Introduction
Vascular tumors, which derive from vascular tissue,
mostly occur in the head, neck and subcutaneous tissue of the limbs
and muscles. Generally, different types of vascular tumors occur in
different age brackets. For example, skin tumors always occur in
young adults, and the peak age of these patients is 20–30 years of
age; while endothelial tumors often appear in older individuals,
mainly in their 40s. Moreover, there are no obvious differences in
this phenomenon between males and females. Large lumps, accompanied
by local pain, dysfunction or other symptoms, are the obvious
pathological features of patients with vascular tumors. Although it
is easy to diagnose the rare vascular tumors by pathological
analysis due to the visible histological features, the boundary
definition between benign and malignant tumors in histology is
still a problem that needs to be addressed. The discrepancies
between clinical judgement and pathological diagnosis often lead to
a difficult clinical decision, which may result in misdiagnosis. In
this context, comprehensive analyses of clinical and pathological
data of rare vascular tumors are necessary.
Vascular tumor specimens were fixed in 10% neutral
buffered formalin, embedded by paraffin, cut into 4-μm-thick
serial sections, and then stained with HE and reticular fiber. For
the immunohistochemical staining, the Envision™ two-step staining
protocol was adopted. Briefly, after antigen retrieval in pH 6.0 10
mmol/l citrate buffer for 20 mins, the sections were prepared by
conventional dewaxing hydration procedures. In this report, the
analysis data of three rare vascular tumor cases that we
encountered in clinical practice are reported. The study was
approved by Ethics Committee of Qilu Children’s Hospital of
Shandong University, Jinan, China. Written informed consent was
obtained from the patient’s family.
Case report
Case 1: Angiosarcoma
The patient was a 47-year-old male who was reported
to have a potential medical B-liver cancer, according to the
clinical symptoms and laboratory tests. However, further
examination was still required to confirm the diagnosis prior to
surgery, since the symptoms and tests were not specific enough. The
postoperative pathology revealed a hepatic angiosarcoma (HAS).
Pathological examination showed the vascular tumors located in the
right lobe of the liver with solid sections, hemorrhage, necrosis
and medium texture. The boundary between tumors and surrounding
liver tissue was unclear, and there were incomplete fibrous
capsules around tumors. The liver biopsy specimen exhibited light
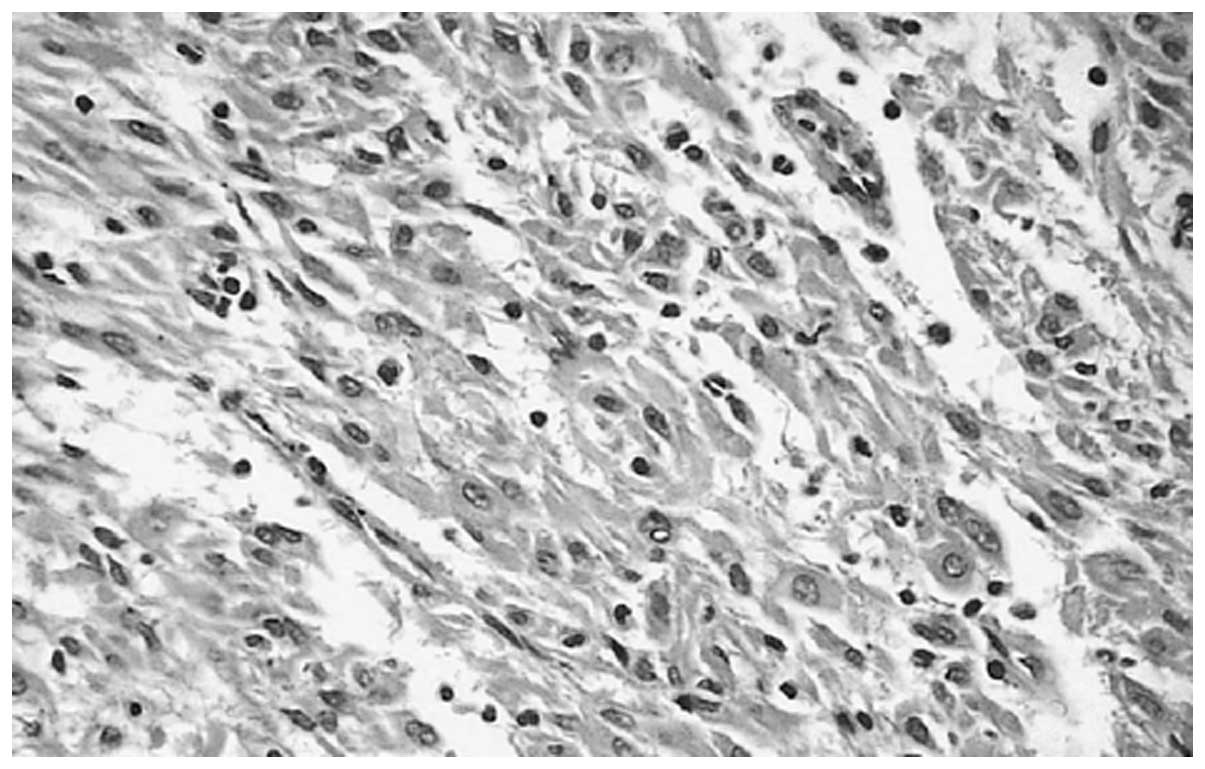
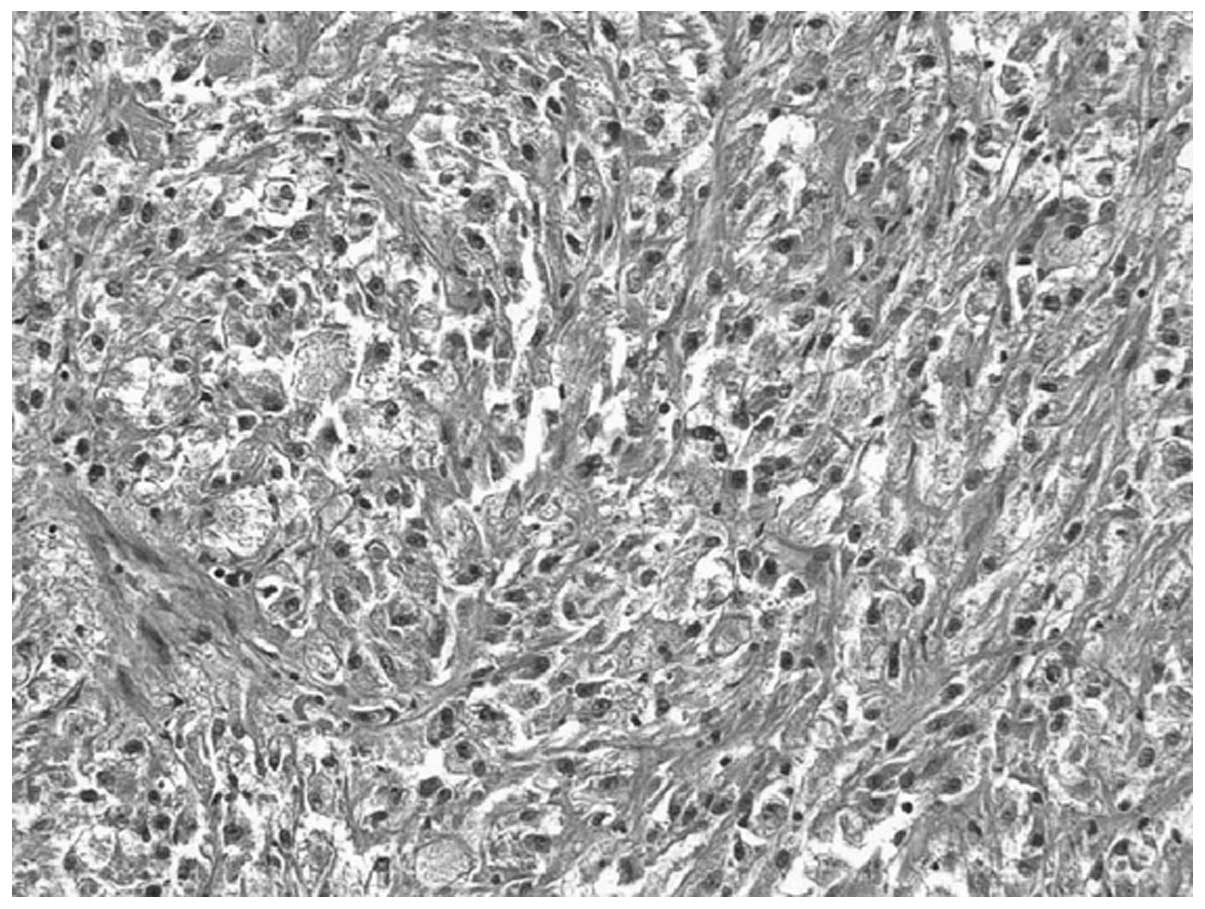
brown spinal cord-like tissue. Microscopic examination showed that
the tumor tissue consisted of irregular tumor blood vessels that
formed mesh compartments, and were lined with one or more layers of
endothelial cells. Endothelial cell proliferations projecting out
of the cavity were papillary (Fig.
1). Hemorrhage and necrosis were observed in the tumors.
Surgically-resected tumor tissue centers on the hardening zone were
rich in fiber and accompanied with hyaline degeneration. There were
many tumor cells in the marginal zone with peripheral enhancement,
which was consistent with the findings from the CT and MRI scans,
and scattered with visible residue and proliferation of small bile
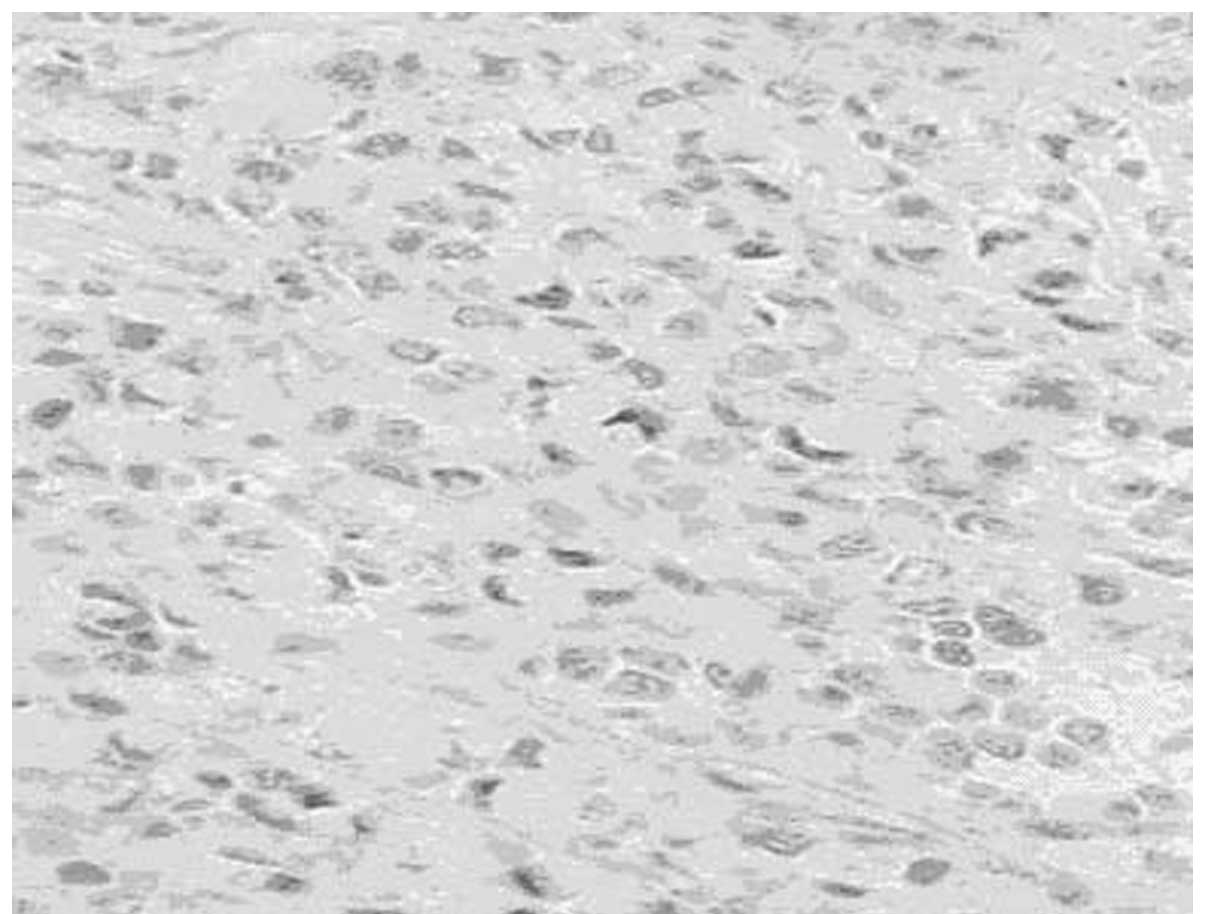
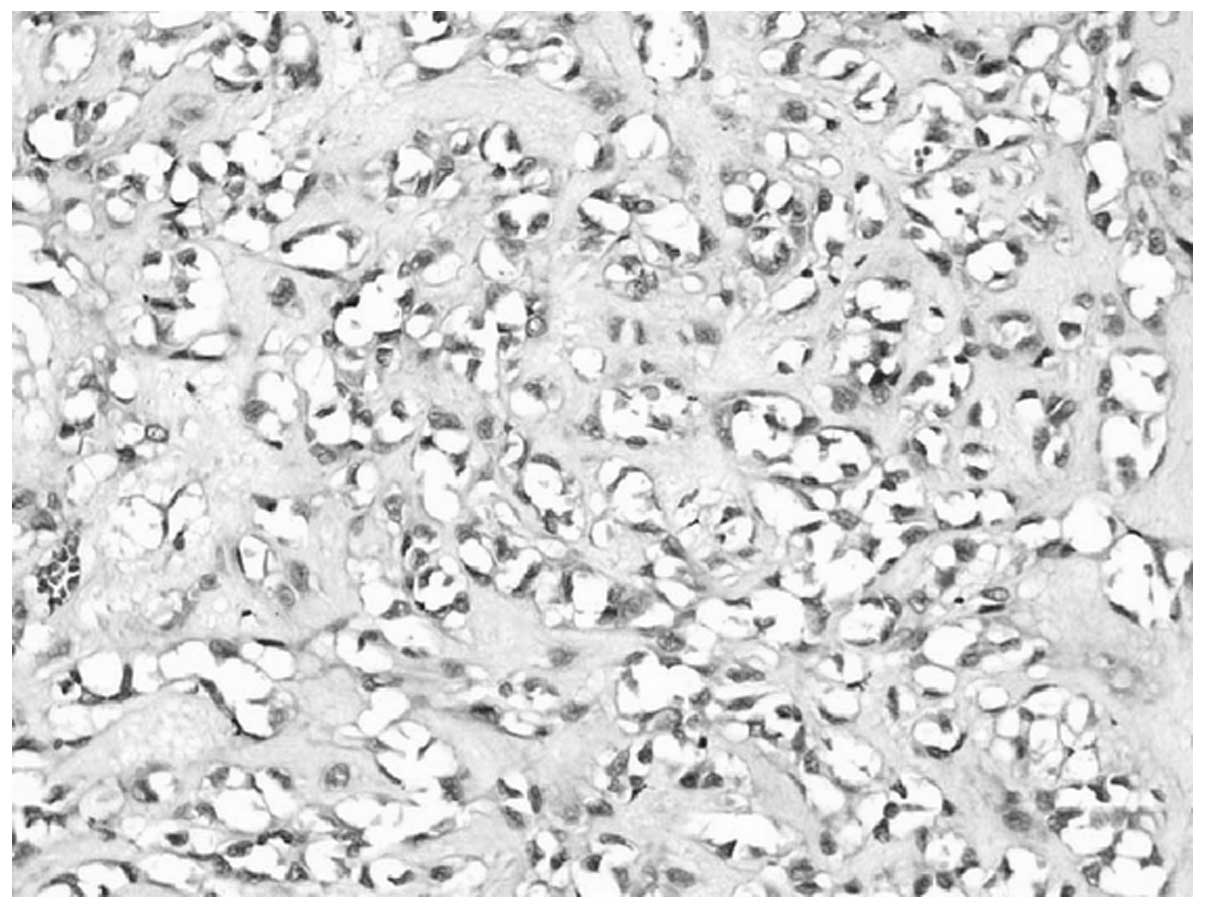
ducts around them. Certain tumor cells had an irregular shape, such
as circular, polygonal or epithelioid, with rich cytoplasm. These
cells demonstrated atypical cell division that was difficult to
see. RBCs, which divide the vessel lumen into single cells, were
observed in the cytoplasm of some tumor cells under closer
examination (Fig. 2).
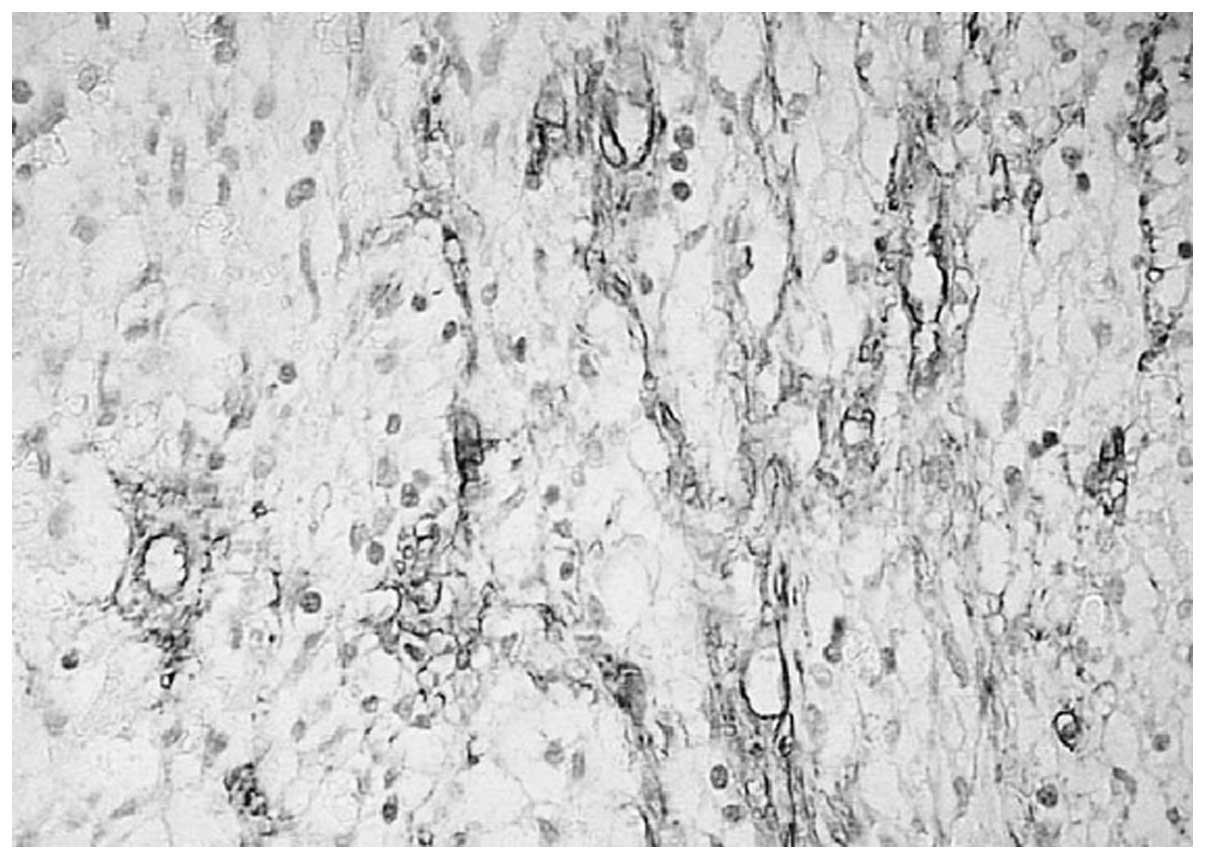
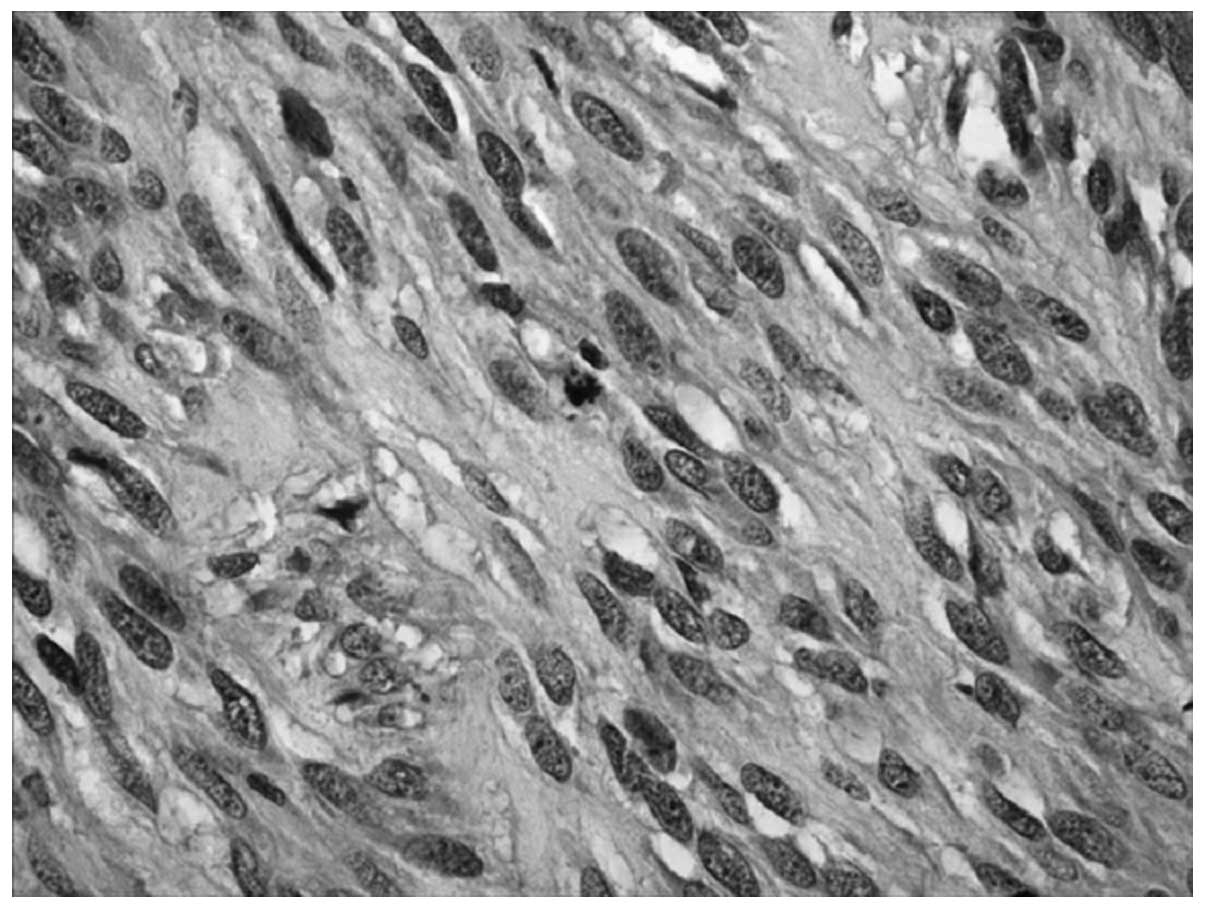
Immunohistochemistry revealed the expression of nestin (Fig. 3).
Case 2: Kaposiform hemangioendothelioma
(KHE)
The patient was a 5-month-old child, who was
hospitalized due to swelling of the right subaxillary for 2 days.
Physical examination showed a 3x4 cm right, subaxillary lump with a
light red surface. B-mode ultrasonic imaging showed an 8×6×2 cm
solid, slightly enhanced echo mass of the armpit with a clear
border. The blood routine results were: WBC, 12.8×109/l;
RBC, 2.15×1012/l; PLT, 42×109/l; and
fibrinogen was decreased by 23 mg/ml. The mass in the deep
subcutaneous tissue was ill-defined and locally associated with
distended lymphatic vessels containing yellow lymph. Pathological
examination showed a section of skin tissue (4.3×3.9×2.1 cm), which
was pale brown, nodular and of medium texture. It partially
consisted of small pieces of sponge-like lumen containing yellow
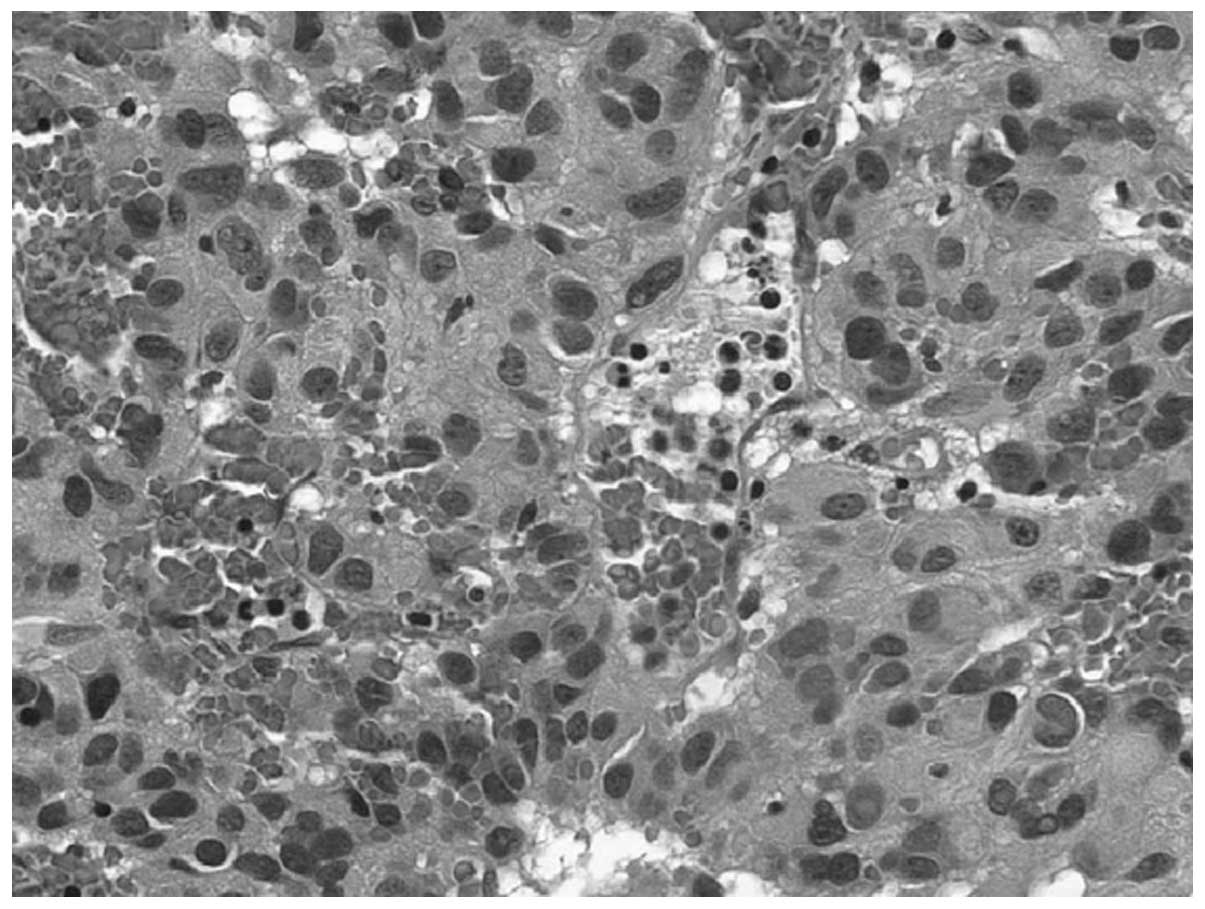
fluid. Microscopic examination showed that the tumor tissue
consisted of fusiform endothelial cells and some round nuclei of
epithelioid form (Fig. 4). The
tumors were nodular in appearance, and contained a small amount of
glomerular-like structures. Endothelial cells of local, crack-like
lumen proliferating in a papillary form and a small transparent
thrombosis were observed. At the edge, there was a crack-like
lymphangioma structure; tumor tissue showed multiple nodular forms
infiltrating to intramuscular. Immunohistochemistry revealed that
spindle tumor cells and vascular endothelial cells all expressed
CD34 and CD31 (Fig. 5).
Case 3: Pleomorphic hyalinizing
angiectatic tumor
The patient was a 30-year-old woman, who was
hospitalized due to a right inguinal lump which had persisted for
more than six months. Physical examination revealed an egg-sized
mass in the right inguinal region, with a clear border, good
activity and no tenderness. The preliminary clinical diagnosis was
skin fibroma. Surgical resection showed the mass was located
subcutaneously with no adhesion to the surrounding tissue.
Pathological examination showed that the tumor was an oval, 6×5×5
cm large, thin and complete capsule, with a solid section, white
color and a slightly hard texture. A small part of the cavity
contained fissures of different sizes, which contained a small
amount of red liquid. Microscopic examination showed that the tumor
was mainly composed of thin-walled blood vessels and fibrous tissue
lumen of different sizes, with hyalinosis of the wall and around
collagen fibers (Fig. 6). Spindle
endothelial cells were lined with expanding blood vessels. There
were a small amount of RBCs in the cavity and a thrombus in part of
the lumen. In between the vessels, there were tumor cells of
multiple forms, including hyperchromatic nuclei and clear
nucleolus. Some of the tumor cells showed marked atypia and giant
tumor cells showing no mitotic activity were observed.
Immunohistochemistry results were as follows: CD34 (+), S-100 (-),
CK (-), SMA (-) (Fig. 7). The
pathological diagnosis was soft tissue pleomorphic hyaline
expansion of tumor blood vessels.
Discussion
Hepatic angiosarcoma (HAS) is an uncommon neoplasm
of endothelial cells of hepatic sinusoids. However, it is the most
common malignant mesenchymal tumor of the liver. It is more common
in males in late adulthood. The etiology of most cases of primary
HAS is unknown; some have been associated with exposure to vinyl
chloride, thorium dioxide, arsenic, anabolic steroids, and diseases
such as hemachromatosis and von Recklinghausen neurofibromatosis
(1). The diagnosis of HAS is often
performed too late due to lack of specific symptoms, laboratory
tests and radiological findings. Therefore, it is necessary to make
more detailed observations of the morphology and immunohistohemical
staining on the basis of the clinical manifestations.
To investigate the value of assisted diagnosis of
the malignant vascular tumors, we conducted a nestin test on
patient 1. Studies show the expression of nestin in vascular
endothelial cells (2,3). The designation ‘nestin’ refers to a
member of the family of intermediate filaments and comes from the
fact that this protein is expressed mainly in neuroepithelial stem
cells and is of vascular origin (4–6). The
result showed positive expression of nestin in the cytoplasm and we
found that the expression of nestin in malignant vascular tumors
was related to the shape change of the tumor. When the tumor cells
were spindle-shaped, strong expression as well as deep stained
cytoplasm was found. When the tumor cells were epithelioid, there
was weak or even no expression of nestin. However, nestin is not
expressed in mature elements and terminal cell differentiation is
associated with loss of immunoreactivity to this protein. It is
possible that spindle cells are in a dedifferentiated, immature
state and are therefore nestin-positive. Thus, the presence of
nestin may indicate dedifferentiation in these tumors as well
(7). Therefore, we should continue
to observe and analyze the assisted diagnostic value of nestin in
malignant vascular tumors in the future. In addition, HAS is often
confused with other spindle tumor cells. However, these tumor cells
were arranged in bundles or interwoven shapes and
immunohistohemical endothelial cell markers were negative.
Kaposiform hemangioendothelioma (KHE) is a rare and
aggressive vascular tumor, which mainly occurs in infants and
children as first proposed in a study by Zukerberg et
al(8). It usually presents
clinically as angioma with thrombocytopenia and low fiber albumin,
namely thrombocytopenic purpura syndrome (Kasabach-Merritt
syndrome, KMS). This feature differentiates this entity from a
juvenile hemangioma that forms the closest differential diagnosis.
It is usually identified in infancy and in the first decade of life
at sites such as extremities and the retroperitoneum, and is
uncommonly found in the head and neck region (9–11). At
times, KHE occurs without KMS (12). It has rarely been documented in the
subaxillary.
Case 2 was identified to be clinically manifested as
KMS and the pathological diagnosis was KHE. The child was treated
with partial resection, corticosteroid and pingyangmycin local
injection for residual tumor, VCR and CTX chemotherapy. As a
result, the tumor shrank and continued to do so for six months. The
child survived with the tumor and PLT rose to 100×109/l.
Enjolras et al(13) believed
that the infant hemangioma associated with KMS was not ordinary
hemangioma; both histological results showed KHE or tufted
hemangioma. Sarkar et al(14) reported 21 cases of KMS and all cases
were diagnosed as KHE, indicating that KHE was the main
pathological form of KMS hemangioma. On the other hand, the
pathological diagnosis of KHE, whether it be thrombocytopenia or
low fibrinogen disease, should be considered in the clinical
analysis. Overall incidence of KHE is not high and there is no
consensus on its treatment. Currently, complete resection of the
tumor is considered as the best treatment method and the prognosis
is improved by increased resection of superficial tumors. Also,
after complete resection, no tumor survived within 2 or 3 years. In
addition, the disease is easily confused with Kaposi’s sarcoma;
both diseases contain spindle cells and slit like blood vessels,
while the latter was infiltrated by inflammatory cells without
glomerular-like structures and commonly occurred in adults.
Pleomorphic hyalinizing angiectatic tumor (PHAT), a
rare neoplasm of unknown origin, an independent entity of soft
tissues, is a type of non-metastasizing tumor (15). Some immunohistochemical and
ultrastructural studies indicate that the neoplastic cells may be a
type of undifferentiated, primitive mesenchymal cell, possibly
related to stromal fibroblasts (16). This disease was firstly described by
Smith et al disease in 1996 (17) and 14 cases were reported. The
disease was mainly found in the trunk, limbs and subcutaneous
tissue of lower limbs in adult women. It generally appeared as a
mass of slow growth (18). The
histological features were thin-walled and expanded blood vessels
that were distributed in clusters; the walls and matrix
transparently degenerated and residual pleomorphic tumor was
visible among blood vessels with the split as low as <1/50 HPF.
Immunohistochemistry showed expression of CD34 and S-100. This was
a low-grade malignant tumor. Out of 14 cases, only 4 cases had
recurrence, and no metastasis was found. The case had the same
features in clinical, histological and immunohistohemical analysis
as mentioned above, which was consistent with this diagnosis. The
most difficult, differential diagnoses of PHAT are neurilemoma and
malignant fibrous histiocytoma (19,20).
Neurilemomas may also occur with angiectatic and hyalinized
vasculature, cells with prominent pseudonuclear inclusions and
pleomorphic cells as observed in ancient or degenerated
neurilemomas; however, they are encapsulated, have characteristic
Antoni A and B zones and are nearly all strongly reactive to S-100
protein. Low mitotic rates, clusters of hyalinized thin-walled
ectatic vessels, and immunoreactivity for CD34 help differentiate
PHAT from malignant fibrous histiocytoma. However, since the study
was limited, the biological behavior remains to be studied.
Meanwhile, due to the complexity and diversity of forms of the
disease’s pathology, a variety of benign and malignant tumors need
to be identified. However, these need to be identified according to
the result that the disease has clear cellulose thin-walled blood
vessels and vascular calm between the more spindle-shaped
interstitial cells of the typical characteristics of
immunohistochemical expression of Vim and VEGF, but not the
expression of SMA, Des, CK and CD31, for example. Subsequently, we
could exclude the other tumors.
Vascular tumors, although occurring relatively
rarely in clinical practice, are a multi-disciplinary disease,
which is easily ignored by clinicians. The clinical manifestations
and the gross specimens of this tumor are often hard, tough and
resemble fish flesh. Mainly by pathological diagnosis of the
vascular tumor, body surface or body cavity for the rapid growth of
tumor recurrence if the transfer should be taken into account the
possibility of such tumors. Signs of suspected malignancy could
puncture cytology during surgery or before the rapid intraoperative
pathological examination. Once diagnosed, resection should be
appropriately extended. For vascular tumors, surgery is the primary
treatment method and radiation therapy has a significant effect in
preventing recurrence. If a comprehensive clinical and pathological
analysis is carried out and an accurate diagnosis is made, early
detection and treatment may reduce the pain of the patients.
Therefore, for a rare vascular tumor, the clinical and pathological
data must be comprehensively analyzed.
References
|
1.
|
Lipshutz G, Brennan T and Warren R:
Thorotrast-induced liver neoplasia: a collective review. J Am Coll
Surg. 195:713–718. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mokry J and Nemecek S: Angiogenesis of
extra-and intraembryonic blood vessels is associated with
expression of nestin in endothelial cells. Folia Biol (Praha).
44:155–161. 1998.PubMed/NCBI
|
|
3.
|
Mokry J and Nemecek S: Cerebral
angiogenesis shows nestin expression in endothelial cells. Gen
Physiol Biophys. 18:25–29. 1999.PubMed/NCBI
|
|
4.
|
Nagle RB: Intermediate filaments: a review
of the basic biology. Am J Surg Pathol. 12:4–16. 1988.PubMed/NCBI
|
|
5.
|
Lendahl U, Zimmerman LB and McKay RD: CNS
stem cells express a new class of intermediate filament protein.
Cell. 60:585–595. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Dahlstrand J, Zimmerman LB, McKay RD and
Lendahl U: Characterization of the human nestin gene reveals a
close evolutionary relationship to neurofilaments. J Cell Sci.
103:589–597. 1992.PubMed/NCBI
|
|
7.
|
Florenes VA, Holm R, Myklebost O, Lendahl
U and Fodstad O: Expression of the neuroectodermal intermediate
filament nestin in human melanomas. Cancer Res. 54:354–356.
1994.PubMed/NCBI
|
|
8.
|
Zukerberg LR, Nickoloff BJ and Weiss SW:
Kaposiform hemangio-endothelioma of infancy and childhood: an
aggressive neoplasm associated with Kasabach-Merritt syndrome and
lymphangiomatosis. Am J Surg Pathol. 17:321–328. 1993.PubMed/NCBI
|
|
9.
|
Lalaji TA, Haller JO and Burgess RJ: A
case of head and neck kaposiform hemangioendothelioma simulating a
malignancy on imaging. Pediatr Radiol. 31:876–878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lyons LL, North PE, Mac-Moune Lai F,
Stoler MH, Folpe AL and Weiss SW: Kaposiform hemangio endothelioma:
a study of 33 cases emphasizing its pathologic, immunophenotypic
and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol.
28:559–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Birchler MT, Schmid S, Holzmann D,
Stallmach T and Gysin C: Kaposiform hemangioendothelioma arising in
the ethmoid sinus of an 8 year old girl with severe epistaxis. Head
Neck. 28:761–764. 2006.PubMed/NCBI
|
|
12.
|
Gruman A, Liang MG, Mulliken JB, et al:
Kaposiform hemangioendothelioma without Kasabach-Merrittt
phenomenon. J Am Dermatol. 52:616–622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Enjolras O, Wassef M, Mazoyer E, et al:
Infants with Kasabach-Merritt syndrome do not have ‘true’
hemangiomas. Pediat. 130:631–640. 1997.
|
|
14.
|
Sarkar M, Mulliken JB, Kozakewich HP,
Robertson RL and Burrows PE: Thrombocytopenic coagulopathy
(Kasabach-Merritt phenomenon) is associated with Kaposiform
hemangio endothelioma and not with common infantile hemangioma.
Plast Reconstr Surg. 100:1377–1386. 1997. View Article : Google Scholar
|
|
15.
|
Christopher DMF, Krishnan UK and Fredrik
M: Pathology and genetics of tumours of soft tissue and bone. World
Health Organization Classification of Tumors. International Agency
for Research on Cancer (IARC) Press; Lyon: pp. 1912002
|
|
16.
|
Folpe AL and Weiss SW: Pleomorphic
hyalinizing angiectatic tumor: analysis of 41 cases supporting
evolution from a distinctive precursor lesion. Am J Surg Pathol.
28:1417–1425. 2004. View Article : Google Scholar
|
|
17.
|
Smith ME, Fisher C and Weiss SW:
Pleomorphic hyalinizing angiectatic tumor of soft parts. A
low-grade neoplasm resembling neurilemoma. Am J Sung Pathol.
20:21–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Peng HC, Huang MT, Chen DJ, Leung TK and
Chu JS: Pleomorphic hyalinizing angiectatic tumor of soft parts. J
Formos Med Assoc. 109:616–620. 2010. View Article : Google Scholar
|
|
19.
|
Fletcher CDM: Soft tissue tumors.
Diagnostic Histopathology of Tumors. Fletcher CDM: 2nd edition.
Churchill Livingstone; London, England: pp. 1473–1540. 2000
|
|
20.
|
Weiss SW and Goldblum JR: Benign soft
tissue tumors and pseudotumors of miscellaneous types. Enzinger and
Weiss’s Soft Tissue Tumors. Weiss SW and Goldblum JR: 4th edition.
Mosby-Year Book, Inc; St. Louis, MO, USA: pp. 1419–1481. 2001
|