Introduction
Colorectal cancer (CRC) is the third most common
cancer worldwide with an estimated one million new cases and half a
million mortalities every year (1).
A significant number of patients with colorectal carcinoma who
undergo apparently curative surgery develop local recurrence or
distant metastasis, leading to shorter survival (2). For this reason, the identification of
factors that accurately predict prognosis in CRC is urgently
required. A deeper insight into the carcinogenesis and the factors
correlated with the aggressiveness of CRC may be necessary for this
requirement.
Several previous studies have revealed that
epithelial-mesenchymal transition (EMT) plays a crucial role in the
progression and aggressiveness of CRC (3,4).
Several genes, called EMT-inducing or master EMT genes, are
essential to the EMT process (5,6). Zinc
finger E-box binding homeobox 1 (ZEB1) encodes a transcription
factor that plays a key role in cancer progression by regulating
EMT in breast, prostate, ovarian and colorectal cancer (7–11).
A previous study showed that ZEB1 was overexpressed
in various CRC cell lines and crucial for the metastasis of CRC
cells (12). Recently, ZEB1 was
also found to be a crucial EMT inducer in human CRC and suppresses
the expression of basement membrane components (13). Notably, EMT-linked loss of basement
membranes indicates metastasis and poor survival in CRC (7,14).
Although a growing number of studies have
demonstrated the function of ZEB1 in experimental systems, no
reports have shown any clinical significance associated with ZEB1
expression in CRC. The aim of this study was therefore to clarify
the clinical significance of ZEB1 expression in CRC.
Materials and methods
Patients and tissue samples
After obtaining adequate informed consent, surgical
specimens of cancer tissue and adjacent normal mucosa were obtained
from 92 patients with primary CRC who underwent surgery without
preoperative treatment at the First Department of General Surgery,
the Affiliated Hospital of North Sichuan Medical College, China,
between January 2005 and May 2008. All tissue samples were
immediately frozen in liquid nitrogen and stored at −80°C until the
extraction of RNA. Clinicopathological information, including age,
gender, tumor size, histological type, depth of invasion, lymph
node metastasis, location, lymphatic invasion and liver metastasis,
was available for all patients. The study was approved by the
medical ethics committee of North Sichuan Medical College.
Quantitative real-time reverse
transcription-PCR
Total RNA was extracted from tumor tissue and
adjacent normal mucosa by homogenizing tissue in TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s
instructions. cDNA was synthesized using an iScript cDNA Synthesis
kit (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative
real-time PCR was performed with an iQ SYBR-Green Supermix
(Bio-Rad). All reactions were run in triplicate on the iCycler IQ
multi-color Detection System (Bio-Rad). The amplification profile
was denatured at 95°C for 10 min, followed by 50 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 1 min. The primers for ZEB1 (226 bp) were
5′-AGCAGTGAAAGAGAAGGGAATGC-3′ (sense) and
5′-GGTCCTCTTCAGGTGCCTCAG-3′ (antisense). The primers for β-actin
(171 bp) were 5′-AGT TGCGTTACACCCTTTCTTGAC-3′ (sense) and 5′-GCTCGC
TCCAACCGACTGC-3′ (antisense). The comparative cycle threshold (CT)
method was applied to quantify the expression levels of miRNAs. The
relative amount of ZEB1 was calculated using the equation
2−ΔCT where ΔCT = (CT
ZEB1 − CT β-actin).
Statistical analysis
The gene expression levels in CRC were compared with
those in normal adjacent mucosa with the use of the Wilcoxon test.
The correlations between the gene expression levels and potential
explanatory variables, including age, gender, tumor size,
histological type, depth of invasion, lymph node metastasis,
location, lymphatic invasion and liver metastasis, were evaluated
with the Chi-square test. The postoperative survival rate was
analyzed with the Kaplan-Meier method, and differences in survival
rates were assessed with the log-rank test. A Cox proportional
hazards model was used for multivariate analysis. All the
statistical analyses were performed using SPSS 16.0 software (SPSS,
Chicago, IL, USA). Two-sided P-values were calculated, and
P<0.05 was considered to indicate a statistically significant
result.
Results
Comparison of ZEB1 expression between CRC
tissue and normal adjacent mucosa
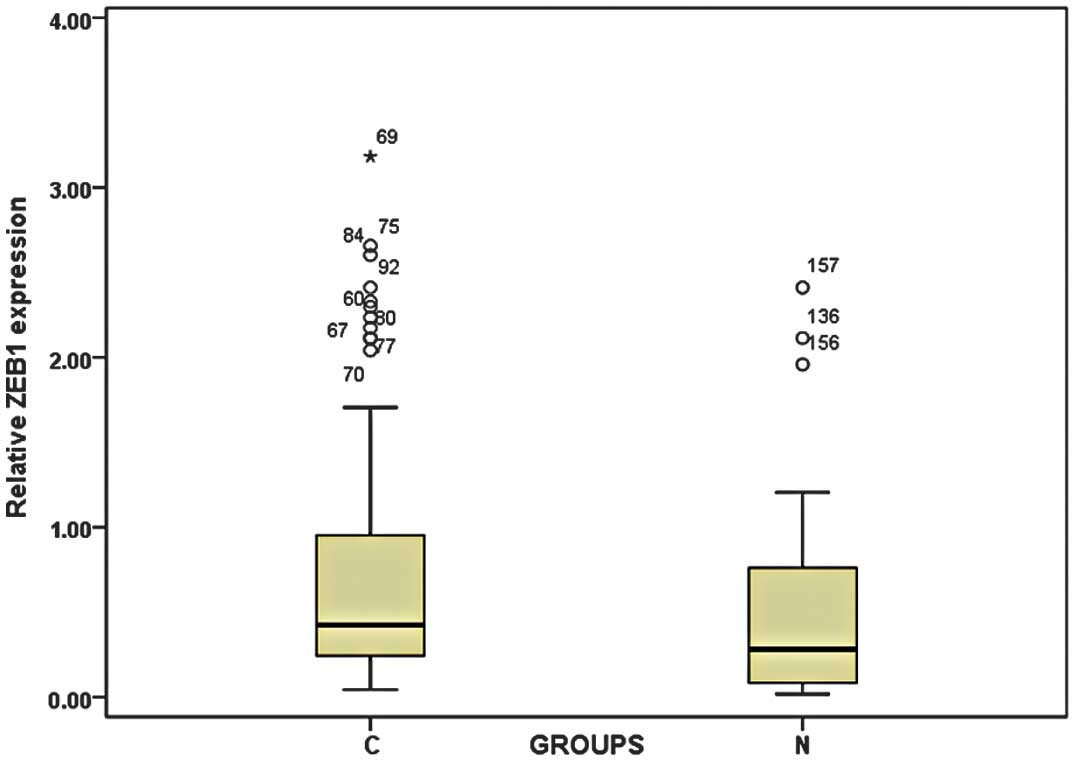
By real-time quantitative RT-PCR, we found that ZEB1
expression levels were significantly higher in cancer tissues from
patients with CRC (0.843±0.693) than in the normal adjacent mucosa
(0.586±0.488; P=0.003; Fig. 1).
Correlation between ZEB1 gene expression
and clinicopatho-logical features of CRC
The expression levels of ZEB1 were categorized as
low or high in relation to the median value. The ZEB1 expression
level was not correlated with age, gender, tumor size, histological
type, depth of invasion, tumor location, lymph node metastasis or
lymphatic invasion. However, the ZEB1 expression level was
correlated with liver metastasis (P=0.043; Table I).
 | Table I.Correlation between ZEB1 expression
and clinico-pathological parameters in 92 colorectal cancer
patients. |
Table I.
Correlation between ZEB1 expression
and clinico-pathological parameters in 92 colorectal cancer
patients.
| Variable | ZEB1 expression
| P-value |
|---|
| Low (n=46) | High (n=46) |
|---|
| Age (years) | 62.8±14.6 | 61.1±11.7 | 0.519 |
| Gender | | | 0.209 |
| Male | 28 | 22 | |
| Female | 18 | 24 | |
| Tumor size (cm) | | | 0.288 |
| ≤5 | 30 | 25 | |
| >5 | 16 | 21 | |
| Histological
type | | | 0.529 |
| Well, moderate | 27 | 24 | |
| Poor, mucinous | 19 | 22 | |
| Depth of
invasion | | | 0.674 |
| T1,T2 | 21 | 19 | |
| T3,T4 | 25 | 27 | |
| Location | | | 0.669 |
| Colon | 19 | 17 | |
| Rectum | 27 | 29 | |
| Lymph node
metastasis | | | 0.058 |
| Absent | 24 | 15 | |
| Present | 22 | 31 | |
| Lymph node
invasion | | | 0.084 |
| Absent | 33 | 25 | |
| Present | 13 | 21 | |
| Liver metastasis | | |
0.043a |
| Absent | 40 | 32 | |
| Present | 6 | 14 | |
Correlation between ZEB1 gene expression
levels and survival
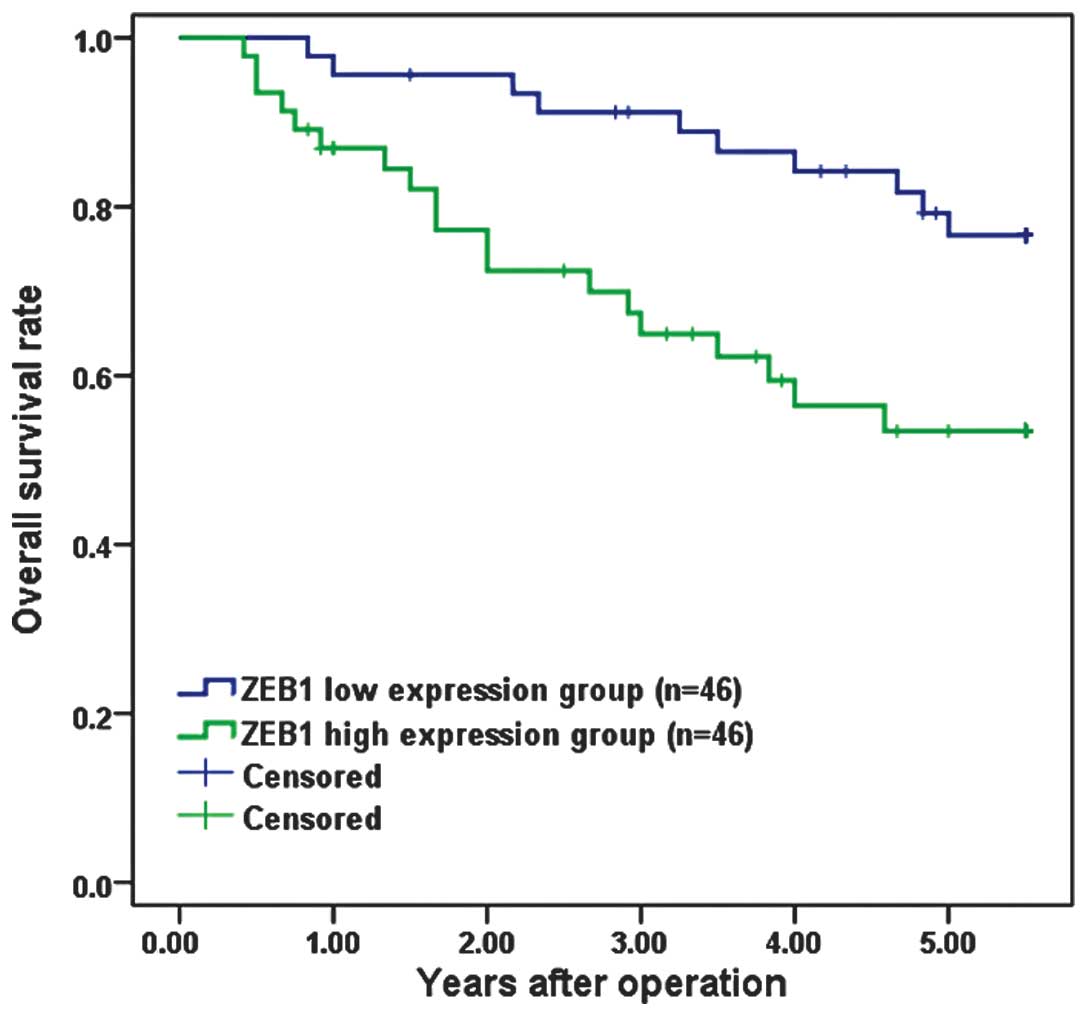
Overall survival curves were plotted according to
ZEB1 mRNA expression level by the Kaplan-Meier method. In the study
group as a whole (92 patients), the overall survival rate was
significantly lower in the patients with high ZEB1 mRNA expression
than in those with low expression (P=0.010; Fig. 2).
Prognostic factors of CRC
Univariate analysis with Cox proportional hazards
model identified seven prognostic factors: histological type, tumor
size, depth of invasion, lymph node invasion, lymphatic invasion,
liver metastasis and ZEB1 expression. The other clinicopathological
features, such as age, gender and location, were not statistically
significant prognostic factors (Table
II). A multivariate analysis of the prognosis factors with a
Cox proportional hazards model confirmed that high ZEB1 expression
was a significant independent predictor of poor survival in CRC
(Table III).
 | Table II.Univariate analysis of
clinicopathological factors for overall survival. |
Table II.
Univariate analysis of
clinicopathological factors for overall survival.
| Variable | n | Hazard ratio | 95% CI | P-value |
|---|
| Age (years) | | | | |
| ≤65 | 40 | 1 | | |
| >65 | 52 | 1.455 | 0.701–3.019 | 0.314 |
| Gender | | | | |
| Male | 50 | 1 | | |
| Female | 42 | 1.274 | 0.608–2.669 | 0.521 |
| Tumor size
(cm) | | | | |
| ≤5 | 55 | 1 | | |
| >5 | 37 | 3.837 | 1.771–8.312 | 0.001a |
| Histological
type | | | | |
| Well,
moderate | 51 | 1 | | |
| Poor,
mucinous | 41 | 2.401 | 1.141–5.052 | 0.021a |
| Depth of
invasion | | | | |
| T1, T2 | 40 | 1 | | |
| T3, T4 | 52 | 3.247 | 1.383–7.625 | 0.007a |
| Location | | | | |
| Colon | 36 | 1 | | |
| Rectum | 56 | 1.674 | 0.807–3.473 | 0.167 |
| Lymph node
metastasis | | | | |
| Absent | 39 | 1 | | |
| Present | 53 | 8.956 | 2.704–29.663 | <0.001a |
| Lymph node
invasion | | | | |
| Absent | 58 | 1 | | |
| Present | 34 | 3.820 | 1.797–8.121 | <0.001a |
| Liver
metastasis | | | | |
| Absent | 72 | 1 | | |
| Present | 20 | 15.427 | 6.342–37.528 | <0.001 |
| ZEB1 | | | | |
| Low | 46 | 1 | | |
| High | 46 | 2.646 | 1.226–5.710 | 0.013a |
 | Table III.Multivariate analysis of
clinicopathological factors for overall survival. |
Table III.
Multivariate analysis of
clinicopathological factors for overall survival.
| Variable | Hazard ratio | 95% CI | P-value |
|---|
| Tumor size (>5
cm/≤5 cm) | 1.779 | 0.736–5.044 | 0.182 |
| Histological type
(poor, muc/well, mod) | 1.852 | 0.841–4.079 | 0.126 |
| Depth of invasion
(T3, T4/T1, T2) | 1.548 | 0.620–3.864 | 0.349 |
| Lymph node
metastasis (present/absent) | 6.165 | 1.733–21.926 | 0.005a |
| Lymphatic invasion
(present/absent) | 2.001 | 0.788–5.080 | 0.144 |
| Liver metastasis
(present/absent) | 4.816 | 1.794–12.929 | 0.002a |
| ZEB1
(high/low) | 2.237 | 1.008–4.968 | 0.048a |
Discussion
Increasing evidence indicates that aberrant
activation of EMT plays a key role in tumor cell invasion and
metastasis. EMT allows the detachment of cells from each other and
increases cell mobility, both of which are necessary for tumor cell
dissemination. A hallmark for EMT is the loss of the cell adhesion
molecule E-cadherin. Several transcription factors have been
described as key inducers of EMT, including members of the Snail
superfamily (Snail1 and Snail2), the basic helix-loop-helix (bHLH)
family (TCF3 and TWIST) and the two zinc-finger E-box-binding
homeobox (ZEB) factors (ZEB1 and ZEB2) (15,16).
Previous data indicate that ZEB1 has emerged as a
key player in cancer progression (8,12,17,18).
Aberrant expression of ZEB1 in endometrial cancers, gastric cancer
and hepatocellular carcinoma has been associated with aggressive
disease, poor differentiation, development of metastases and poor
clinical prognosis (19–21). However, ZEB1 expression in CRC
tissues and its correlation with the clinical pathology of CRC are
rarely reported.
The overexpression of ZEB1 has been observed in
prostate cancer, gastric cancer, osteosarcoma and hepatocellular
carcinoma, suggesting an important role in tumorigenesis (11,20–22). A
previous study also showed that ZEB1 was overexpressed in various
CRC cell lines (12). In this
study, we confirmed that ZEB1 gene expression was higher in CRC
tissue than in adjacent normal mucosa, consistent with the results
of previous studies.
A previous study demonstrated that high ZEB1
expression in hepatocellular carcinoma was correlated with advanced
TNM stage, tumor size, intrahepatic metastasis and vascular
invasion (21). Okugawa et
al reported that increased ZEB1 expression in gastric cancer
was not clearly associated with histological type, tumor size,
lymph node metastasis and hepatic metastasis, and elevated ZEB1
expression was shown to be significantly correlated with peritoneal
dissemination (20). However, the
correlation between ZEB1 expression level and clinicopathological
behavior of CRC is unclear. In the present study, ZEB1 expression
was shown to be associated only with liver metastases, suggesting
that ZEB1 is involved in the carcinogenesis, development,
progression and metastasis of CRC. The inconsistent results may be
due to different roles of ZEB1 in different tumors.
More importantly, we proved that ZEB1 expression was
significantly associated with overall survival of patients with
CRC. In support of this, the Kaplan-Meier analysis of overall
survival showed that patients whose tumors had higher ZEB1
expression tend to have a significantly worse overall survival,
indicating that a high ZEB1 level is a marker of poor prognosis for
patients with CRC. Moreover, Cox proportional hazards model showed
that ZEB1 was a marker of poor overall survival independent of the
known clinical prognostic indicators such as lymph node and liver
metastases. Therefore, it could constitute a molecular prognostic
marker for these patients, identifying those patients who are more
likely to have a higher risk of mortality; thus, good candidates to
receive more aggressive treatment.
The precise molecular mechanisms behind the altered
expression of ZEB1 in CRC are unclear. To the best of our
knowledge, this is the first study to describe the significance of
ZEB1 to liver metastasis and prognosis of CRC patients. ZEB1, which
is also known as TCF8 or ΔEF1, is a member of the zinc finger
family (22). It had been found
that ZEB1 inhibits the expression of multiple genes in a variety of
cell lines. In hematopoietic cells, ZEB1 negatively regulated the
expression levelss of IL-2, CD4, the heavy chain of immunoglobulin
μ and GATA-3 (23–25). In mesenchymal cells, ZEB1 inhibited
the expression of the p73 gene (26). Recent studies found that ZEB1 also
inhibited the expression of epithelial cadherin (E-cadherin), and
thus plays an important role in the carcinogenesis, progression,
invasion and metastasis of a variety of tumors (27,28).
Putzke et al reported that the overexpression of ZEB-1 in
prostate cancer cells inhibited the expression of E-cadherin
protein, thereby promoting the invasion and metastasis of prostate
cancer cells (27). The
overexpression of ZEB1 was also found to promote the invasion and
metastasis of lung cancer cells (29). In addition, ZEB1 promotes the
epithelialmesenchymal transformation of liver cancer cells
(30). Taken together, these
studies may explain why ZEB1 overexpression is associated with poor
prognosis of CRC patients. Additional studies to investigate the
molecular mechanisms of both the cause and effects of altered
expression of ZEB1 in the development and/or progression of CRC are
essential.
In conclusion, we have shown that ZEB1 expression
was increased in clinical CRC specimens and associated with liver
metastasis and poor prognosis. This study further demonstrated that
ZEB1 was an independent prognostic factor of patients with CRC.
These findings suggest that ZEB1 may be a potential diagnostic and
therapeutic target in patients with CRC.
Acknowledgements
This study was in part supported by
the Scientific Research Program of Sichuan Provincial Health
Department of China (110319).
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Kobayashi H, Mochizuki H, Sugihara K, et
al: Characteristics of recurrence and surveillance tools after
curative resection for colorectal cancer: a multicenter study.
Surgery. 141:67–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Brabletz T, Hlubek F, Spaderna S, et al:
Invasion and metastasis in colorectal cancer:
epithelial-mesenchymal transition, mesenchymal-epithelial
transition, stem cells and beta-catenin. Cells Tissues Organs.
179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: A cancer researcher’s conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009.PubMed/NCBI
|
|
7.
|
Spaderna S, Schmalhofer O, Hlubek F, et
al: A transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Eger A, Aigner K, Sonderegger S, et al:
DeltaEF1 is a transcriptional repressor of E-cadherin and regulates
epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ohira T, Gemmill RM, Ferguson K, et al:
WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci U
S A. 100:10429–10434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chua HL, Bhat-Nakshatri P, Clare SE, et
al: NF-kappaB represses E-cadherin expression and enhances
epithelial to mesenchymal transit ion of mammary epithelial cells:
Potential involvement of ZEB-1 and ZEB-2. Oncogene. 26:711–724.
2007. View Article : Google Scholar
|
|
11.
|
Graham TR, Zhau HE, Odero-Marah VA, et al:
Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives
epithelial-to-mesenchymal transition in human prostate cancer
cells. Cancer Res. 68:2479–2488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Spaderna S, Schmalhofer O, Wahlbuhl M, et
al: The transcriptional repressor ZEB1 promotes metastasis and loss
of cell polarity in cancer. Cancer Res. 68:537–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sánchez-Tilló E, Lázaro A, Torrent R, et
al: ZEB1 represses E-cadherin and induces an EMT by recruiting the
SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 29:3490–3500.
2010.PubMed/NCBI
|
|
14.
|
Xiong H, Hong J, Du W, et al: Roles of
STAT3 and ZEB1 proteins in E-cadherin down-regulation and human
colorectal cancer epithelial-mesenchymal transition. J Biol Chem.
287:5819–5832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Waldmann J, Feldmann G, Slater EP, et al:
Expression of the zinc-finger transcription factor Snail in
adrenocortical carcinoma is associated with decreased survival. Br
J Cancer. 99:1900–1907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Drake JM, Strohbehn G, Bair TB, et al:
ZEB1 enhances transendothelial migration and represses the
epithelial phenotype of prostate cancer cells. Mol Biol Cell.
20:2207–2217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Takeyama Y, Sato M, Horio M, et al:
Knockdown of ZEB1, a master epithelial-to-mesenchymal transition
(EMT) gene, suppresses anchorage-independent cell growth of lung
cancer cells. Cancer Lett. 296:216–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Singh M, Spoelstra NS, Jean A, et al: ZEB1
expression in type I vs type II endometrial cancers: a marker of
aggressive disease. Mod Pathol. 21:912–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Okugawa Y, Toiyama Y, Tanaka K, et al:
Clinical significance of zinc finger E-box binding homeobox 1
(ZEB1) in human gastric cancer. J Surg Oncol. 106:280–285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhou YM, Cao L, Li B, et al:
Clinicopathological significance of ZEB1 protein in patients with
hepatocellular carcinoma. Ann Surg Oncol. 19:1700–1706. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shen A, Zhang Y, Yang H, et al:
Overexpression of ZEB1 relates to metastasis and invasion in
osteosarcoma. J Surg Oncol. 105:830–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Stridh P, Thessen Hedreul M, Beyeen AD, et
al: Fine-mapping resolves Eae23 into two QTLs and implicates ZEB1
as a candidate gene regulating experimental neuroinflammation in
rat. PLoS One. 5:e127162010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hidaka T, Nakahata S, Hatakeyama K, et al:
Down-regulation of TCF8 is involved in the leukemogenesis of adult
T-cell leukemia/lymphoma. Blood. 112:383–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Genetta T, Ruezinsky D and Kadesch T:
Displacement of an E-box-binding repressor by basic
helix-loop-helix proteins: Implications for B-cell specificity of
the immunoglobulin heavy-chain enhancer. Mol Cell Biol.
14:6153–6163. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Fontemaggi G, Gurtner A, Strano S, et al:
The transcriptional repressor ZEB regulates p73 expression at the
crossroad between proliferation and differentiation. Mol Cell Biol.
21:8461–8470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Putzke AP, Ventura AP, Bailey AM, et al:
Metastatic progression of prostate cancer and e-cadherin regulation
by zeb1 and SRC family kinases. Am J Pathol. 179:400–410. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Argast GM, Krueger JS, Thomson S, et al:
Inducible expression of TGFbeta, Snail and Zeb1 recapitulates EMT
in vitro and in vivo in a NSCLC model. Clin Exp Metastasis.
28:593–614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Roy BC, Kohno T, Iwakawa R, et al:
Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of
human lung cancer cells. Lung Cancer. 70:136–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kim T, Veronese A, Pichiorri F, et al: p53
regulates epithelialmesenchymal transition through microRNAs
targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. 2011. View Article : Google Scholar : PubMed/NCBI
|