Introduction
Anaplastic large cell lymphoma (ALCL) is a
peripheral T-cell-derived malignancy, representing 2–3% of all
lymphoid neoplasms, according to estimates by the World Health
Organization (WHO) (1). It was
first recognized by Stein et al(2) in 1985, who reported the consistent
expression of the Ki-1 antigen (later designated as CD30) in tumors
with frequent cohesive proliferation of large pleomorphic cells.
Two different types are recognized as systemic forms, the
anaplastic lymphoma kinase (ALK)-positive and ALK-negative ALCL.
Regarding genetic and clinical features, the former is
characterized by the deregulated expression of chimeric proteins
expressing the intracytoplasmic domain of the ALK gene, with a
strong and uniform expression of CD30 and ALK proteins (3). Since ALK(−) ALCL lacks distinctive
immunophenotypic features and appears to have a prognosis similar
to peripheral T-cell lymphoma not otherwise specified (PTCL-NOS),
it should be considered a subtype of PTCL-NOS (4–6).
Previously, it was suggested that ALK(−) ALCL should continue to be
separated from ALK(+) ALCL (6). In
the WHO classification, ALK(−) ALCL was regarded only as a
provisional entity (1).
Evaluation of suspected tissue by histopathologists
is crucial for the diagnosis and accurate classification of ALCL.
Flow cytometric immunophenotyping (FCI) is widely performed on bone
marrow aspirates and peripheral blood specimens, and is commonly
used to diagnose and classify leukemias, myeloproliferative
disorders and autoimmune lymphoproliferative disorders as well as
to assess residual disease in leukemias. Combined use of cytology
and FCI analysis of fine needle aspiration (FNA) samples has been
found to contribute to an improved classification of non-Hodgkin
lymphomas (NHL) into WHO categories (7,8).
However, the majority of previous studies on diagnosing lymphoma
with FCI have focused primarily on common T- or B-cell NHL. Since
ALCL is a distinct subtype of lymphoma and ALK expression is not
available for a considerable subset of the cases analyzed, only a
limited number of reports studying the role of FCI in tissue
diagnosis of ALCL are available (9,10). In
the present study, we reviewed the FCI and morphology results of
lymph node biopsy specimens from 15 patients with ALK(+) ALCL to
investigate its pathological features in children and to evaluate
the role of flow cytometry in diagnosing ALK(+) ALCL.
Materials and methods
Subject selection
Lymph node tissues obtained from biopsy specimens of
patients with a suspected diagnosis of lymphoma were studied. The
specimens were obtained from the main surgical centers in the
Children’s Hospital of Zhejiang University School of Medicine,
China, from January 2004 to March 2012. Each sample was divided in
two; one fresh sample was sent to the Hematology-Oncology
Laboratory for FCI analysis and the other to the Department of
Pathology for cytomorphological analysis. Samples with insufficient
material for the two diagnostic processes were excluded and sent
solely to the histopathology department.
A total of 121 specimens were analyzed with FCI and
cytomorphological analysis. According to the pathological features
and immunohistochemical results, samples were definitively
diagnosed as follows: 27 patients with reactive hyperplasia
disease; 4 patients with tuberculosis; 11 patients with necrotic
lymphadenitis; 7 patients with Hodgkin lymphoma (HD); 25 patients
with B-NHL; 32 patients with T-lymphoblastic lymphoma (LBL) and 15
patients with ALCL. The patients with ALCL consisted of 10 males
and 5 females with a male to female ratio of 2:1. The median age of
this cohort was 10.3 years (range 2.0–15.0). All 15 ALCL patients
presented varying degrees of lymphadenopathy at diagnosis. Ten
cases had systemic disease (involving ≥2 lymph nodes or extranodal
areas, most commonly in the liver, spleen and mediastinum) and 5
cases had the disease limited to ≤1 lymph node with a diameter of
2–6 cm. Nine patients presented fever and 2 of these experienced
pleural effusions. The median follow-up time was 17 months (range,
6 months to 3 years) and 3 patients succumbed during this
period.
Pathological and immunohistochemical
analysis
The lymph node biopsy specimens were fixed in 10%
neutral-buffered formalin and sent to the pathology laboratory
immediately. Once the samples were dehydrated, they were embedded
in paraffin. Unstained 4 μm sections were cut from each
tissue block. Hematoxylin and eosin (H&E), periodic acid-Schiff
(PAS) and immunohistochemical staining were carried out for the
histopathological study. Macroscopic and microscopic examinations
were performed by at least two experienced pathologists. Sections
were stained immunohistochemically using the ChemMate™ Dako
EnVision™/horseradish peroxidase (HRP) two-step system
(DakoCytomation, Glostrup, Denmark). Antibodies were used to detect
the expression of CD1a, CD3, CD20, CD43, CD79a, CD30, CD15, CD68,
ALK, epithelial membrane antigen (EMA) and Epstein-Barr virus
(EBV). Antibodies were obtained from DakoCytomation (Denmark).
Appropriate positive and negative controls were set.
FCI analysis
FCI and histopathological studies were performed
separately and in a blinded manner. Fresh biopsy tissue samples
were sliced and disaggregated through a mesh <100 μm and
the cells were suspended in phosphate-buffered saline (PBS)
containing 0.1% sodium azide. Specimens were analyzed for various
antigens using a flow cytometer (FACSCalibur™, Becton Dickinson,
San Jose, CA, USA). Data acquisition and analysis were performed
using CellQuest software (Becton Dickinson). A minimum of 10,000
events were acquired for analysis. Immunophenotyping determination
was performed by four-color immunofluorescent staining using the
commercially available fluorescently-labeled monoclonal antibodies:
mouse IgG1, mouse IgG2a, CD1a, CD2, CD3, CD4, CD5, CD7, CD8, CD10,
CD11c, CD19, CD20, CD22, CD23, CD25, CD30, CD34, CD38, CD45, CD56,
CD69, CD103, CD138, human leukocyte antigen (HLA)-DR, CD45RA,
CD45RO, FMC7, T cell receptor (TCR) α/β, TCRγ/δ, Cμ, SmIgM,
κ and λ (Becton Dickinson). In particular, CD1a, CD2, CD3, CD4,
CD5, CD7, CD8, CD25, CD30, CD45, CD69, CD56, TCRα/β, TCRγ/δ, CD45RA
and CD45RO were used for T-cell lymphoproliferative disease, while
CD10, CD11c, CD19, CD20, CD22, CD23, CD34, CD38, CD103, CD138,
FMC7, HLA-DR, Cμ, SmIgM and surface κ and λ light chains
were used for B-cell lymphoproliferative disease.
Statistical analysis
The diagnostic sensitivity and specificity of FCI
were calculated by the Chi-square test. Comparison of the mean
flourescence intensity (MFI) of antigen expressions between normal
and neoplastic cells was performed by the Wilcoxon signed-rank
test. Statistical analyses were performed using the Statistical
Package for the Social Sciences (SPSS) software, version 12.0 (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Pathological findings
According to the pathological features and
immunohistochemical results, and with reference to the new WHO
classification, 15 cases were diagnosed as ALK(+) ALCL. Of these,
10 were common type, 4 were lymphohistiocytic type and 1 was
neutrophil-rich type. Their morphological variants and
immunohistochemical results are shown in Table I.
 | Table I.Morphological subforms and
immunohistochemical results of 15 cases with ALCL. |
Table I.
Morphological subforms and
immunohistochemical results of 15 cases with ALCL.
| Case no. | Gender | ALK | CD3 | CD43 | CD30 | CD15 | CD68 | CD1a | EMA | EBV | Histological
subtype |
|---|
| 1 | M | + | − | + | + | − | − | − | − | − | Common |
| 2 | M | + | + | + | + | − | − | − | − | − | Common |
| 3 | M | + | − | − | + | − | − | − | − | − | Common |
| 4 | M | + | + | − | + | − | − | − | + | − | Common |
| 5 | F | + | − | + | + | − | − | − | − | − | Common |
| 6 | M | + | + | − | + | − | − | − | + | − | Common |
| 7 | M | + | − | − | + | − | − | − | − | − | Common |
| 8 | M | + | + | + | + | − | − | − | − | − | Common |
| 9 | F | + | + | − | + | − | − | − | + | − | Common |
| 10 | M | + | − | − | + | − | − | − | − | − | Common |
| 11 | M | + | + | − | + | − | + | − | + | − |
Lymphohistiocytic |
| 12 | M | + | + | + | + | + | + | − | + | − |
Lymphohistiocytic |
| 13 | F | + | − | − | + | − | + | − | + | − |
Lymphohistiocytic |
| 14 | F | + | + | + | + | − | + | − | + | − |
Lymphohistiocytic |
| 15 | F | + | + | + | + | + | − | − | − | − |
Neutrophil-rich |
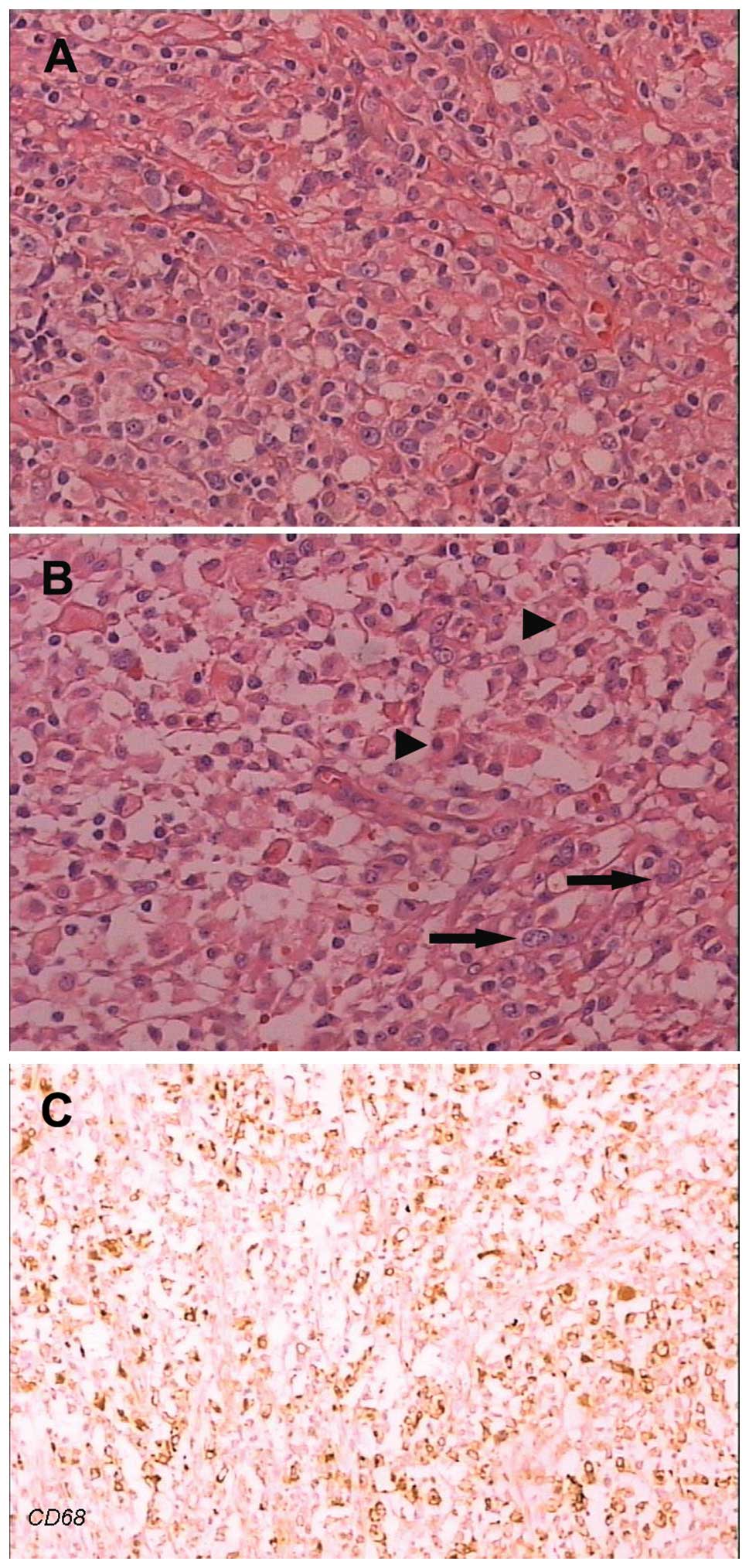
In the 10 cases with common type ALCL, paraffin
sections revealed that the lymph node structure had been destroyed
completely or partially with a diffuse proliferation of neoplastic
cells and fibrosis. Neoplastic cells were polymorphous with
irregular nuclei (horseshoe, folded and multinucleated) and
compartmentalized into nests by thin bands of fibrous tissue.
Multinucleated cells with Reed-Sternberg-like appearance were
observed (Fig. 1A). In the 4 cases
with lymphohistiocytic-type ALCL, paraffin sections revealed that
the polymorphous neoplastic cells dispersed but did not form nests.
A number of mature lymphocytes and histiocytes were evident in the
background. In 2 cases, necrosis and fewer neoplastic cells were
observed (Fig. 1B and C).
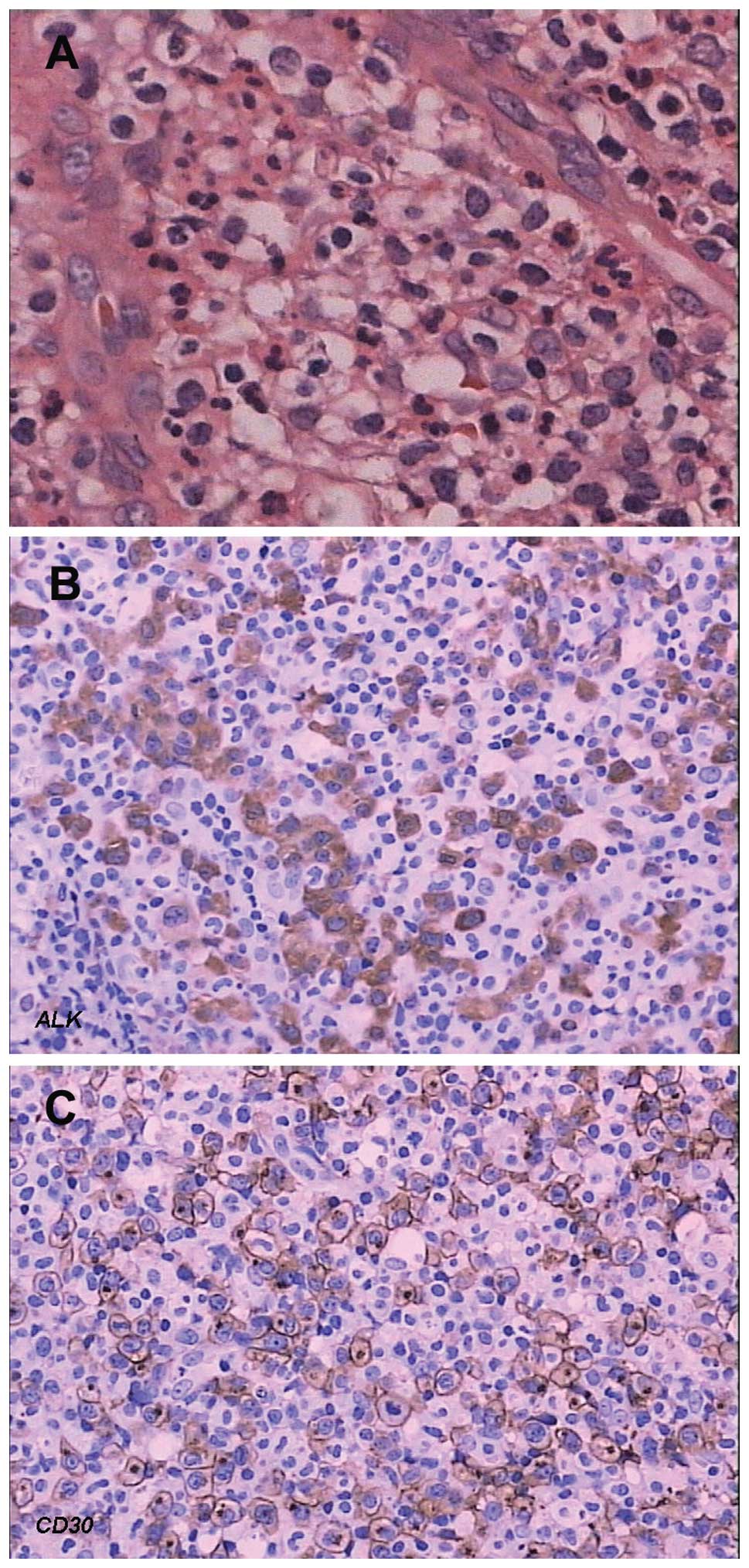
The patient with neutrophil-rich type ALCL presented
fever and pleural effusions. Paraffin sections revealed that the
polymorphous neoplastic cells with irregular nuclei dispersed. The
neoplastic cells were intermixed with a large number of mature
neutrophils and a local small abscess was observed (Fig. 2A). Immunohistochemical stain of
neoplastic cells was positive for ALK and CD30 (Fig. 2B and C). B-cell markers, including
CD79a and CD20 were negative in all cases.
FCI results
Of the 15 cases with ALCL analyzed in this study, 13
(10 common, 2 lymphohistiocytic and 1 neutrophil-rich type) were
diagnosed as ALCL with FCI. These 13 cases were CD30-positive and
immunophenotypically aberrant with respect to expression of the
T-cell antigens CD2, CD3, CD4, CD5 and CD7. CD30-positive
neoplastic cells accounted for a small proportion of the total
cells with FCI, with a median of 19.3% (range, 7.9–31.8%). The
frequently expressed T-cell antigens were CD4 (84.6%), CD2 (76.9%),
CD7 (61.5%), CD3 (53.8%) and CD5 (38.4%). Dim expression of CD3 was
observed in ALCL cases compared with that in the background
reactive T cells (median MFI, 293 vs. 95, P=0.018). CD25 was
brightly expressed in the neoplastic cells in 10 of the 13 ALCL
cases analyzed (76.9%); however, it was almost not expressed in
background reactive T cells. CD45 was positive in all cases. CD8
and B-cell markers, including CD19, CD20, CD22, CD23, FMC7 and
surface immunoglobulin were negative in all cases. In the majority
of cases, the neoplastic cells demonstrated high forward and high
side scatter, similar to monocytes or granulocytes on a dot plot
(Fig. 3). In 3 common type cases,
the neoplastic cells were concentrated in the large lymphocyte
region (Fig. 4).
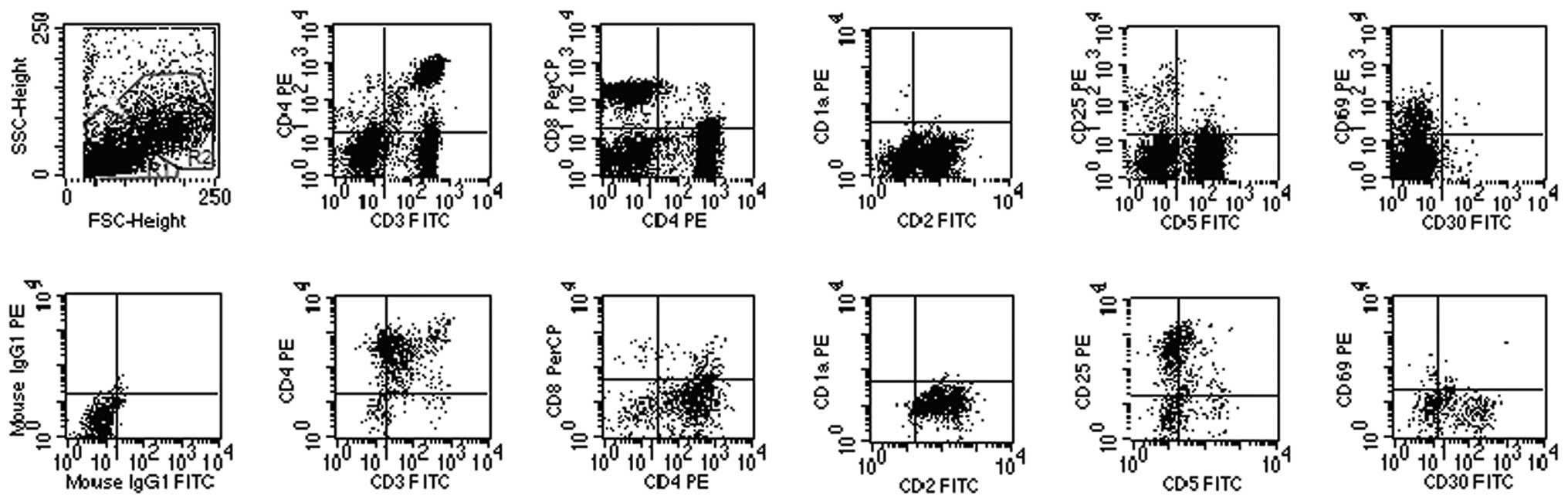 | Figure 3.Flow cytometric analysis of lymph node
biopsy specimen of ALCL case 6. Forward scatter (FSC) and side
scatter (SSC) biparametric dot plot showing two lymphocytic
populations (R1 and R2 regions). The cells in the R1 region with
normal phenotype (CD2+, CD3+,
CD5+, CD69dim, CD1a−,
CD25− and CD30−) and normal CD4/CD8 ratio
were suggested as normal mature T cells by FCI (top panel). Flow
cytometry detected a population of T lymphocytes (19.2%; R2
regions) with an abnormal phenotype (CD3dim,
CD2+, CD4+, CD30+,
CD25+, CD5−, CD69−,
CD1a− and CD8−; bottom panel) and high
FSC/SSC, similar to monocytes and granulocytes, suggesting ALCL
cell origin. ALCL, anaplastic large cell lymphoma; FCI, flow
cytometry immunophenotyping. |
 | Figure 4.Flow cytometric analysis of lymph node
biopsy specimen of ALCL case 9. The neoplastic cells are
concentrated in the large lymphocyte region with an abnormal
immunophenotype (CD3dim, CD2+,
CD7+, CD4+, CD30+,
CD5−, CD25−, CD56−,
CD69−, CD1a− and CD8−). ALCL,
anaplastic large cell lymphoma. |
In the remaining 2 specimens with lymphohistiocytic
type presenting extensive necrosis (cases 13 and 14), FCI revealed
a low percentage (0.60 and 1.5%, respectively) of CD30-positive
cells, thus FCI was not able to establish a diagnosis due to the
relative rarity of the CD30-positive cells in the specimens.
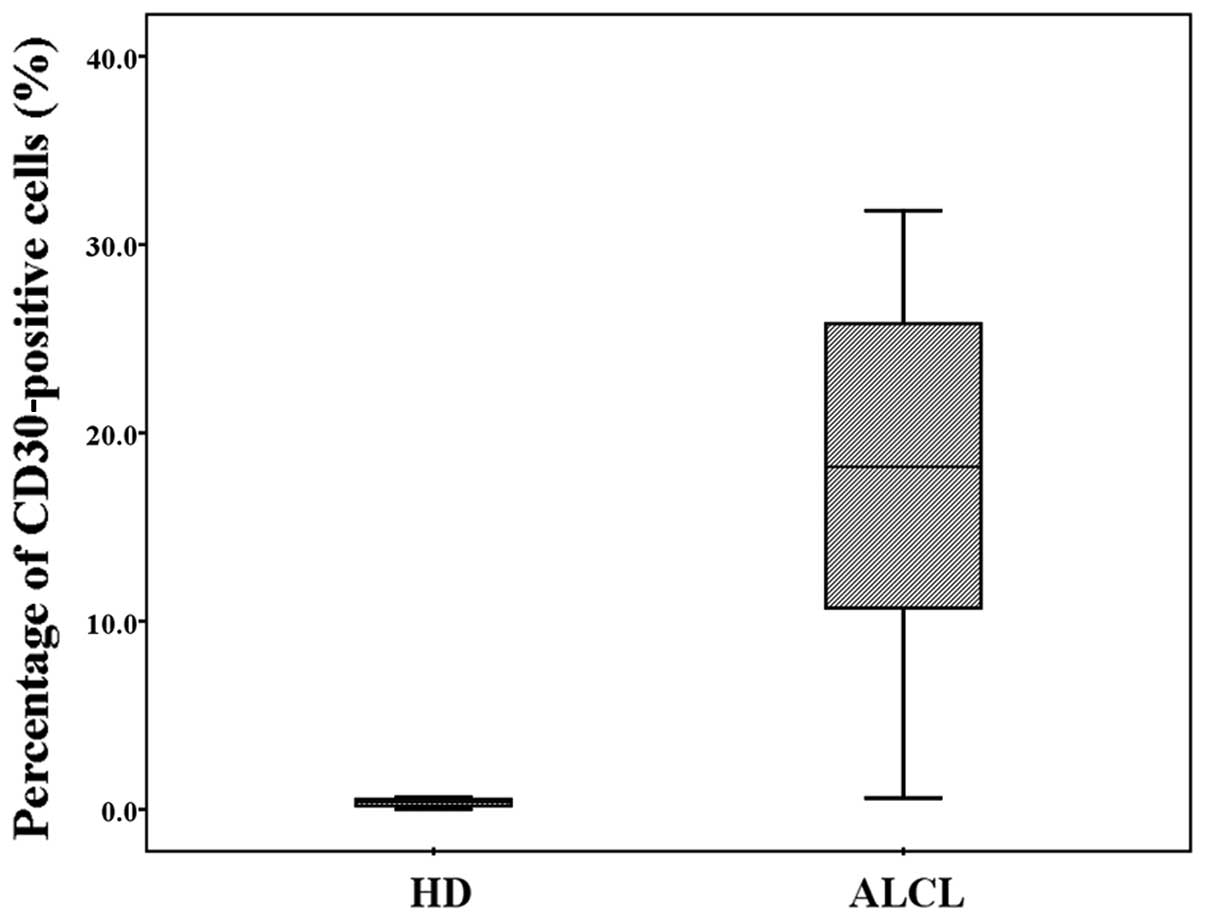
In 99 cases of non-ALCL diseases, there were 27
cases of reactive hyperplasia, 4 cases of tuberculosis, 11 cases of
necrotic lymphadenitis, 25 cases of B-NHL and 32 cases of T-LBL.
FCI analysis did not show the presence of cells with coexpression
of CD30 and T-cell antigens. In the remaining 7 cases with HD, FCI
revealed a low percentage (median, 0.52% with a range of
0.01–0.65%) of CD30-positive cells with a dim expression, but did
not establish a diagnosis for these cases due to the relative
rarity of the CD30-positive cells in the specimens. The percentage
of CD30-positive cells among patients with HD and ALCL is shown in
Fig. 5.
In this study, the estimated sensitivity of FCI for
diagnosing ALCL in lymph node samples was 86.7% (13/15 cases) with
a specificity of 100%. When the specimens with necrosis were
excluded from the analysis, the sensitivity increased to 100%,
suggesting that FCI is a reliable approach for the diagnosis of
ALCL when a neoplastic cell clone presents >5% cells with
coexpression of CD30 and any of the T-cell antigens, identified by
flow cytometry.
Discussion
ALCL is a rare disease in children, accounting for
10–15% of all childhood NHL (11).
The majority of ALCLs in children and adolescents are ALK-positive.
In our cohort, the male to female ratio was 2:1. Previous studies
demonstrated that there may be a male predominance, particularly in
ALK(+) cases, in which the male to female ratio is ∼3:1 (12).
Histologically, several ALCL variants have been
described. Morphological variants of ALCL include the following
types: common, lymphohistiocytic, small cell and rare subforms,
including neutrophil-rich types (13). Of these variants, the common,
lymphohistiocytic and small cell types are the most frequently
encountered. The ‘horseshoe’ or ‘wreath’ cell is considered the
cytologic hallmark of this disease. Histological characteristics
may have a high potential for future risk stratification and
treatment (14).
Since ALCL demonstrates a broad morphological
spectrum, definitive diagnosis and differential diagnosis of ALCL
from other forms of lymphoma and reactive lymphadenopathy are
difficult. Unlike other lymphomas, the tumor masses comprise normal
reactive T cells and neoplastic cells in ALCL and in a number of
cases only a careful search is likely to reveal the presence of
neoplastic cells. Diagnosis of common type ALK(+) ALCL has become
straightforward owing to the widespread availability of reliable
anti-ALK antibodies. However, ALK(+) ALCL includes a morphological
spectrum with small cell and lymphohistiocytic variants that
represent ∼10–20% of cases and is easily confused with reactive
lymphadenopathy (15). In our
study, the paraffin sections of two cases of lymphohistiocytic ALCL
demonstrated varying degrees of necrosis, which causes the
misdiagnosis of inflammation. Scattered neoplastic cells were
observed with a careful search and recognized by an
immunohistochemical stain of CD30- and ALK-positive cells. In a
neutrophil-rich type patient who was misdiagnosed with an
inflammatory disease in a local hospital, the polymorphous
neoplastic cells with irregular nuclei dispersed and were
intermixed with a large number of mature neutrophils. CD30- and
ALK-positive results suggest a diagnosis of ALCL in this case. The
neutrophil-rich variant of anaplastic large cell lymphoma (NR-ALCL)
is a rare type of NHL. Diagnosis by lymph node biopsy is difficult
owing to the rarity of this tumor, its resemblance to HD and other
NHL, its similarity to an infectious process and its occasional
confusion with metastatic carcinoma and melanoma (16). Numerous ALCL cases present lymph
node sclerosis and may be misdiagnosed as HD in which CD30 is also
positive (17). As ALCL is a
peripheral T-cell-derived malignancy, an immunohistochemical stain
for T cell-specific markers, CD15, EMA and ALK protein may be
useful in the differential diagnosis between ALCL and HD. In
addition, the nodal ALCL should be differentiated from metastatic
undifferentiated carcinoma, malignant melanoma, Langerhans cell
histiocytosis and soft tissue sarcoma. Therefore, lymphadenopathy
in children with abnormal cells in a background of inflammation
should be considered as ALCL. Immunohistochemical staining of ALK
and CD30 is a useful approach to confirm the diagnosis of ALCL.
Flow cytometry provides rapid analysis of multiple
characteristics of separate cell populations based on their sizes,
cytoplasmic characteristics and antigens expressed. Lymph node
specimens are now routinely submitted for flow cytometric analysis
in patients with suspected lymphoma and are considered to be
standard practice in a number of centers. By comparison, relatively
few studies have addressed the use of flow cytometry in the
evaluation of ALCL. In the present study, we analysed 15 samples
from patients with ALK(+) ALCL with FCI, 13 of which were
consistent with pathological results. The sensitivity and
specificity of FCI for diagnosing ALCL were 86.7 and 100%,
respectively. The samples were CD30-positive and
immunophenotyically aberrant with respect to T-cell antigen
expression (CD2, CD3, CD4, CD5 and CD7), which is in concordance
with the previous study (9). CD3
antigen was often expressed at a dim intensity compared with
background normal T cells and such a marked contrast in the same
FCI plot contributes to the diagnosis of ALCL. The frequent
expression of CD25 in ALCL suggests that this antigen is a
potentially useful marker in the immunophenotypic diagnosis of ALCL
and a potential therapeutic target (18).
ALCL should also be differentiated from HD when
diagnosed using FCI. Unlike ALCL in which the neoplastic cells
dispersed in lymph node tissue, the Reed-Sternberg (RS) cells of HD
usually scatter, where they constitute <1% and frequently
<0.01% of the total cells (19).
Therefore, routine analysis of 10,000 events by FCI is not able to
bring a population that represents 0.01% of the total cells well
within range of sensitivity of clinical cytometry, thus FCI is not
able to establish a diagnosis in routine practice. By comparison,
neoplastic cells have a relatively higher proportion of total cells
in lymph node tissue with ALCL and are rapidly detected with FCI.
We suggest that when CD45-, CD30- and T-cell marker-positive
neoplastic cells constitute at least 5% of the total cells, a
diagnosis of ALCL may be made using FCI.
Unlike other PTCL-NOS in which the neoplastic cells
are usually small- to medium-sized, the majority of ALCL cells are
large and show increased FSC and SSC. Therefore they are
concentrated largely or entirely outside the lymphocyte gate,
similar to monocytes or granulocytes. Conventional gating for
lymphoma cells with low FSC and SSC may lead to false-negativity
and careful analysis is required since the proportion of the
aberrant population with a diagnostic value may be extremely small.
Flexible gating strategies are important in the diagnosis of ALCL.
In this study, 2 specimens (lymphohistiocytic type) with extensive
necrosis were not confirmed as ALCL by flow cytometry due to the
lack of analyzable cells present in the specimens. Similarly,
Muzzafar et al(10) were
unable to identify neoplastic cells by flow cytometry in 4 of 23
(17.4%) adults with ALCL. There are a number of technical factors
and potential pitfalls that make ALCL diagnosis by flow cytometry
particularly challenging. False-negative FCI results in ALCL may be
due to the necrosis and apoptosis commonly associated with such
tumors and to the fragility of the large atypical neoplastic cells,
which may be easily disrupted during sample processing (20). Sampling issues may also be involved
due to the neoplastic cells focally distributed in the lymph node
(21). Clinical application of FCI
for ALCL may be expanded, with the exception of diagnosing ALCL in
lymph node biopsy specimens. Our previous study revealed that in
pleural effusion from a case with ALCL, which was considered
negative for ALCL by morphological examination, FCI detected a
minor proportion (9.3%) of aberrant T-cell population with high
FSC/SSC, a positive expression of CD4, CD7, CD2, CD45RO and CD30
and a negative expression of CD5 and CD69 (22). Moreover, Damm-Welk et al
considered that FCI using antibodies against ALK and CD30,
sensitively and specifically detects circulating ALCL cells in bone
marrow or blood (23). Therefore,
FCI holds a clear advantage over morphological examination in such
circumstances.
In summary, ALK(+) ALCL is a distinct subset of NHL
morphologically and immunophenotypically. FCI may be used as an
adjunct to histopathological examination for reliable diagnosis of
pediatric ALCL with high specificity and sensitivity. It is rapid
and suitable for emergency situations, allowing for therapeutic
decisions to be made promptly. However, flexible gating strategies
and careful analysis are required to identify neoplastic cells with
FCI.
Acknowledgements
This study was supported in part by
grants from the Zhejiang Provincial Fund of Education Bureau (no.
Y200908359) and the Fund of Zhejiang Province Innovation Team for
Early Screening and Intervention of Birth Defects (no. 2010R50045).
The authors thank P. Chen, B. Qian and N. Zhao at the Division of
Hematology-Oncology Laboratory for their excellent technical
support.
References
|
1.
|
Campo E, Swerdlow SH, Harris NL, Pileri S,
Stein H and Jaffe ES: The 2008 WHO classification of lymphoid
neoplasms and beyond: evolving concepts and practical applications.
Blood. 117:5019–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Stein H, Mason DY, Gerdes J, et al: The
expression of the Hodgkin’s disease associated antigen Ki-1 in
reactive and neoplastic lymphoid tissue: evidence that
Reed-Sternberg cells and histiocytic malignancies are derived from
activated lymphoid cells. Blood. 66:848–858. 1985.
|
|
3.
|
Falini B: Anaplastic large cell lymphoma:
pathological, molecular and clinical features. Br J Haematol.
114:741–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Medeiros LJ and Elenitoba-Johnson KS:
Anaplastic large cell lymphoma. Am J Clin Pathol. 127:707–722.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
ten Berge RL, Oudejans JJ, Ossenkoppele GJ
and Meijer CJ: ALK-negative systemic anaplastic large cell
lymphoma: differential diagnostic and prognostic aspects - a
review. J Pathol. 200:4–15. 2003.PubMed/NCBI
|
|
6.
|
Savage KJ, Harris NL, Vose JM, et al:
ALK-anaplastic large-cell lymphoma is clinically and
immunophenotypically different from both ALK+ ALCL and peripheral
T-cell lymphoma, not otherwise specified: report from the
International Peripheral T-Cell Lymphoma Project. Blood.
111:5496–5504. 2008.PubMed/NCBI
|
|
7.
|
Sethuraman C, Simmerson M, Vora AJ and
Cohen MC: Flowcytometric immunophenotyping in the diagnosis of
pediatric lymphoma: how reliable is it and how can we optimize its
use? J Pediatr Hematol Oncol. 32:298–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Barrena S, Almeida J, Del Carmen
Garcia-Macias M, et al: Flow cytometry immunophenotyping of
fine-needle aspiration specimens: utility in the diagnosis and
classification of non-Hodgkin lymphomas. Histopathology.
58:906–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kesler MV, Paranjape GS, Asplund SL,
McKenna RW, Jamal S and Kroft SH: Anaplastic large cell lymphoma: a
flow cytometric analysis of 29 cases. Am J Clin Pathol.
128:314–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Muzzafar T, Wei EX, Lin P, Medeiros LJ and
Jorgensen JL: Flow cytometric immunophenotyping of anaplastic large
cell lymphoma. Arch Pathol Lab Med. 133:49–56. 2009.PubMed/NCBI
|
|
11.
|
Wright D, McKeever P and Carter R:
Childhood non-Hodgkin lymphomas in the United Kingdom: findings
from the UK Children’s Cancer Study Group. J Clin Pathol.
50:128–134. 1997.
|
|
12.
|
Jacobsen E: Anaplastic large-cell
lymphoma, T-/null-cell type. Oncologist. 11:831–840. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stein H, Foss HD, Durkop H, et al: CD30(+)
anaplastic large cell lymphoma: a review of its histopathologic,
genetic, and clinical features. Blood. 96:3681–3695. 2000.
|
|
14.
|
Lamant L, McCarthy K, d’Amore E, et al:
Prognostic impact of morphologic and phenotypic features of
childhood ALK-positive anaplastic large-cell lymphoma: Results of
the ALCL99 study. J Clin Oncol. 29:4669–4676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kinney MC, Higgins RA and Medina EA:
Anaplastic large cell lymphoma: twenty-five years of discovery.
Arch Pathol Lab Med. 135:19–43. 2011.PubMed/NCBI
|
|
16.
|
Creager AJ, Geisinger KR and Bergman S:
Neutrophil-rich Ki-1-positive anaplastic large cell lymphoma: a
study by fine-needle aspiration biopsy. Am J Clin Pathol.
117:709–715. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Deutsch YE, Tadmor T, Podack ER and
Rosenblatt JD: CD30: an important new target in hematologic
malignancies. Leuk Lymphoma. 52:1641–1654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Costa V, Oliva T and Norton L: Successful
treatment with daclizumab of refractory anaplastic lymphoma.
Pediatr Blood Cancer. 53:1130–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Swerdlow SH, Campo E, Harris NL, et al:
WHO Classification of Tumours of Haematopoietic and Lymphoid
Tissues. IARC Press; Lyon: pp. 326–329. 2008
|
|
20.
|
Craig FE and Foon KA: Flow cytometric
immunophenotyping for hematologic neoplasms. Blood. 111:3941–3967.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
El-Sayed AM, El-Borai MH, Bahnassy AA and
El-Gerzawi SM: Flow cytometric immunophenotyping (FCI) of lymphoma:
correlation with histopathology and immunohistochemistry. Diagn
Pathol. 3:432008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shen H, Tang Y, Xu X, et al: Rapid
detection of neoplastic cells in serous cavity effusions in
children with flow cytometry immunophenotyping. Leuk Lymphoma.
53:1509–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Damm-Welk C, Schieferstein J, Schwalm S,
Reiter A and Woessmann W: Flow cytometric detection of circulating
tumour cells in nucleophosmin/anaplastic lymphoma kinase-positive
anaplastic large cell lymphoma: comparison with quantitative
polymerase chain reaction. Br J Haematol. 138:459–466. 2007.
View Article : Google Scholar
|