Introduction
Gastric cancer involves tumors of the digestive
system and has the highest incidence and mortality rate in China
(1). The overall 5-year mortality
rate of patients is more than 80% worldwide. Gastric cancer has the
second highest mortality rate of all types of cancer in the world
(2) and therefore seriously
threatens human health. Currently, it is thought that tumor stromas
play a significant regulatory role in the balance of the tumor-host
interface microenvironment (3,4). As
stroma cells are non-variant cells and their genetic compositions
are relatively stable, they are easily studied and have gradually
become a significant focus of research (5). Cancer-associated fibroblasts (CAFs)
are the main cellular component of tumor stromas. Previous research
has suggested that CAFs have different phenotypes and biological
characteristics from normal fibroblasts (NFs) and alterations to
these phenotypes are mostly associated with the infiltration and
metastasis of tumors (6,7). However, at present little is known
about the role of CAFs in gastric cancer invasion and metastasis.
Therefore, human gastric CAFs were isolated and cultured in
vitro to establish a model of interaction of CAFs with
SGC-7901. Their influences on the migration and invasion abilities
of SGC-7901 gastric cancer cells was further analyzed. This study
is likely to aid in understanding the infiltration and metastasis
mechanisms of gastric cancer cells and may provide a reference for
clinical treatment.
Materials and methods
Cells
The gastric cancer cell line SGC-7901 was provided
by Dr Chen Xi, Department of General Surgery, First College of
Clinical Medicine, Harbin Medical University and cultured in
high-sugar DMEM containing 10% fetal bovine serum in an incubator
containing 5% CO2 at 37°C. All experimental cells were
in the logarithmic growth phase.
Primary culture of CAFs
The fresh specimen from radical correction of
gastric cancer was taken from the operating room and sheared into
small blocks 1 mm in diameter on an aseptic bench and then placed
uniformly onto the culture face of a disposable culture flask with
an elbow straw (the interval between the blocks was 5 mm).
High-sugar DMEM (2 ml) containing 20% fetal bovine serum was added,
the culture flask was then inverted and cultured for 1 h in the
incubator containing 5% CO2 at 37°C. Subsequently, the
culture flask was overturned. The culture liquid was replaced on
the third day and then every subsequent three days. The soonest
cells were seen to appear was following 5 to 7 days. After ∼2 to 3
weeks, cells covered the whole base of the flask. The enzyme
digestion method was used to purify the cells. Following the first
passage, the medium was replaced with high-sugar DMEM containing
10% fetal bovine serum to conduct a conventional culture. The
third-generation purified cells were used to conduct preliminary
identification. In addition, the 4th to 10th generations of cells
were used for subsequent tests.
Identification of human gastric CAFs
The cover glass with cells was fixed with 95%
alcohol for 30 min. The first antibodies included cytokeratin,
vimentin, smooth muscle actin (SMA) and fibroblast activation
protein (FAP). The PV6000 general-type two step method was used
according to the reagent instructions. In addition, the negative
and positive control groups were set. If pale brown or brown
granules appeared in the cytoplasm, the specimen was judged as
positive.
Preparation of CAF conditioned medium
(FCM)
Human gastric CAFs were inoculated into a 50-ml
culture flask and a conventional culture was carried out for 48 h.
Subsequently, the medium was replaced with 5 ml DMEM containing 5
g/l BSA without serum to continuously culture for 48 h. The
supernatant was collected, centrifuged at 1,000 rpm, filtered with
a 0.22-μm membrane for sterilization and stored at −20°C for
use. At the same time, DMEM containing 5 g/l BSA without serum was
set as the control.
Cell scratch test
Matrigel and DMEM without serum were mixed at a
ratio of 1:2 and added to a 96-well plate at 30 μl/well
(these processes were completed on ice). Artificially remodeled
basement membrane was prepared and air dried on the super-clean
bench. Subsequently, 50 μl medium containing 10 g/l BSA
without serum was added into each well and incubated at 37°C for 30
min in order to hydrolyze the basement membrane. SGC7901 cells and
CAFs were added to a 96-well plate (10,000 cells/well) with 5
parallel samples in each group. Conventional culture was conducted
until a monolayer of cells formed. Subsequently, the cells were
scratched into a linear shape along the bottom of culture plate
with a 10-μl sterile tip head, the relative distance of the
scratch area was recorded under the microscope. Subsequently, the
cells were washed with the culture liquid without serum and the
variously conditioned culture liquid was used to continuously
culture for 72 h. In addition, the scratch widths were observed at
24, 48 and 72 h and images were captured. According to the original
distance of the damaged cell area, the relative migration distance
of cells was calculated. For each group, tests were repeated three
times. Calculation formula: relative migration distance = 1 −
(real-time scratch width/original scratch width).
Transwell migration test
FCM and monolayer cultured CAFs were respectively
added to a 24-well plate and DMEM containing 5 g/l BSA without
serum was taken as the control. In each group, three repeated wells
were set. An SGC-7901 cell suspension (100 μl) with a
density of 5.0×104 cells/ml was added to a Transwell
cell and a conventional culture was conducted for 24 h. After the
cells were washed with distilled water and dried with air, crystal
violet staining was conducted. Under the microscope, upper, lower,
central, left and right 5 view fields were selected to count the
number of transmembrane cells. The mean value represented the
migratory cell number.
Transwell invasion test
Similar to the migration test, Matrigel was used to
cover the basement membrane of the Transwell cell prior to the
test. The inoculation density of the SGC-7901 gastric cancer cells
was 1×105/ml. Following crystal violet staining, the
transmembrane cell number was counted under the microscope.
Statistical analysis
SPSS software was used for statistical analysis and
measurement data were expressed as mean ± SD. A t-test was used to
compare the mean values between the two groups, and analysis of
variance was used for the comparison of mean values among more than
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of CAFs
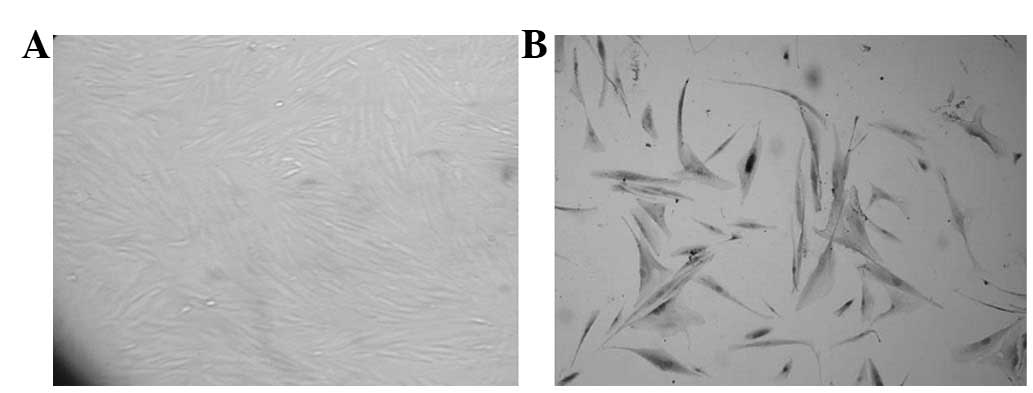
Inverted microscope observation revealed that
purified CAFs were fibroblast-like cells, their sizes were
different and their shapes presented as a fusiform or irregular
triangle. Also, there were various lengths of multiple processes.
The nucleus was at the center of cell body, presenting as a
circular or elliptical shape. The cells were arranged in a radial
or vertical shape and partial cells were disordered. Polarity,
contact inhibition and density inhibition disappeared (Fig. 1A) and there were apparent
overlapping phenomena. The immunocytochemical staining results
revealed that the purified third-generation CAFs cells all
expressed vimentin, FAP and SMA and cytokeratin was not expressed
(Fig. 1B).
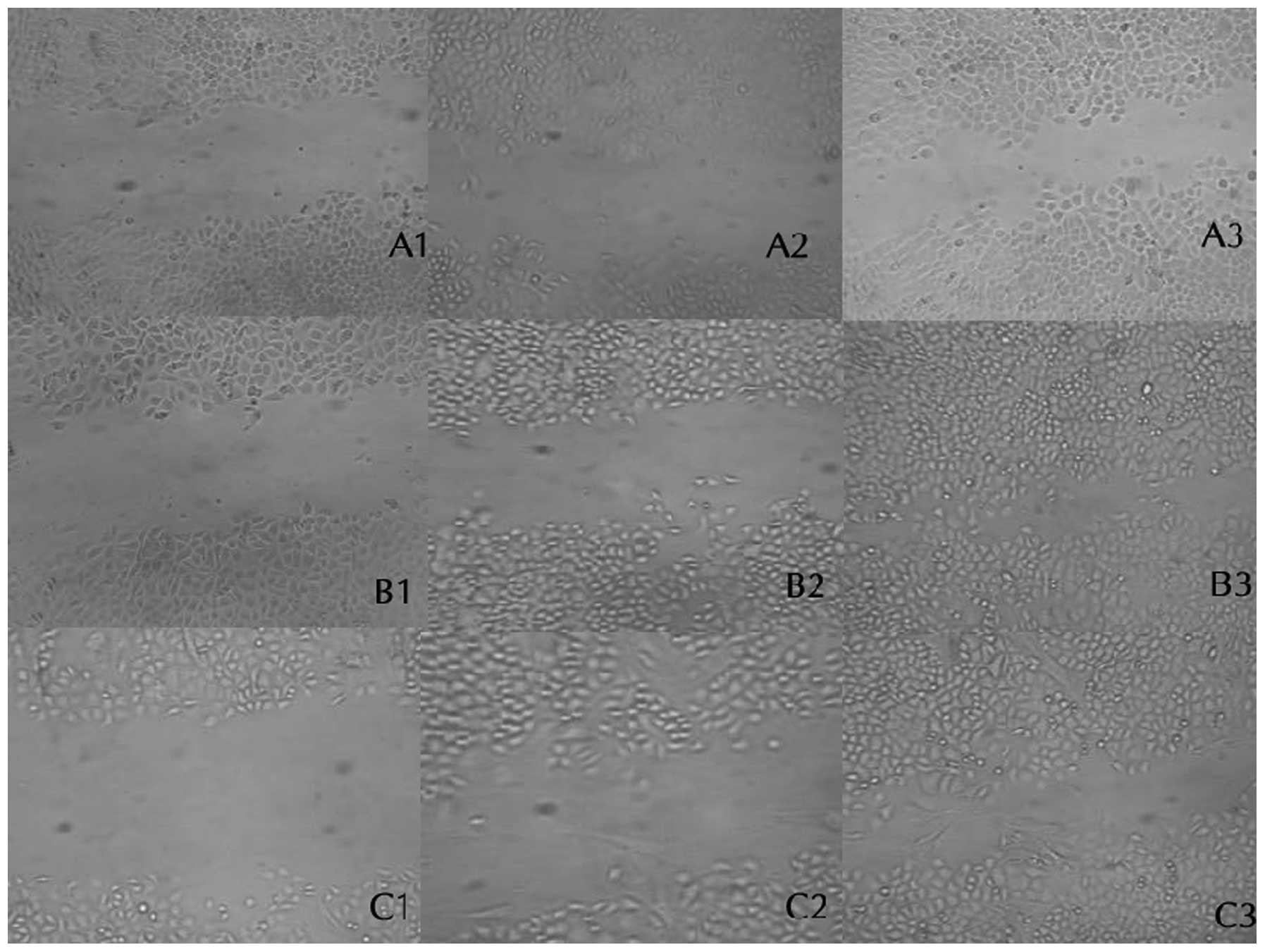
CAFs promotes the transverse migration of
SGC-7901 cells
SGC-7901 cells and CAFs were co-cultured. SGC-7901
cells were respectively cultured in FCM or DMEM without serum for
24, 48 and 72 h. Under the inverted microscope, the relative
distance of cells migrating to a damaged area was measured and
calculated. CAFs had a more marked transverse migration ability
than SGC-7901 cells and the migration distance of SGC-7901 cells of
the CAFs and FCM group significantly increased. Therefore, CAFs
promoted the transverse migration of SGC-7901 gastric cancer cells
(Fig. 2, Table I).
 | Table I.Scratch test results. |
Table I.
Scratch test results.
| Scratch width after
culture (mean + SD)
|
|---|
| Group | 24 h | 48 h | 72 h |
|---|
| CAFs | 0.23±0.06a | 0.69±0.05a,b | 0.75±0.03a |
| FCM | 0.24±0.05a | 0.60±0.04a,b | 0.78±0.04a,b |
| Control | 0.11±0.03 | 0.18±0.05 | 0.27±0.04 |
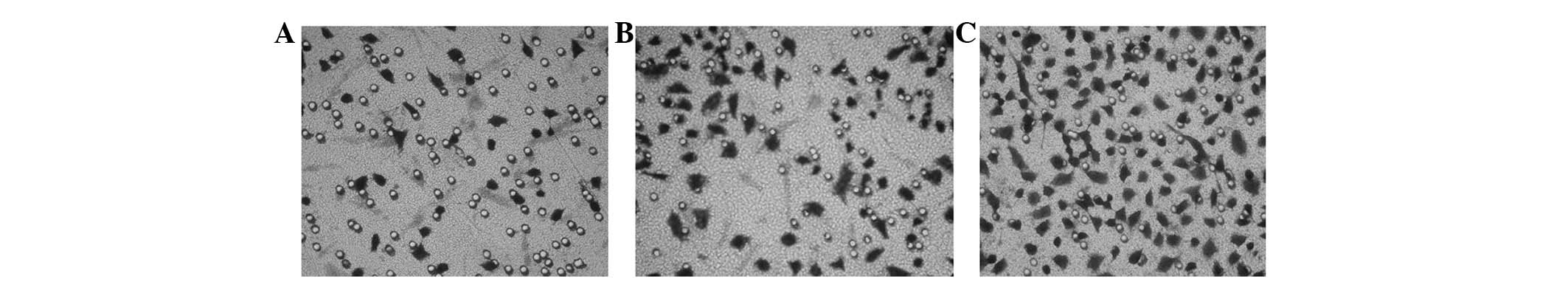
CAFs promotes the longitudinal migration
of SGC-7901 cells
Transwell cells were used to co-culture CAFs and
SGC-7901 cells for 24 h. The transmembrane cell number of SGC-7901
cells significantly increased, indicating that CAFs were able to
promote the longitudinal migration of SGC-7901 cells and this
effect was induced by FCM repeatedly (Fig. 3, Table
II).
 | Table II.Effect of CAFs on invasiveness and
migration capability of SGC7901 cells. |
Table II.
Effect of CAFs on invasiveness and
migration capability of SGC7901 cells.
| Group | Invasiveness
experiment | Migration
experiment |
|---|
| CAFs |
98.67±13.49a,b | 34.40±4.63a,b |
| FCM | 78.47±10.59a | 26.93±3.99a |
| Control | 40.54±12.55 | 13.33±9.65 |
CAFs promotes the invasion ability of
SGC-7901 cells
SGC-7901 cells, CAFs and Matrigel were co-cultured
and the transmembrane cell number of SGC-7901 cells significantly
increased. FCM application simulated the effect of CAFs to a
certain extent. Thus, CAFs promoted SGC-7901 invasion and the
interaction of CAFs with SGC-7901 cells significantly promoted
SGC-7901 invasion (Table II).
Discussion
CAFs specifically refer to the myofibroblasts of the
host’s malignant tumor. They were first identified in the stroma of
solid tumors accompanied by connective tissue hyperplasia,
including breast carcinoma and pancreatic cancer. In 1999, Olumi
et al cultured prostate cancer stroma fibroblasts and named
them CAFs (8,9). Studies have revealed that CAFs
participate in the synthesis, deposition and reconstruction
processes of the tumor extracellular matrix and a variety of
paracrine growth factors, proteases and their inhibitors. They also
induce the immunological escape of tumor cells and thus promote
cytogeny and the development of tumors (10–12).
Previous research has suggested that CAFs in human
gastric cancer tissues not only express fibroblast-labeled
vimentin, but also the myofibroblast markers SMA and FAP. This
phenotypic change of CAFs was positively correlated with a number
of clinical and pathological indicators of gastric cancer (13). In the current study, an
immunocytochemical technique was used to detect the expression of
vimentin, FAP and SMA in human gastric CAFs cultured in
vitro. The result revealed that human gastric CAFs cultured
in vitro had phenotypic characteristics similar to those of
CAFs in vivo, which was different from rat gastric CAFs
isolated and cultured in vitro(14). This indicates that the study of
human gastric CAFs may better simulate the progression of human
gastric cancer.
Animal experiments have shown that in most tumor
models, the interaction of tumor cells with the extracellular
matrix influences the growth rate, infiltration range and distant
metastasis ability of tumor cells (15). There are four steps in the invasion
of cancer cells to the extracellular matrix: mutal separation of
tumor cells, adhesion to matrix components, degradation of the
extracellular matrix and movement of tumor cells. In this study,
the cell scratch, Transwell migration and invasion tests were
applied and Matrigel was used to simulate the effect of the
extracellular matrix. CAFs or their conditioned medium was used to
induce SGC-7901 migration or invasion and simulate the interaction
of tumor cells with their surrounding CAFs and extracellular
matrix. The migration and invasiveness of SGC-7901 greatly enhance
under the action of CAFs. The application of FCM repeated this
process, indicating that CAFs induced tumor cells to degrade the
extracellular matrix by secreting soluble factors and caused
infiltration and metastasis. In addition, it was revealed that
under the conditions of CAFs, tumor cells and extracellular matrix
co-culture, CAFs had a more marked migration ability than tumor
cells. Human gastric cancer CAFs accompany invasive tumor cells
in vivo, and they are primarily distributed at the front of
neoplasm invasiveness as well as at the tumor-interstitium
interface (11). Therefore, it is
speculated that CAFs present invasion ability earlier than tumor
cells and induce extracellular matrix reconstruction and stroma
formation by secreting a variety of growth factors and proteases.
The newly formed stroma damages the continuity of the normal
structure and creates a pathway for the invasion of tumor
cells.
CAFs promote tumor infiltration and metastasis by
the following possible routes: i) sectrion of a variety of growth
factors including TGF-β1 and matrix metalloproteinases and
participation in tumor progression (12,16,17);
ii) promotion of angiogenesis (18); iii) inhibition of the body’s
antitumor immunity (19,20). In the current study, CAFs highly
expressed SMA and FAP. FAP is a glycoprotein on the cell surface,
with the dual activity of collagenase and dipeptidyl peptidase and
it degrades multiple substrates of dipeptidase, gelatin and type -
collagen in the extracellular matrix. Therefore, it is conducive to
invasion of tumor cells. For tumor cells transfected with FAP,
tumor formation rate and invasion ability in the nude rat body
significantly increase. They also inhibit the effect of FAP in
tumor tissues and significantly improve the tumor sensitivity to
chemotherapy and survival rate of rats with cancer (21–23).
Future research should include the effect of FAP in gastric cancer
tissues, targeted inhibition of FAP and the possibility of reducing
or inhibiting the influence of CAFs on the invasive ability of
tumor cells. It is likely to be useful in elucidating the
occurrence, development, infiltration and metastasis mechanisms of
cancer cells to further clarify the cellular signal transduction
mechanism associated with the interaction of CAFs with tumor
cells.
This study used human gastric CAFs to establish the
in vitro model and simulate the in vivo tumor
microenvironment to further confirm that CAFs promote the migration
and invasion of tumor cells. It also provides theoretical and
practical bases for researching the mechanism of action of CAFs and
their role in future tumor treatment.
References
|
1.
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006.
|
|
2.
|
Terry MB, Gaudet MM and Gammon MD: The
epidemiology of gastric cancer. Semin Radiat Oncol. 12:111–127.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Elenbaas B and Weinberg RA: Heterotypic
signaling between epithelial tumor cells and fibroblasts in
carcinoma formation. Exp Cell Res. 264:169–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bhowmick NA, Neilson EG and Moses HL:
Stromal fibroblasts in cancer initiation and progression. Nature.
432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
7.
|
Allinen M, Beroukhim R, Cai L, et al:
Molecular characterization of the tumor microenvironment in breast
cancer. Cancer Cell. 6:17–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dolznig H, Schweifer N, Puri C, Kraut N,
Rettig WJ, Kerjaschki D and Garin-Chesa P: Characterization of
cancer stroma markers: in silico analysis of an mRNA expression
database for fibroblast activation protein and endosialin. Cancer
Immun. 5:102005.PubMed/NCBI
|
|
9.
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
10.
|
Tlsty TD: Stromal cells can contribute
oncogenic signals. Semin Cancer Biol. 11:97–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Desmoulière A, Guyot C and Gabbiani G: The
stroma reaction myofibroblast: a key player in the control of tumor
cell behavior. Int J Dev Biol. 48:509–517. 2004.PubMed/NCBI
|
|
12.
|
Radisky DC and Przybylo JA: Matrix
metalloproteinase-induced fibrosis and malignancy in breast and
lung. Proc Am Thorac Soc. 5:316–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wang LF, Wang RF and Wang ZC: Changes in
protein expression and significance of tumor-associated fibroblasts
in gastric cancer. World Chin J Digestol. 15:2263–2267. 2007.
|
|
14.
|
Guo X, Oshima H, Kitmura T, Taketo MM and
Oshima M: Stromal fibroblasts activated by tumor cells promote
angiogenesis in mouse gastric cancer. J Biol Chem. 283:19864–19871.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Geng JS, Song HT and Wang WR: Diversity of
invasiveness and matrix metalloproteinase expression profile of
human gastric carcinoma xenografted in different tissue
environments. Chin J Pathology. 33:53–56. 2004.(In Chinese).
|
|
16.
|
Zhang YW, Deng H, Chen PS and Chen LL:
Increased expression of matrix metalloproteinase inducer though
interaction between fibroblasts and colonic cancer cell. Chin J
Pathology. 36:764–767. 2007.(In Chinese).
|
|
17.
|
Kuperwasser C, Chavarria T, Wu M, et al:
Reconstruction of functionally normal and malignant human breast
tissues in mice. Proc Natl Acad Sci USA. 101:4966–4971. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Liu F, Lang R, Wei J, et al: Increased
expression of SDF-1/CXCR4 is associated with lymph node metastasis
of invasive micropapillary carcinoma of the breast. Histopathology.
54:741–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Barth PJ and Westhoff CC: CD34+
fibrocytes: morphology, histogenesis and function. Curr Stem Cell
Res Ther. 2:221–227. 2007.
|
|
20.
|
Fassnacht M, Lee J, Milazzo C, Boczkowski
D, Su Z, Nair S and Gilboa E: Induction of CD4(+) and CD8(+) T-cell
responses to the human stromal antigen, fibroblast activation
protein: implication for cancer immunotherapy. Clin Cancer Res.
11:5566–5571. 2005.
|
|
21.
|
Lee J, Fassnach M, Nair S, Boczkowski D
and Gilboa E: Tumor immunotherapy targeting fibroblast activation
protein, a product expressed in tumor-associated fibroblasts.
Cancer Res. 65:11156–11163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tahtis K, Lee F, Wheatley JM, et al:
Expression and targeting of human fibroblast activation protein in
a human skin/severe combined immunodeficient mouse breast cancer
xenograft model. Mol Cancer Ther. 2:720–737. 2003.
|
|
23.
|
Loeffler M, Krüger JA, Niethammer AG and
Reisfeld RA: Targeting tumor-associated fibroblasts improves cancer
chemo-therapy by increasing intratumoral drug uptake. J Clin
Invest. 116:1955–1962. 2006. View
Article : Google Scholar : PubMed/NCBI
|