Introduction
It is widely accepted that prostaglandin
E2 (PGE2) is important in the pathogenesis of
tumors. Early evidence was indirectly provided by epidemiological
and laboratory studies, which revealed that cyclooxygenase-2
(COX-2) inhibitors are capable of suppressing the incidence of
colorectal and breast cancer (1–3). By
contrast, COX-2 overexpression was observed in a series of types of
human cancer (4–6), while mice with COX-2 overexpression
were found to be more susceptible to carcinogenesis (7). As a key enzyme in the synthesis of
PGE2, COX-2 is considered to act via PGE2.
Certain studies have directly shown that PGE2 is
involved in several crucial aspects of malignant tumors, including
proliferation, invasion, migration and angiogenesis (8–11).
COX-2 expression has also been demonstrated to be
present in human melanoma tissue, whereas it has been revealed to
be absent in benign melanocytic nevi and normal epithelium
(12). Certain melanoma cell lines
tested were found to produce PGE2, which was suppressed
by NS398, a specific inhibitor of COX-2 (12). The roles of PGE2 in
melanoma have not been extensively investigated. PGE2
suppression has been demonstrated to have no effects on melanoma
cell proliferation, but to reduce cell invasion, indicating that
PGE2 may be implicated in melanoma progression (12). Recently, several studies from one
laboratory provided further evidence for the involvement of
PGE2 in melanoma metastasis. It was demonstrated that
green tea catechin, grape seed proanthocyanidins and berberine are
capable of suppressing melanoma cell invasion and migration by
inhibition of COX-2, PGE2 and PGE2 receptors
(13–15).
In the present study, we identified a novel role of
PGE2 in melanoma. We found that via EP4/p38 MAPK
signaling, endogenous PGE2 upregulates the expression of
macrophage chemoattractant protein-1 (MCP-1), an important
chemoattractant to macrophages, suggesting that PGE2 may
be involved in macrophage recruitment in melanoma. Macrophage
infiltration is often present in melanoma tissues, and studies have
revealed that macrophages facilitate melanoma angiogenesis
(16) and invasion (17). However, MCP-1 is also able to
recruit natural killer (NK) cells and cytotoxic lymphocytes (CTLs),
which exert an inhibitory effect on melanoma cells (18). Overall, these data imply that
PGE2 may play more complex roles in melanoma than
previously known.
Materials and methods
Melanoma cells and specimens
Melanoma cell lines, A2058 and MeWo, were purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum, 100 mg/ml penicillin and 100 mg/ml streptomycin at
37°C in an incubator with 5% CO2. Eighteen melanoma
specimens were obtained with consent from patients undergoing
surgery at the First Hospital of China Medical University
(Liaoning, China). This study was approved by the Ethics Committee
of China Medical University.
Real-time reverse transcription PCR
Total RNA was isolated from cells and tissues by
TRIzol (Takara, Dalian, China) and reverse transcribed by the
PrimeScript RT Reagent kit (Takara) according to the manufacturer’s
instructions. Primer sequences for COX-2 and MCP-1 were as
described in a previous study (19). Real-time PCR was performed using
SYBR Premix Ex Taq II (Takara) in an Applied Biosystems 7500
Fast Real-Time PCR system (Applied Biosystems/Life Technologies
Corporation; Carlsbad, CA, USA). The housekeeping gene GAPDH was
used as an internal control. Gene expression was quantified by the
comparative CT method, normalizing CT values of the target gene to
those of GAPDH and calculating relative expression values.
Western blot analysis
Cells were lysed with sample buffer containing 50
mmol/l Tris-HCl (pH 6.8), 100 mmol/l dithiothreitol (DTT), 2% SDS,
0.1% bromophenol blue and 10% glycerol. Protein (20 μg) was
separated in a 10% sodium dodecyl sulfate (SDS)/acrylamide gel and
was then transferred to a nitrocellulose membrane, which was
blocked at 4°C in phosphate-buffered saline (PBS) supplemented with
0.1% Tween and 10% milk powder. The membrane was then incubated
with the primary antibody (1:1,000) at 4°C overnight. After the
membrane was washed three times with PBS, the corresponding second
antibody was added (1:2000) for 1 h at room temperature. Primary
antibodies for COX-2, EP4, phosphorylated p38 MAPK, β-actin and the
corresponding secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The human β-actin gene
was used as an internal control.
Enzyme-linked immunosorbant asssay
(ELISA)
Concentrations of PGE2 and MCP-1 in cell
culture supernatants (2×105/well) were measured using
Quantikine ELISA kits (Boster Biological Technology, Ltd.; Wuhan,
China) according to the manufacturer’s instructions. The detection
limit of the assay was 4 pg/ml.
RNA interference
The COX-2 siRNA and nonsilencing control siRNA
plasmids were provided by Takara. Cells were seeded into a 24-well
plate at a density of 5×105/well for 24 h, and were then
transfected with siRNA plasmids using Lipofectamine 2000
(Invitrogen Life Technologies; Carlsbad, CA, USA) according to the
manufacturer’s instructions. Silencing of COX-2 expression in cells
following transfection was verified by western blot analysis.
Macrophage migration assay
Macrophages (2×105) were added to the
upper chamber of a transwell insert with an 8-μm pore size
membrane. Conditioned medium from melanoma cells was added to the
lower chamber. Cells were allowed to migrate for 6 h at 37°C and 5%
CO2, then fixed and stained with Diff-Quick stain
(International Reagents Corp., Kobe, Japan) according to the
manufacturer’s recommendations. The number of migrated cells in
five random microscopy fields per well were counted. Experiments
were performed in triplicate.
Statistical analyses
A Spearman’s rank correlation was used to analyze
the correlation between MCP-1 and COX-2 expression in melanoma
specimens. Differences in the protein content of the cell culture
supernatant, the cell mRNA level and macrophage migration were
evaluated using a Student’s t-test or a one-way analysis of
variance (ANOVA). Statistical analyses were conducted using the
Statistical Package for the Social Sciences (SPSS) software,
version 13.0 (SPSS Inc.; Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
MCP-1 expression is correlated with COX-2
expression in melanoma tissue
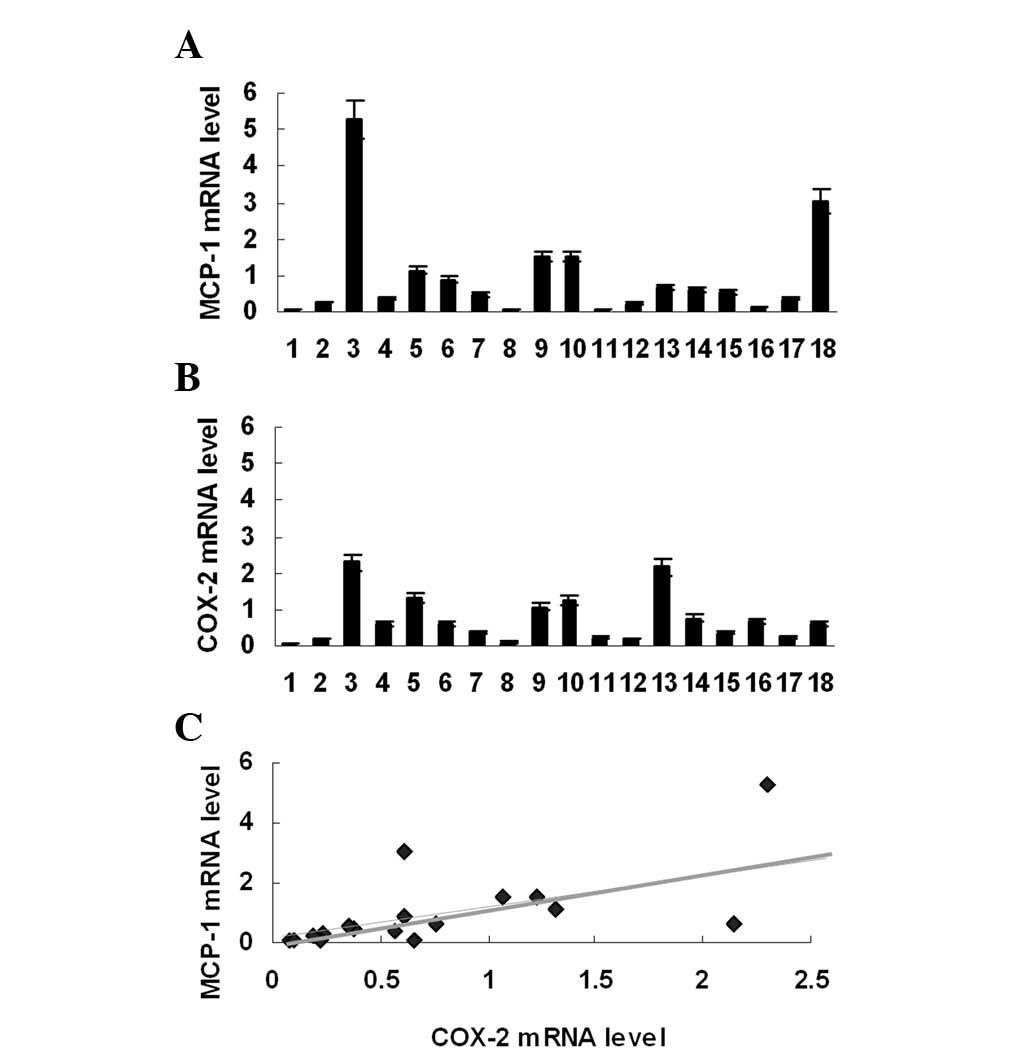
MCP-1 mRNA expression was examined in 18 melanoma
specimens. Real-time PCR revealed that all specimens expressed
MCP-1 mRNA (Fig. 1A). However,
MCP-1 mRNA expression in melanoma was heterogeneous; the highest
mRNA expression level was >100-fold greater than the lowest. In
order to analyze the correlation of MCP-1 expression with
PGE2 production, COX-2 mRNA expression was subsequently
investigated in the same group of melanoma specimens. It was found
that COX-2 mRNA was also expressed in these specimens (Fig. 1B) and that the levels of COX-2 mRNA
were positively correlated with those of MCP-1 mRNA (Fig. 1C).
PGE2 production is dependent
on COX-2 expression and NFκB activation in melanoma cells
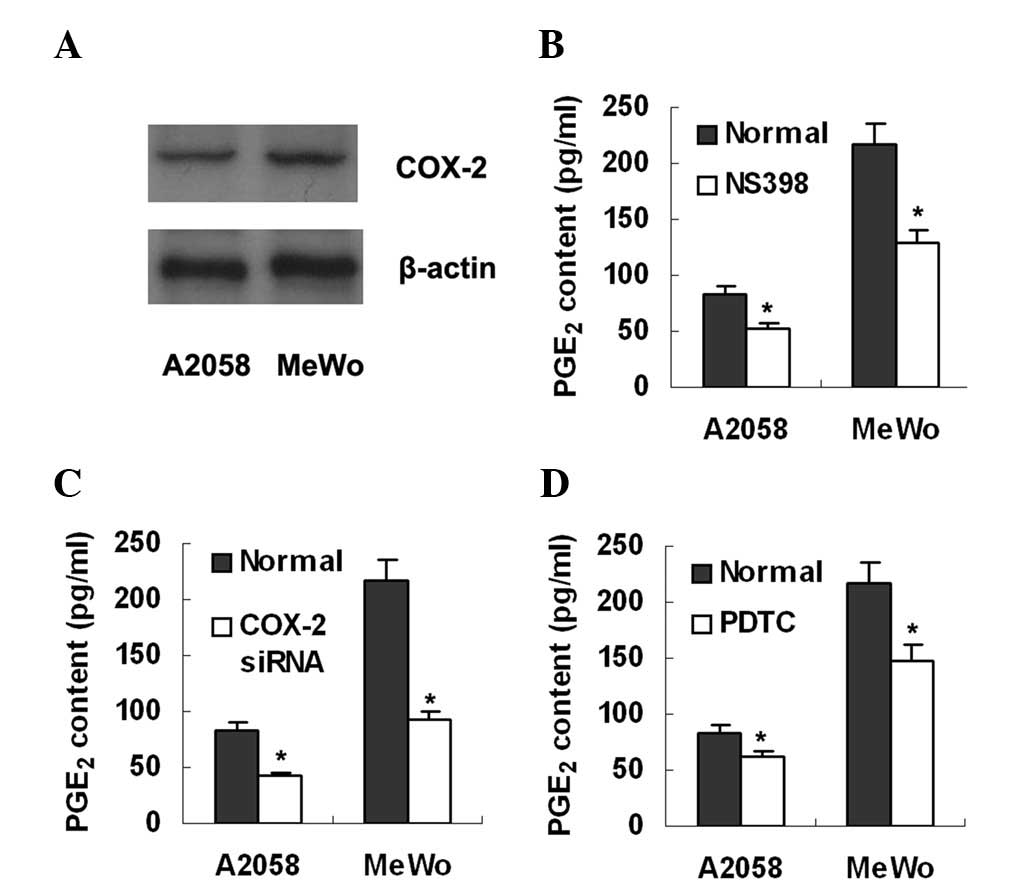
Western blot analysis and ELISA were used to detect
COX-2 expression and PGE2 production, respectively, in
two melanoma cell lines, A2058 and MeWo. The results showed that
both cell lines expressed COX-2 protein (Fig. 2A) and secreted PGE2 into
culture supernatant at 84 and 216 pg/ml for A2508 and MeWo cell
lines, respectively (Fig. 2). To
further explore whether the production of endogenous
PGE2 is dependent on COX-2 expression, the cell lines
were treated with a COX-2 inhibitor, NS398 (Sigma; St Louis, MO,
USA; 100 μmol/l). ELISA detection revealed that the
PGE2 levels in the culture supernatant had decreased in
the two cell lines (Fig. 2B).
Similarly, treatment with COX-2 siRNA was also demonstrated to
reduce PGE2 production in the two cell lines (Fig. 2C). To determine whether NFκB is
involved in PGE2 production, we treated the melanoma
cells with an NFκB inhibitor, PDTC (Sigma; 100 μmol/l). It
was observed that PGE2 production was significantly
inhibited (Fig. 2D).
MCP-1 expression is downregulated by
inhibition of endogenous PGE2 production
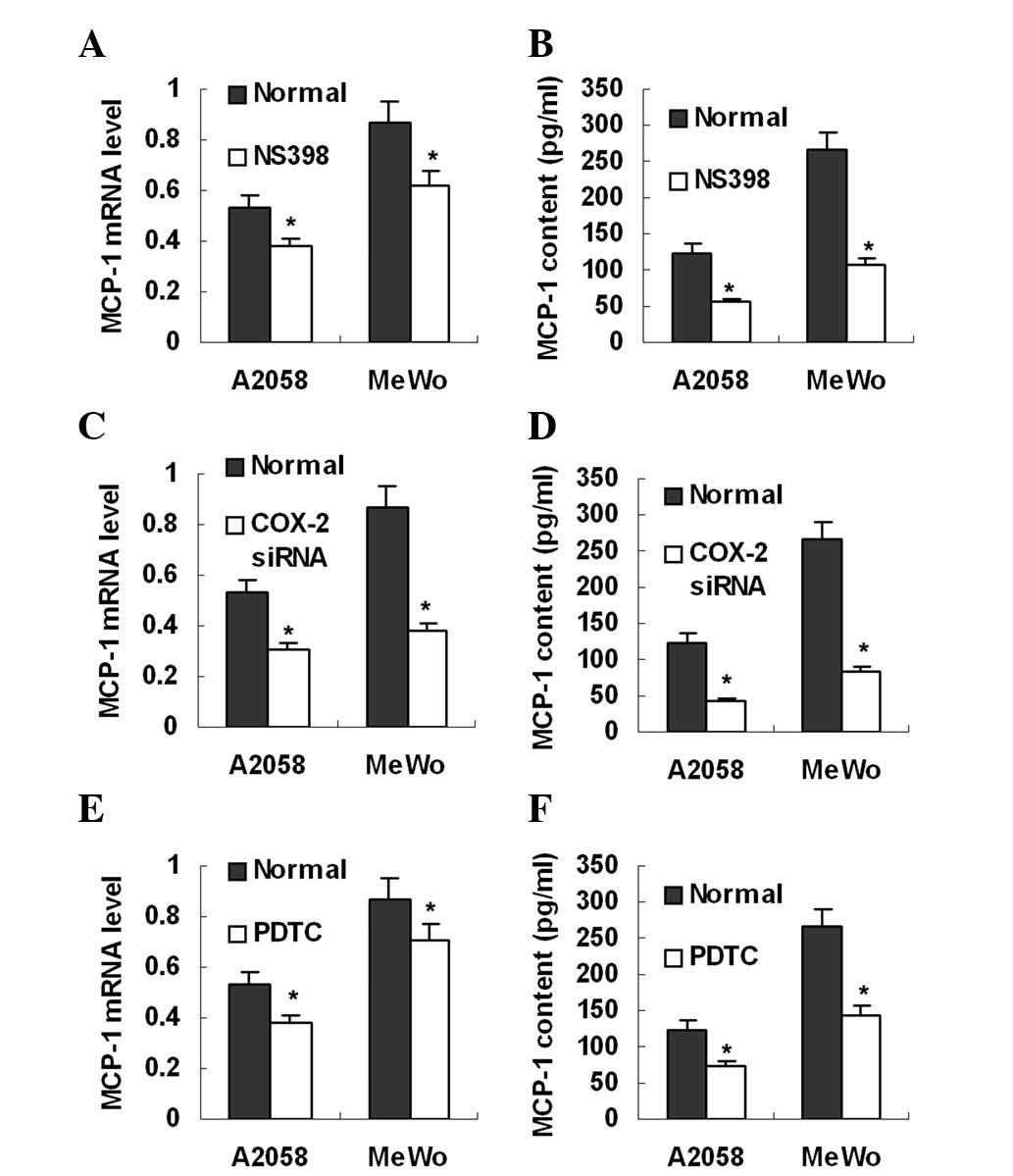
Real-time PCR and ELISA revealed that the two
melanoma cell lines expressed MCP-1 mRNA (Fig. 3) and secreted MCP-1 protein
(Fig. 3), 124 pg/ml for A2058 and
266 pg/ml for MeWo, into the culture supernatant. To investigate
whether endogenous PGE2 is involved in MCP-1 expression,
the melanoma cell lines were treated with NS398 and COX-2 siRNA,
respectively. It was found that treatment with either NS398 or
COX-2 siRNA markedly inhibited MCP-1 mRNA expression and protein
production in the melanoma cell lines (Fig. 3A–D). In addition, MCP-1 expression
in these cell lines was also reduced by the NFκB inhibitor, PDTC
(Fig. 3E and F). These data
indicate that inhibition of endogenous PGE2 production
suppresses MCP-1 expression in melanoma cells.
MCP-1 expression is upregulated by
endogenous and exogenous PGE2
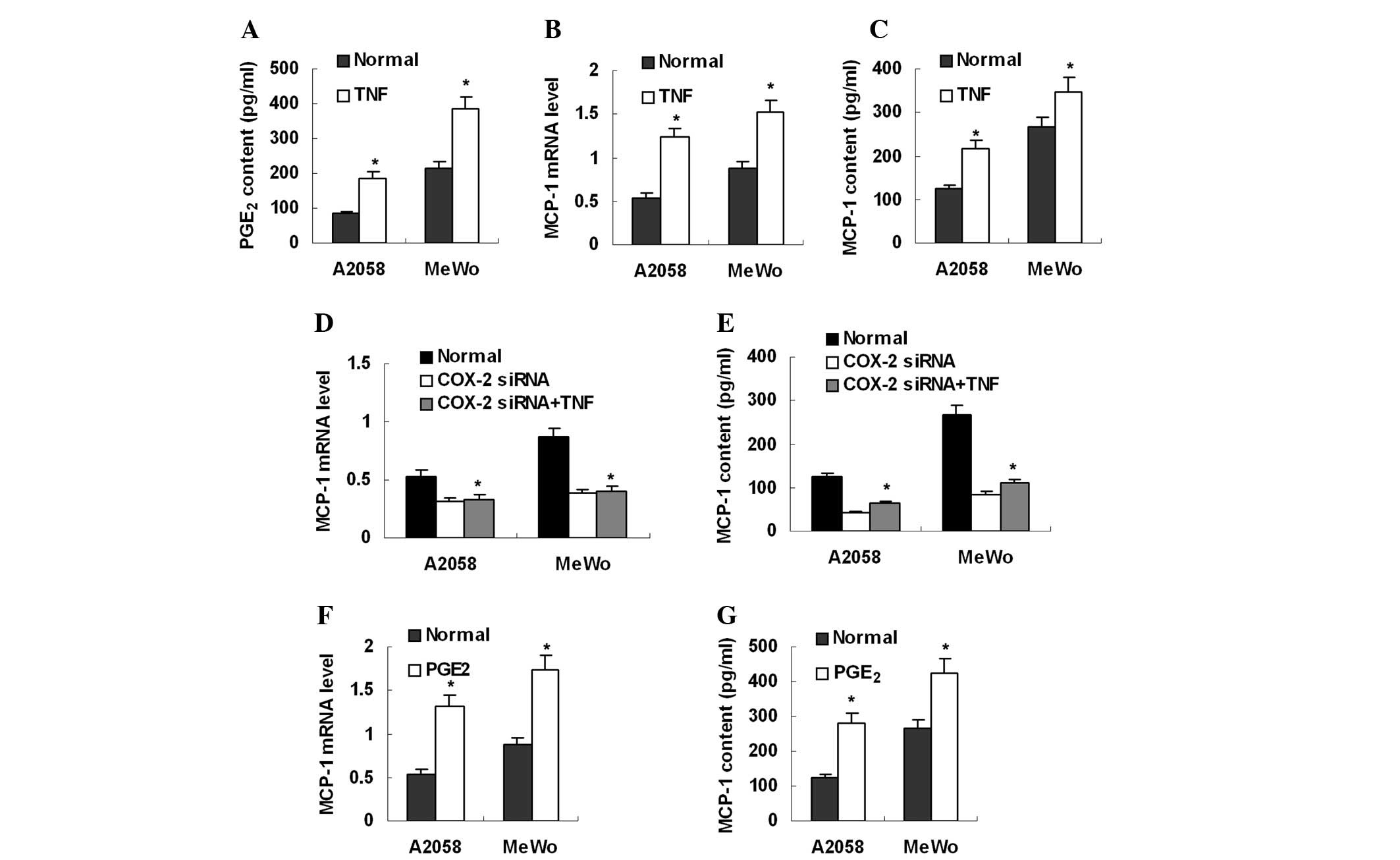
To stimulate endogenous PGE2 production,
mealnoma cell lines were treated with TNF-α (Sigma; 100 ng/ml), a
strong PGE2 inducer. As expected, TNF-α treatment
significantly increased PGE2 production (Fig. 4A). MCP-1 expression was
simultaneously elevated in these cells (Fig. 4B and C). However, TNF-α treatment
was not able to stimulate MCP-1 expression in melanoma cells
pre-treated with COX-2 siRNA (Fig. 4D
and E). These data suggest that PGE2 mediates
TNF-α-induced MCP-1 upregulation. Furthermore, it was investigated
whether exogenous PGE2 was capable of enhancing MCP-1
expression in melanoma cells. After treatment with exogenous
PGE2 (Sigma; 100 μmol/l) for 8 h, melanoma cells
were found to express more MCP-1 mRNA and secreted more MCP-1
protein into culture supernatant (Fig.
4F and G).
EP4/p38 MAPK signaling is involved in
MCP-1 upregulation by PGE2
The PGE2 receptor EP4 was observed to be
expressed in the two melanoma cell lines by western blot analysis
(Fig. 5A). To further analyze the
role of EP4 in the PGE2-MCP-1 axis, the cell lines were
treated with an EP4 antagonist, AH23848 (100 μmol/l). ELISA
detection revealed that MCP-1 production was significantly
inhibited by AH23848 (Fig. 5B). In
addition, AH23848 blocked the induction of MCP-1 caused by TNF-α or
exogenous PGE2 (Fig.
5B). Overall, these data suggest that PGE2
stimulates MCP-1 expression via EP4 in an autocrinal or paracrinal
manner.
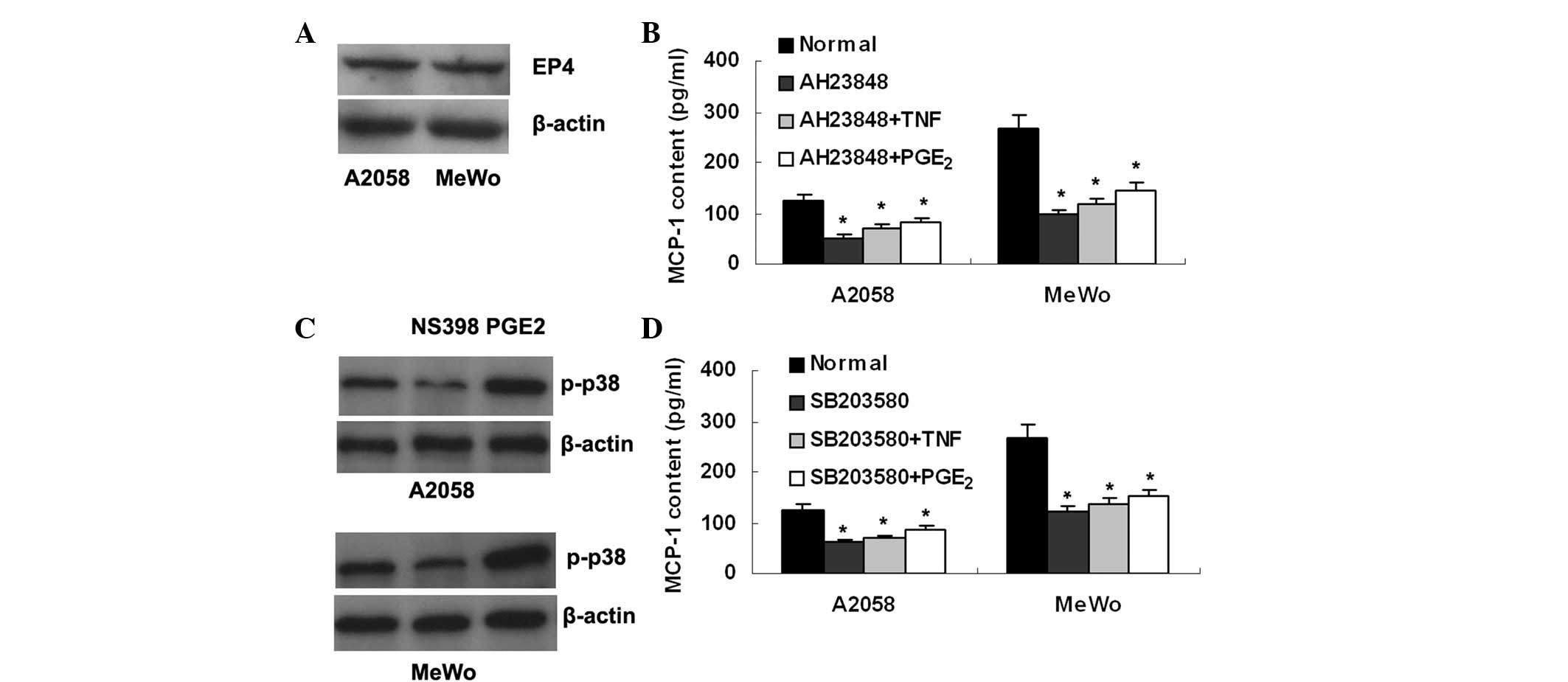 | Figure 5.EP4/p38 MAPK signaling is involved in
MCP-1 upregulation by PGE2. (A) The PGE2
receptor, EP4, is shown in A2058 and MeWo melanoma cells by western
blot analysis. (B) The EP4 antagonist, AH23848, reduces MCP-1
production in melanoma cells, and blocks the increased MCP-1
production induced by TNF-α or exogenous PGE2. (C)
Expression of p-p38 MAPK is suppressed by NS398, whereas it is
enhanced by exogenous PGE2 in the melanoma A2058 and
MeWo cells. (D) The p38 MAPK inhibitor, SB203580, decreases MCP-1
production in melanoma cells and abrogates the increased MCP-1
production induced by TNF-α or exogenous PGE2.
*P<0.01 vs. normal. MCP-1, macrophage chemoattractant
protein-1; PGE2, prostaglandin E2. |
Next we attempted to identify the intracellular
molecule mediating PGE2/EP4 signaling in the
upregulation of MCP-1. It was found that expression of
phosphorylated p38 MAPK (p-p38) was suppressed by NS398, whereas it
was enhanced by exogenous PGE2, in mealnoma cells
(Fig. 5C). Furthermore, the p38
MAPK inhibitor SB203580 was revealed to suppress MCP-1 production
in melanoma cells, and as with AH23848, SB203580 abrogated MCP-1
upregulation caused by TNF-α or exogenous PGE2 (Fig. 5D). Overall, these data suggest that
p38 MAPK may be an intracellular signal molecule linking
PGE2/EP4 with MCP-1.
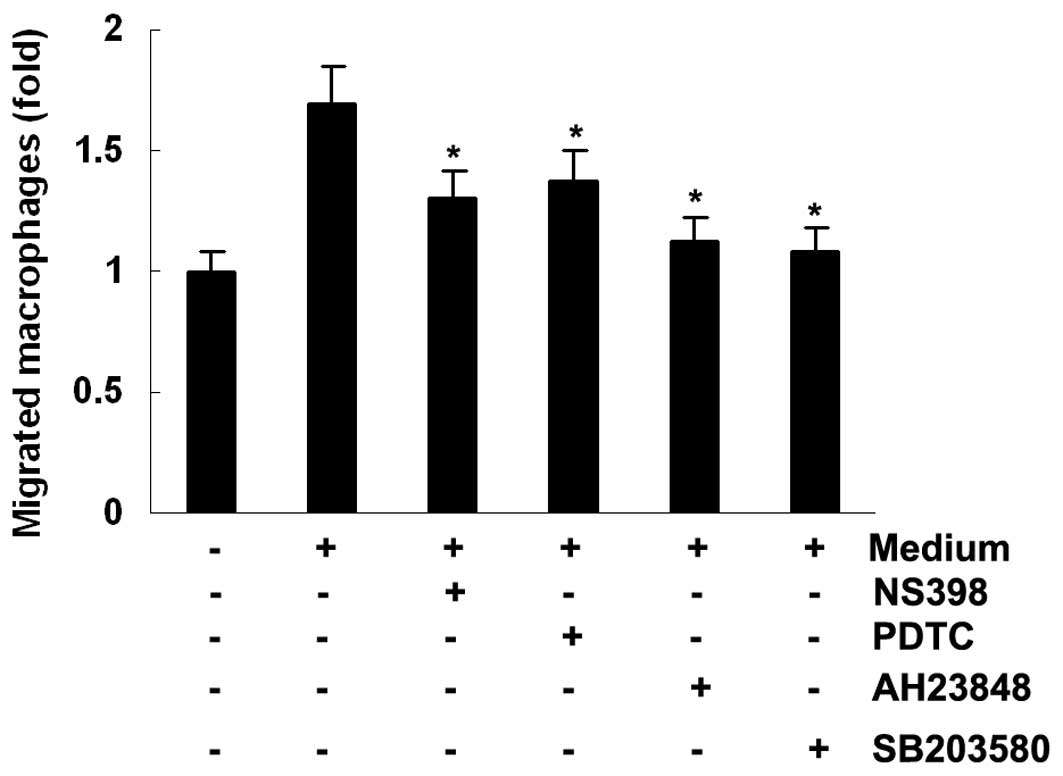
Macrophage migration assays
To investigate whether MCP-1 expression is
associated with macrophage chemotaxis, macrophage migration was
analyzed in transwell inserts in vitro. On addition of
conditioned medium from the MeWo melanoma cell line to the lower
chamber, the number of migrated macrophages increased 1.7-fold.
However, the increased macrophage migration was suppressed by
conditioned medium from melanoma cells treated with NS398, PDTC,
AH23848 and SB203580 (Fig. 6).
Discussion
It has been well established that the normal level
of PGE2 is indispensable to the maintenance of the
functional integrity of the gastrointestinal tract, kidney and
cardiovascular system, whereas excessive production of
PGE2 is frequently linked to certain inflammatory
conditions, including rheumatoid arthritis and osteoarthritis
(20). As a known pro-inflammatory
mediator, PGE2 is responsible for inflammatory pain and
fever, the most common signs of inflammatory disease. However,
PGE2 also exhibits anti-inflammatory properties, as it
has been observed to inhibit the production of inflammatory
cytokine TNF-α by macrophages (21,22).
In contrast to the potentially dual roles of PGE2 in
inflammation, PGE2 is observed to play a single
pro-tumor role in the pathogenesis of human cancer (23).
In the present study, two melanoma cell lines were
found to secrete a detectable amount of PGE2 into the
culture supernatant. Furthermore, our study revealed that
PGE2 secretion was suppressed by a COX-2 inhibitor or
COX-2 siRNA. These data indicate that production of endogenous
PGE2 in melanoma cells is dependent on COX-2 expression.
The mechanisms underlying COX-2 overexpression in melanoma cells
are largely unknown. In physiological conditions, COX-2 was not
expressed in the majority of tissues. However, COX-2 expression may
be induced significantly by certain stimuli, including inflammatory
cytokines and growth factors. In the current study, PGE2
production was observed to be markedly suppressed by an NFκB
inhibitor, suggesting that NFκB activation may play an important
role in COX-2 expression as well as in PGE2 production
in melanoma.
The roles of PGE2 in melanoma have not
been widely investigated, despite several recent studies
demonstrating that PGE2 is involved in melanoma invasion
and migration (13–15). In this study, several lines of
evidence demonstrate that PGE2 is a potential inducer of
MCP-1 expression in melanoma. Firstly, the level of COX-2 mRNA was
positively correlated with that of MCP-1 mRNA in the melanoma
specimens. Secondly, MCP-1 expression was downregulated
significantly in melanoma cells treated with a COX-2 inhibitor or a
COX-2 siRNA plasmid to reduce PGE2 production. Thirdly,
pre-treatment with a COX-2 inhibitor clearly abrogated
TNF-α-induced MCP-1 upregulation in melanoma cells. Finally, MCP-1
expression was elevated in melanoma cells treated with exogenous
PGE2. The involvement of PGE2 in MCP-1
regulation has also been observed in other organs by two recent
animal studies. It has been demonstrated that overproduction of
PGE2 was able to upregulate MCP-1 expression in the
stomach (19). However, inhibition
of PGE2 production by a COX-2 inhibitor or by COX-2
knockdown significantly reduced MCP-1 expression in several organs
in mice (24).
There are four G protein-coupled receptors (GPCRs)
that bind extracellular PGE2 molecules, EP1-4. EP4 is
extensively involved in tumor growth, angiogenesis and metastasis
(25–27). The intracellular signaling pathway
initiated by PGE2/EP4 is mainly mediated by cAMP/protein
kinase A (PKA) (27). In addition,
phosphatidylinositol 3-kinase/protein kinase B (PI3K/PKB) (28) and extracellular-signal regualted
kinase (ERK) (29) have been
implicated in this process. In melanoma, Vaid et al
demonstrated that PGE2/EP4-ERK signaling is correlated
with melanoma invasion and migration (14). In the present study, we revealed
that EP4/p38 MAPK signaling is involved in PGE2-induced
MCP-1 upregulation in an auto-crinal manner, based on the findings
that the EP4 antagonist and p38 MAPK inhibitor blocked MCP-1
induction and macrophage migration toward MCP-1. These data suggest
that PGE2/EP4 may play distinct roles in melanoma via
different intracellular signals. Our results are consistent with
another study, which indicated that EP4 mediates MCP-1 upregulation
and consequent macrophage recruitment in the stomach (19).
The recruitment of tumor-associated macrophages
(TAMs) has been recognized as one of the hallmarks of human cancer.
TAMs have positive roles in carcinogenesis, via the production of
growth factors, cytokines, chemokines and extracellular matrix
degrading enzymes. TAMs are derived from circulating monocytes,
which are attracted to tumor tissues by chemokines, including
MCP-1. As one of the most important macrophage attractants, MCP-1
is overexpressed in a number of types of human cancer (30) and is produced by tumor cells, T
lymphocytes, endothelial cells or other stromal cells in the tumor
microenvironment (30). However,
the mechanisms underlying MCP-1 overexpression in human tumors
remain unclear, in particular in melanoma. The present study
demonstrated that endogenous PGE2 may be an important
stimulator for MCP-1 expression, which is speculated to be involved
in macrophage infiltration in melanoma. This is due to the in
vitro finding that MCP-1-conditioned melanoma medium enhanced
macrophage migration; however, the increased migration was
substantially suppressed when MCP-1 expression in melanoma cells
was downregulated by inhibitors of the PGE2/EP4/p38 MAPK
signaling pathway.
In addition to the pro-tumor role caused by TAMs,
MCP-1 is also capable of exerting an inhibitory effect on
tumorigenesis, partly via the recruitment of NK cells and CTLs.
This is the case with melanoma, as MCP-1 expression has been
observed to be associated with the recruitment of CTLs and NK cells
in vitro and in vivo(31). Additionally, animal studies have
demonstrated that MCP-1-induced CTL recruitment promotes melanoma
cell apoptosis (32), whereas MCP-1
deficiency, which is associated with a decreased infiltration of
CTLs and NK cells, enhances melanoma growth and lung metastasis
(18). These results indicate that
PGE2, as an endogenous stimulator for MCP-1, may play
dual, or even more complex, roles in melanoma.
Acknowledgements
This study was supported by a project
from the Division of Education, Liaoning, China (2009A743).
References
|
1.
|
Thun MJ, Namboodiri MM and Heath CW Jr:
Aspirin use and reduced risk of fatal colon cancer. N Engl J Med.
325:1593–1596. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Kawamori T, Rao CV, Seibert K and Reddy
BS: Chemopreventive activity of celecoxib, a specific
cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer
Res. 58:409–412. 1998.PubMed/NCBI
|
|
3.
|
Harris RE, Alshafie GA, Abou-Issa H and
Seibert K: Chemoprevention of breast cancer in rats by celecoxib, a
cyclooxygenase 2 inhibitor. Cancer Res. 60:2101–2103.
2000.PubMed/NCBI
|
|
4.
|
Half E, Tang XM, Gwyn K, Sahin A, Wathen K
and Sinicrope FA: Cyclooxygenase-2 expression in human breast
cancers and adjacent ductal carcinoma in situ. Cancer Res.
62:1676–1681. 2002.PubMed/NCBI
|
|
5.
|
Hida T, Yatabe Y, Achiwa H, Muramatsu H,
Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T and Takahashi
T: Increased expression of cyclooxygenase 2 occurs frequently in
human lung cancers, specifically in adenocarcinomas. Cancer Res.
58:3761–3764. 1998.PubMed/NCBI
|
|
6.
|
Okami J, Yamamoto H, Fujiwara Y, Tsujie M,
Kondo M, Noura S, Oshima S, Nagano H, Dono K, Umeshita K, Ishikawa
O, et al: Overexpression of cyclooxygenase-2 in carcinoma of the
pancreas. Clin Cancer Res. 5:2018–2024. 1999.PubMed/NCBI
|
|
7.
|
Oshima H, Oshima M, Inaba K and Taketo MM:
Hyperplastic gastric tumors induced by activated macrophages in
COX-2/mPGES-1 transgenic mice. EMBO J. 23:1669–1678. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Krysan K, Reckamp KL, Dalwadi H, Sharma S,
Rozengurt E, Dohadwala M and Dubinett SM: Prostaglandin
E2 activates mitogen-activated protein kinase/Erk
pathway signaling and cell proliferation in non-small cell lung
cancer cells in an epidermal growth factor receptor-independent
manner. Cancer Res. 65:6275–6281. 2005.
|
|
9.
|
Han C and Wu T: Cyclooxygenase-2-derived
prostaglandin E2 promotes human cholangiocarcinoma cell
growth and invasion through EP1 receptor-mediated activation of the
epidermal growth factor receptor and Akt. J Biol Chem.
280:24053–24063. 2005.PubMed/NCBI
|
|
10.
|
Buchanan FG, Wang D, Bargiacchi F and
DuBois RN: Prostaglandin E2 regulates cell migration via
the intracellular activation of the epidermal growth factor
receptor. J Biol Chem. 278:35451–35457. 2003.PubMed/NCBI
|
|
11.
|
Chang SH, Liu CH, Conway R, Han DK,
Nithipatikom K, Trifan OC, Lane TF and Hla T: Role of prostaglandin
E2-dependent angiogenic switch in cyclooxygenase
2-induced breast cancer progression. Proc Natl Acad Sci USA.
101:591–596. 2004.
|
|
12.
|
Denkert C, Köbel M, Berger S, Siegert A,
Leclere A, Trefzer U and Hauptmann S: Expression of cyclooxygenase
2 in human malignant melanoma. Cancer Res. 61:303–308.
2001.PubMed/NCBI
|
|
13.
|
Singh T and Katiyar SK: Green tea
catechins reduce invasive potential of human melanoma cells by
targeting COX-2, PGE2 receptors and
epithelial-to-mesenchymal transition. PLoS One. 6:e252242011.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vaid M, Singh T and Katiyar SK: Grape seed
proanthocyanidins inhibit melanoma cell invasiveness by reduction
of PGE2 synthesis and reversal of
epithelial-to-mesenchymal transition. PLoS One. 6:e215392011.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Singh T, Vaid M, Katiyar N, Sharma S and
Katiyar SK: Berberine, an isoquinoline alkaloid, inhibits melanoma
cancer cell migration by reducing the expressions of
cyclooxygenase-2, prostaglandin E2 and prostaglandin
E2 receptors. Carcinogenesis. 32:86–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Torisu H, Ono M, Kiryu H, Furue M, Ohmoto
Y, Nakayama J, Nishioka Y, Sone S and Kuwano M: Macrophage
infiltration correlates with tumor stage and angiogenesis in human
malignant melanoma: possible involvement of TNFalpha and IL-1alpha.
Int J Cancer. 85:182–188. 2000. View Article : Google Scholar
|
|
17.
|
Varney ML, Johansson SL and Singh RK:
Tumour-associated macrophage infiltration, neovascularization and
aggressiveness in malignantmelanoma: role of monocyte chemotactic
protein-1 and vascular endothelial growth factor-A. Melanoma Res.
15:417–425. 2005. View Article : Google Scholar
|
|
18.
|
Nakasone Y, Fujimoto M, Matsushita T,
Hamaguchi Y, Huu DL, Yanaba M, Sato S, Takehara K and Hasegawa M:
Host-derived MCP-1 and MIP-1α regulate protective anti-tumor
immunity to localized and metastatic B16 melanoma. Am J Pathol.
180:365–374. 2012.
|
|
19.
|
Oshima H, Hioki K, Popivanova BK, Oguma K,
Van Rooijen N, Ishikawa TO and Oshima M: Prostaglandin
E2 signaling and bacterial infection recruit
tumor-promoting macrophages to mouse gastric tumors.
Gastroenterology. 140:596–607.e7. 2011.
|
|
20.
|
Siegle I, Klein T, Backman JT, Saal JG,
Nüsing RM and Fritz P: Expression of cyclooxygenase 1 and
cyclooxygenase 2 in human synovial tissue: differential elevation
of cyclooxygenase 2 in inflammatory joint diseases. Arthritis
Rheum. 41:122–129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Ratcliffe MJ, Walding A, Shelton PA,
Flaherty A and Dougall IG: Activation of E-prostanoid4 and
E-prostanoid2 receptors inhibits TNF-alpha release from human
alveolar macrophages. Eur Respir J. 29:986–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fennekohl A, Sugimoto Y, Segi E, Maruyama
T, Ichikawa A and Püschel GP: Contribution of the two Gs-coupled
PGE2-receptors EP2-receptor and EP4-receptor to the
inhibition by PGE2 of the LPS-induced TNFalpha-formation
in Kupffer cells from EP2-or EP4-receptor-deficient mice. Pivotal
role for the EP4-receptor in wild type Kupffer cells. J Hepatol.
36:328–334. 2002.PubMed/NCBI
|
|
23.
|
Wang MT, Honn KV and Nie D:
Cyclooxygenases, prostanoids, and tumor progression. Cancer
Metastasis Rev. 26:525–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Fujita M, Kohanbash G, Fellows-Mayle W,
Hamilton RL, Komohara Y, Decker SA, Ohlfest JR and Okada H: COX-2
blockade suppresses gliomagenesis by inhibiting myeloid-derived
suppressor cells. Cancer Res. 71:2664–2674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chell SD, Witherden IR, Dobson RR,
Moorghen M, Herman AA, Qualtrough D, Williams AC and Paraskeva C:
Increased EP4 receptor expression in colorectal cancer progression
promotes cell growth and anchorage independence. Cancer Res.
66:3106–3113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zhang Y and Daaka Y: PGE2
promotes angiogenesis through EP4 and PKA Cγ pathway. Blood.
118:5355–5364. 2011.
|
|
27.
|
Timoshenko AV, Xu G, Chakrabarti S, Lala
PK and Chakraborty C: Role of prostaglandin E2 receptors
in migration of murine and human breast cancer cells. Exp Cell Res.
289:265–274. 2003.PubMed/NCBI
|
|
28.
|
Sheng H, Shao J, Washington MK and DuBois
RN: Prostaglandin E2 increases growth and motility of
colorectal carcinoma cells. J Biol Chem. 276:18075–18081.
2001.PubMed/NCBI
|
|
29.
|
Pozzi A, Yan X, Macias-Perez I, Wei S,
Hata AN, Breyer RM, Morrow JD and Capdevila JH: Colon carcinoma
cell growth is associated with prostaglandin E2/EP4
receptor-evoked ERK activation. J Biol Chem. 279:29797–29804. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Conti I and Rollins BJ: CCL2 (monocyte
chemoattractant protein-1) and cancer. Semin Cancer Biol.
14:149–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Harlin H, Meng Y, Peterson AC, Zha Y,
Tretiakova M, Slingluff C, McKee M and Gajewski TF: Chemokine
expression in melanoma metastases associated with CD8+
T-cell recruitment. Cancer Res. 69:3077–3085. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Zhang T, Somasundaram R, Berencsi K,
Caputo L, Gimotty P, Rani P, Guerry D, Swoboda R and Herlyn D:
Migration of cytotoxic T lymphocytes toward melanoma cells in
three-dimensional organotypic culture is dependent on CCL2 and
CCR4. Eur J Immunol. 36:457–467. 2006. View Article : Google Scholar : PubMed/NCBI
|