CIK cells against tumor cells in the 3-D tumor
culture model was slower than that in the 2-D tumor cell culture
models. We observed that CIK cells migrated in the collagen matrix,
surrounded the tumor spheroids, invaded the tumor spheroids and
finally killed the tumor cells. Therefore, the cytotoxic process of
immune cells in the engineered 3-D tumor culture mode may be
similar to that in vivo.
Antigen-specific T cells have strong cytotoxicity
against tumor cells. CEA is expressed by BGC823 tumor cells, and
the CEA/CD3 bi-specific single-chain antibody inhibits the growth
of CEA+ tumor cells in vivo(30). We compared the cytotoxicity of CIK,
DC-CIK and CIK-CEA/CD3-bscAb cells in the engineered 3-D tumor
culture model. We found that DC-CIK cells displayed early and
strong cytotoxicity against the tumor cells, although the
cytotoxicity of these immune effector cells was comparable at 48 h
post-interaction in the engineered 3-D tumor culture cell model.
Therefore, the engineered 3-D tumor culture model may be used for
evaluating the cytotoxicity of different types of immune cells
in vitro.
In conclusion, we used collagen as the matrix to
establish an engineered gastric tumor 3-D culture model, in which
gastric tumor cells formed spheroids. Furthermore, our data
indicated that the established engineered 3-D tumor culture model
was used for effectively evaluating the cytotoxicity of different
types of activated immune cells in vitro.
Acknowledgements
This study was supported by grants
from the National High Technology Research and Development Program
of China (2006AA02Z4B9) and the Technology Innovation Foundation of
PLA General Hospital (08CXLXB04).
References
|
1.
|
Santini MT and Rainaldi G:
Three-dimensional spheroid model in tumor biology. Pathobiology.
67:148–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Smalley KS, Lioni M and Herlyn M: Life
isn’t flat: taking cancer biology to the next dimension. In Vitro
Cell Dev Biol Anim. 42:242–247. 2006.
|
|
3.
|
Zaman MH, Trapani LM, Sieminski AL,
Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA and
Matsudaira P: Migration of tumor cells in 3D matrices is governed
by matrix stiffness along with cell-matrix adhesion and
proteolysis. Proc Natl Acad Sci USA. 103:10889–10894. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami
S, Stanley ER, Segall JE, Pollard JW and Condeelis J: Direct
visualization of macrophage-assisted tumor cell intravasation in
mammary tumors. Cancer Res. 67:2649–2656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Vamvakidou AP, Mondrinos MJ, Petushi SP,
Garcia FU, Lelkes PI and Tozeren A: Heterogeneous breast tumoroids:
An in vitro assay for investigating cellular heterogeneity and drug
delivery. J Biomol Screen. 12:13–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tang J, Cui J, Chen R, Guo K, Kang X, Li
Y, Gao D, Sun L, Xu C, Chen J, Tang Z and Liu Y: A
three-dimensional cell biology model of human hepatocellular
carcinoma in vitro. Tumour Biol. 32:469–479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Rodday B, Hirschhaeuser F, Walenta S and
Mueller-Klieser W: Semiautomatic growth analysis of multicellular
tumor spheroids. J Biomol Screen. 16:1119–1124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Santini MT, Rainaldi G and Indovina PL:
Multicellular tumour spheroids in radiation biology. Int J Radiat
Biol. 75:787–799. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Oktem G, Bilir A, Selvi N, Yurtseven ME,
Vatansever S, Ates U, Uysal A and Omay SB: Chemotherapy influences
inducible nitric oxide synthase (iNOS) and endothelial nitric oxide
synthase (eNOS) activity on 3D breast cancer cell line. Oncol Res.
16:195–203. 2006.PubMed/NCBI
|
|
10.
|
Ho WJ, Pham EA, Kim JW, Ng CW, Kim JH,
Kamei DT and Wu BM: Incorporation of multicellular spheroids into
3-D polymeric scaffolds provides an improved tumor model for
screening anticancer drugs. Cancer Sci. 101:2637–2643. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Higashiyama M, Okami J, Maeda J, Tokunaga
T, Fujiwara A, Kodama K, Imamura F and Kobayashi H: Differences in
chemosensitivity between primary and paired metastatic lung cancer
tissues: In vitro analysis based on the collagen gel droplet
embedded culture drug test (CD-DST). J Thorac Dis. 4:40–47.
2012.PubMed/NCBI
|
|
12.
|
Horning JL, Sahoo SK, Vijayaraghavalu S,
Dimitrijevic S, Vasir JK, Jain TK, Panda AK and Labhasetwar V: 3-D
tumor model for in vitro evaluation of anticancer drugs. Mol Pharm.
5:849–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhao Y, Xu Y, Zhang B, Wu X, Xu F, Liang
W, Du X and Li R: In vivo generation of thick, vascularized hepatic
tissue from collagen hydrogel-based hepatic units. Tissue Eng Part
C Methods. 16:653–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Guo XM, Zhao YS, Chang HX, Wang CY, E LL,
Zhang XA, Duan CM, Dong LZ, Jiang H, Li J, Song Y and Yang XJ:
Creation of engineered cardiac tissue in vitro from mouse embryonic
stem cells. Circulation. 113:2229–2237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yamada KM and Cukierman E: Modeling tissue
morphogenesis and cancer in 3D. Cell. 130:601–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gottfried E, Kunz-Schughart LA, Andreesen
R and Kreutz M: Brave little world: spheroids as an in vitro model
to study tumor-immune-cell interactions. Cell Cycle. 5:691–695.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kim JW, Ho WJ and Wu BM: The role of the
3D environment in hypoxia-induced drug and apoptosis resistance.
Anticancer Res. 31:3237–3245. 2011.PubMed/NCBI
|
|
18.
|
Sakai S, Inamoto K, Liu Y, Tanaka S, Arii
S and Taya M: Multicellular tumor spheroid formation in duplex
microcapsules for analysis of chemosensitivity. Cancer Sci.
103:549–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Loessner D, Stok KS, Lutolf MP, Hutmacher
DW, Clements JA and Rizzi SC: Bioengineered 3D platform to explore
cell-ECM interactions and drug resistance of epithelial ovarian
cancer cells. Biomaterials. 31:8494–8506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Egeblad M, Rasch MG and Weaver VM: Dynamic
interplay between the collagen scaffold and tumor evolution. Curr
Opin Cell Biol. 22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Xu F and Burg KJ: Three-dimensional
polymeric systems for cancer cell studies. Cytotechnology.
54:135–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Olioso P, Giancola R, Di Riti M, Contento
A, Accorsi P and Iacone A: Immunotherapy with cytokine induced
killer cells in solid and hematopoietic tumours: a pilot clinical
trial. Hematol Oncol. 27:130–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
June CH: Adoptive T cell therapy for
cancer in the clinic. J Clin Invest. 117:1466–1476. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhong R, Teng J, Han B and Zhong H:
Dendritic cells combining with cytokine-induced killer cells
synergize chemotherapy in patients with late-stage non-small cell
lung cancer. Cancer Immunol Immunother. 60:1497–1502. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhou P, Liang P, Dong B, Yu X, Han Z and
Xu Y: Phase I clinical study of combination therapy with microwave
ablation and cellular immunotherapy in hepatocellular carcinoma.
Cancer Biol Ther. 11:450–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kim HM, Lim J, Park SK, Kang JS, Lee K,
Lee CW, Lee KH, Yun MJ, Yang KH, Han G, Kwon SW, Kim Y and Han SB:
Antitumor activity of cytokine-induced killer cells against human
lung cancer. Int Immunopharmacol. 7:1802–1807. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hu Y, He Y, Srivenugopal KS, Fan S and
Jiang Y: In vitro antitumor cytotoxic T lymphocyte response induced
by dendritic cells transduced with ΔNp73α recombinant adenovirus.
Oncol Rep. 18:1085–1091. 2007.
|
|
28.
|
Korpos E, Wu C, Song J, Hallmann R and
Sorokin L: Role of the extracellular matrix in lymphocyte
migration. Cell Tissue Res. 339:47–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Sant S, Hancock MJ, Donnelly JP, Iyer D
and Khademhosseini A: Biomimetic gradient hydrogels for tissue
engineering. Can J Chem Eng. 88:899–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lutterbuese R, Raum T, Kischel R,
Lutterbuese P, Schlereth B, Schaller E, Mangold S, Rau D, Meier P,
Kiener PA, Mulgrew K, Oberst MD, Hammond SA, Baeuerle PA and Kufer
P: Potent control of tumor growth by CEA/CD3-bispecific
single-chain antibody constructs that are not competitively
inhibited by soluble CEA. J Immunother. 32:341–352. 2009.
View Article : Google Scholar : PubMed/NCBI
|