Introduction
Recently, in addition to conventional cytotoxic
antitumor drugs, molecular-targeted drugs that target genes
involved in the biological properties of cancer cells, such as
growth, invasion, progression and metastasis, have been developed.
Notably, the epidermal growth factor receptor (EGFR) has been
demonstrated to be overexpressed in various types of cancer,
including non-small-cell lung cancer (NSCLC), and to be involved in
the growth of cancer cells. The therapeutic potential of gefitinib,
which targets EGFR tyrosine kinase, was investigated in a
placebo-controlled phase III study (ISEL) in patients with advanced
or recurrent NSCLC who had received up to 2 regimens. Although the
superiority of gefitinib relative to the placebo was not
demonstrated (1), a subset analysis
revealed that this drug significantly prolonged the survival of
non-smokers and Asian individuals. These results were similar to
those of a phase II clinical study conducted prior to the ISEL
study (2). A prospective study has
demonstrated that a greater antitumor effect of gefitinib was
observed in patients with tumors carrying an EGFR mutation
(3,4). In a phase III study (IPASS) of
patients with previously untreated NSCLC, conducted mainly in Asian
countries, the usefulness of gefitinib therapy was compared with
that of carboplatin plus paclitaxel therapy. A subset analysis
revealed that in EGFR mutation-positive patients, the gefitinib
therapy significantly prolonged progression-free survival (PFS) as
compared with the carboplatin plus paclitaxel therapy. By contrast,
in patients who were EGFR mutation-negative, the carboplatin plus
paclitaxel therapy was found to significantly prolong the PFS
(5). Based on these results,
gefitinib is considered to be less effective in EGFR
mutation-negative patients.
Erlotinib, as with gefitinib, is an EGFR tyrosine
kinase inhibitor (EGFR-TKI). In a phase III study (BR.21) that
compared erlotinib with a placebo in the second- or third-line
treatment of NSCLC patients who were not responding to standard
chemotherapy, erlotinib was confirmed to significantly prolong
overall survival, PFS and the time to deterioration of lung
cancer-related symptoms (cough, dyspnea and pain) as a QOL measure
(6). Successful results were also
achieved in a combined analysis of two phase II clinical studies
(JO16565 and JO18396) conducted in Japan. The objective response
and disease control rates were 28% [95% confidence interval (CI),
20.0–37.9] and 49% (39.2–59.0), respectively, while the time to
progression was 10.7 weeks (8.1–18.3) and the overall survival time
was 13.8 months (11.4–18.1) (7).
While erlotinib is demonstrated to be effective in EGFR
mutation-positive patients (8,9), as is
gefitinib, it is also suggested to be effective in EGFR
mutation-negative patients (8,10).
However, the aforementioned findings were obtained
in clinical studies; in clinical practice, a large number of
patients who are excluded from clinical studies due to their
characteristics are also treated. Recently, increasing numbers of
advanced or recurrent NSCLC patients have received ≥2 regimens of
chemotherapy due to the advances in chemotherapy, but adequate
information is not available from clinical studies of the
conventional second-line treatment. EGFR-TKIs in particular, which
are orally administered, are frequently used for lung cancer
treatment as their toxicity is typically low, although severe
adverse reactions to the drug, including interstitial lung disease,
have been demonstrated. However, studies investigating their
efficacy and safety in Japanese individuals under conditions of
actual use are limited (11).
Hence, we set out to investigate the usefulness of erlotinib in
lung cancer treatment by collecting and analyzing data from all
patients receiving erlotinib, irrespective of their individual
characteristics.
Patients and methods
Patients
Fourteen sites (17 departments) in Ibaraki (area,
6095 km2; population, ∼3 million) were involved in the
current study, which included patients who were treated with
erlotinib at these sites between December 2007 and December 2010.
All patients demonstrated histological or cytological evidence of
NSCLC. Histopathological diagnoses were defined by the World Health
Organization (WHO) classification system, and patients were staged
according to the Union for International Cancer Control (UICC)
tumor node metastasis (TNM) staging system.
The patient characteristics, efficacy and safety
were evaluated using patient data extracted from the database of
each site. Tumor responses were classified as a complete response
(CR), a partial response (PR), stable disease (SD), progressive
disease (PD) or not evaluable (NE), according to the response
evalution criteria in solid tumors (RECIST), version 1.1.
The present observational study conformed to the
Ethical Guidelines for Clinical Studies issued by the Ministry of
Health, Labor and Welfare of Japan.
Statistical analysis
The patient survival time was calculated from the
day of erlotinib therapy intitiation to the death or latest
follow-up time of the patient. The survival rate was analyzed by
the Kaplan-Meier method and comparisons were performed using the
log-rank test. P<0.005 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The final data set consisted of 307 patients. The
patient characteristics are shown in Table I. The median age of the patients was
67 years (range, 32–91) and 34.2% of patients were female. Of the
patients, 75.9% had adenocarcinoma, while the performance status
(PS) was 0 or 1, 2, and 3 or 4 in 67.4, 20.5 and 12.1% of patients,
respectively. In total, 17.9% of patients were EGFR
mutation-positive and 27.7% were negative. Of the patients, 34.5%
had never smoked and 63.2% had a history of smoking, while 70.4%
received erlotinib as a third-line or subsequent treatment.
 | Table I.Characteristics of 307 patients. |
Table I.
Characteristics of 307 patients.
| Characteristic | Number | Percentage |
|---|
| Age (years) | | |
| Median | 67 | |
| Range | 32–91 | |
| Gender | | |
| Male | 202 | 65.8 |
| Female | 105 | 34.2 |
| Histological
type | | |
| Adenocarcinoma | 233 | 75.9 |
| Squamous cell
carcinoma | 42 | 13.7 |
| Other | 32 | 10.4 |
| Tumor stage | | |
| I–IIIA | 23 | 7.5 |
| IIIB | 43 | 14.0 |
| IV | 177 | 57.7 |
| Postoperative
recurrence | 64 | 20.8 |
| PS | | |
| 0 | 82 | 26.7 |
| 1 | 125 | 40.7 |
| 2 | 63 | 20.5 |
| 3 | 30 | 9.8 |
| 4 | 7 | 2.3 |
| Smoking history | | |
| Non-smoker | 106 | 34.5 |
| Former smoker | 178 | 58.0 |
| Current smoker | 16 | 5.2 |
| Unknown | 7 | 2.3 |
| EGFR mutation | | |
| Positive | 55 | 17.9 |
| Negative | 85 | 27.7 |
| Unknown | 167 | 54.4 |
| Metastatic focus | | |
| Lung | 121 | 39.4 |
| Liver | 48 | 15.6 |
| Lymph node | 124 | 40.4 |
| Bone | 105 | 34.2 |
| Adrenal | 6 | 2.0 |
| Other | 79 | 25.7 |
| Treatment line | | |
| First-line | 20 | 6.5 |
| Second-line | 71 | 23.1 |
| Third-line | 76 | 24.8 |
| Fourth-line or
subsequent | 140 | 45.6 |
Therapeutic effect
The tumor response rate was 11.1% (CR, 1.3%; PR,
9.8%) in the patients receiving erlotinib. SD was observed in 35.2%
of patients and the disease control rate was 46.3%.
Toxicity
The most common adverse event was skin disorder,
which occurred in 177 patients (57.7%); 154 (50.2%) patients
developed a grade 1 or 2 skin disorder. The second most common
adverse event was diarrhea; occurring in 53 (17.3%) patients; 51
(16.6%) patients developed diarrhea of grade 1 or 2. Liver disorder
was observed in 18 (5.9%) patients, none of which were grade 5.
Interstitial lung disease was observed in 21 (6.8%) patients, two
(0.7%) of whom developed an interstitial lung disease of grade
5.
Survival analysis
Of the 307 patients, 242 (78.8%) did not survive to
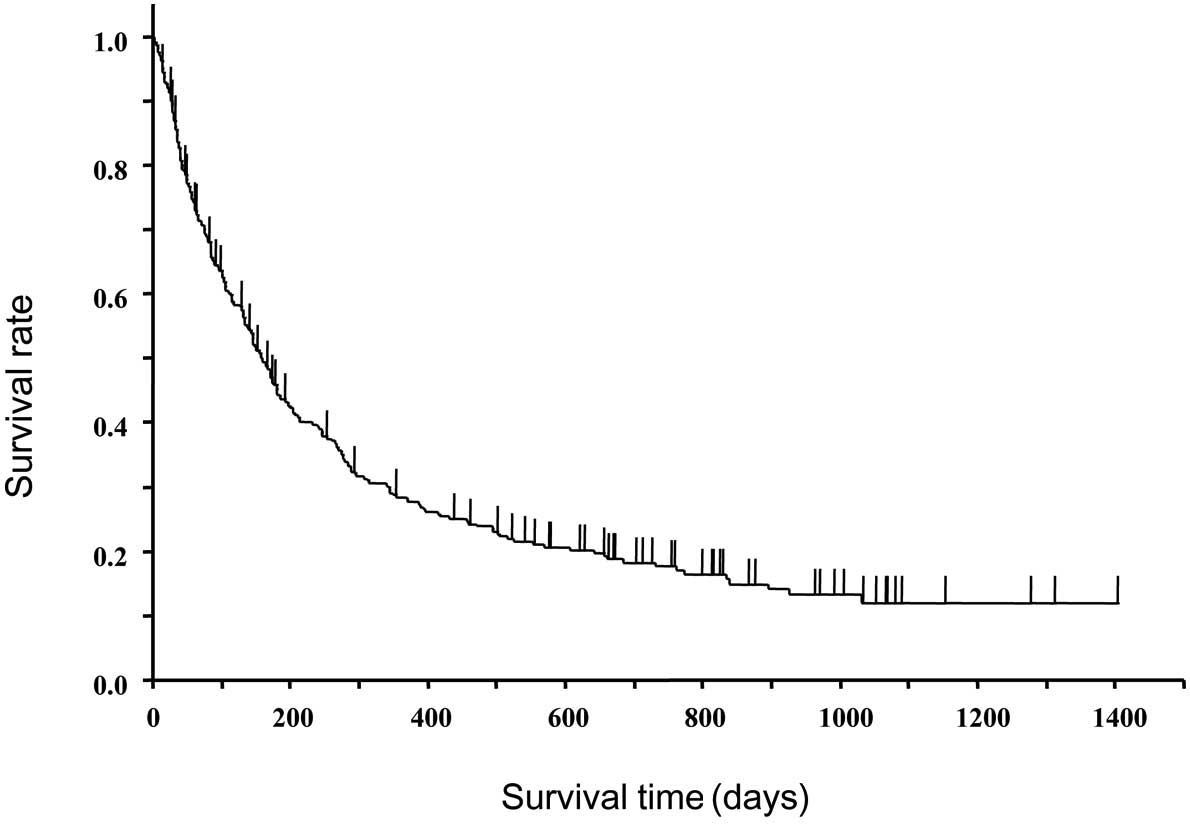
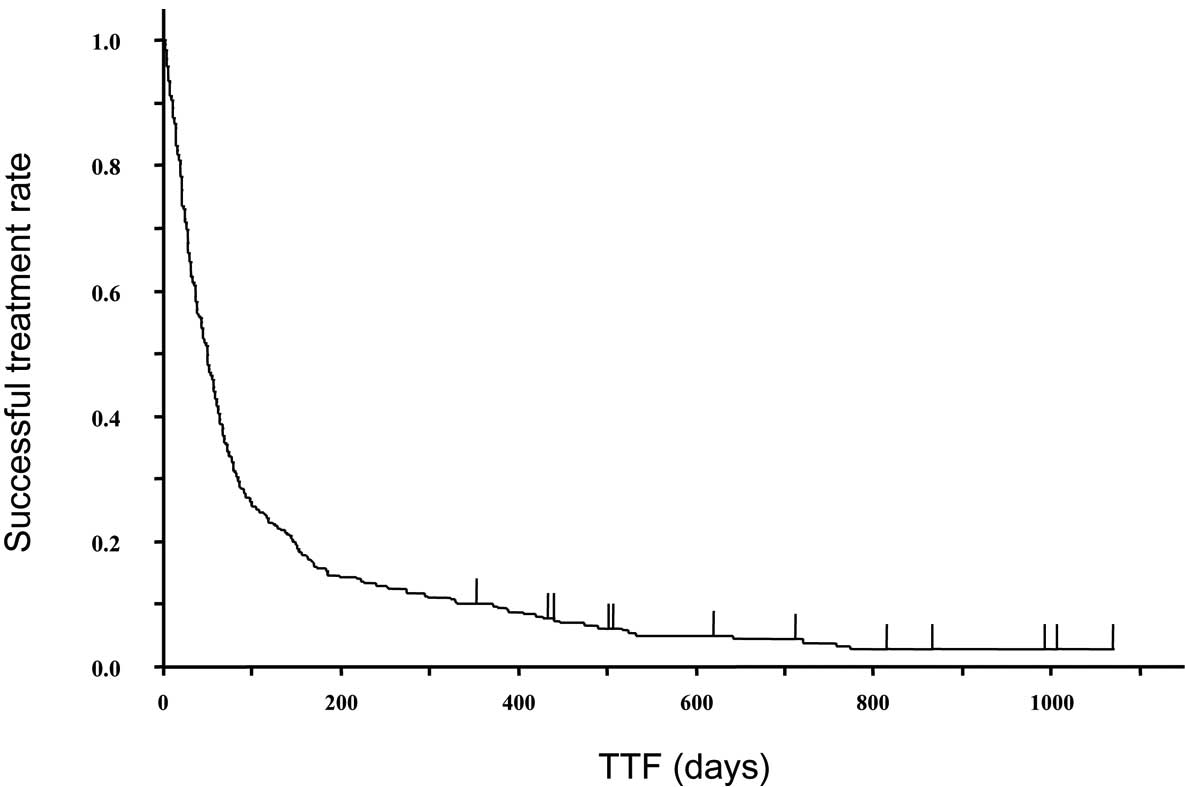
the point of analysis. Figs. 1 and
2 show the survival curve and time
to treatment failure (TTF) of the 307 patients treated with
erlotinib. The median TTF and survival time were 1.6 months (95%
CI, 41–57 days) and 5.3 months (134–181 days) in all patients,
respectively. In male and female patients, the median TTFs were 1.5
months (95% CI, 36–56 days) and 1.7 months (40–68 days), and the
median survival times were 4.9 months (127–180 days) and 5.8 months
(131–270 days), respectively; no significant differences were
observed in both of these measurements (P=0.05 and P=0.1008,
respectively).
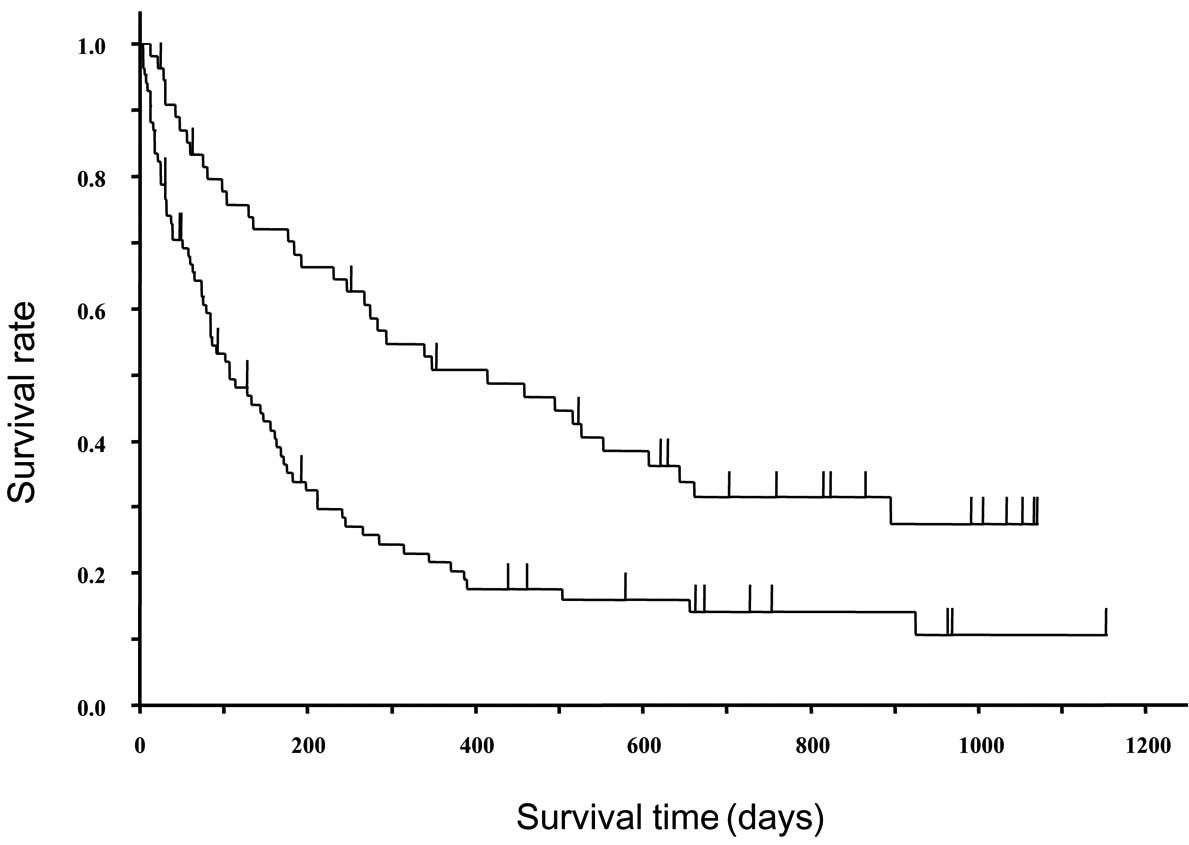
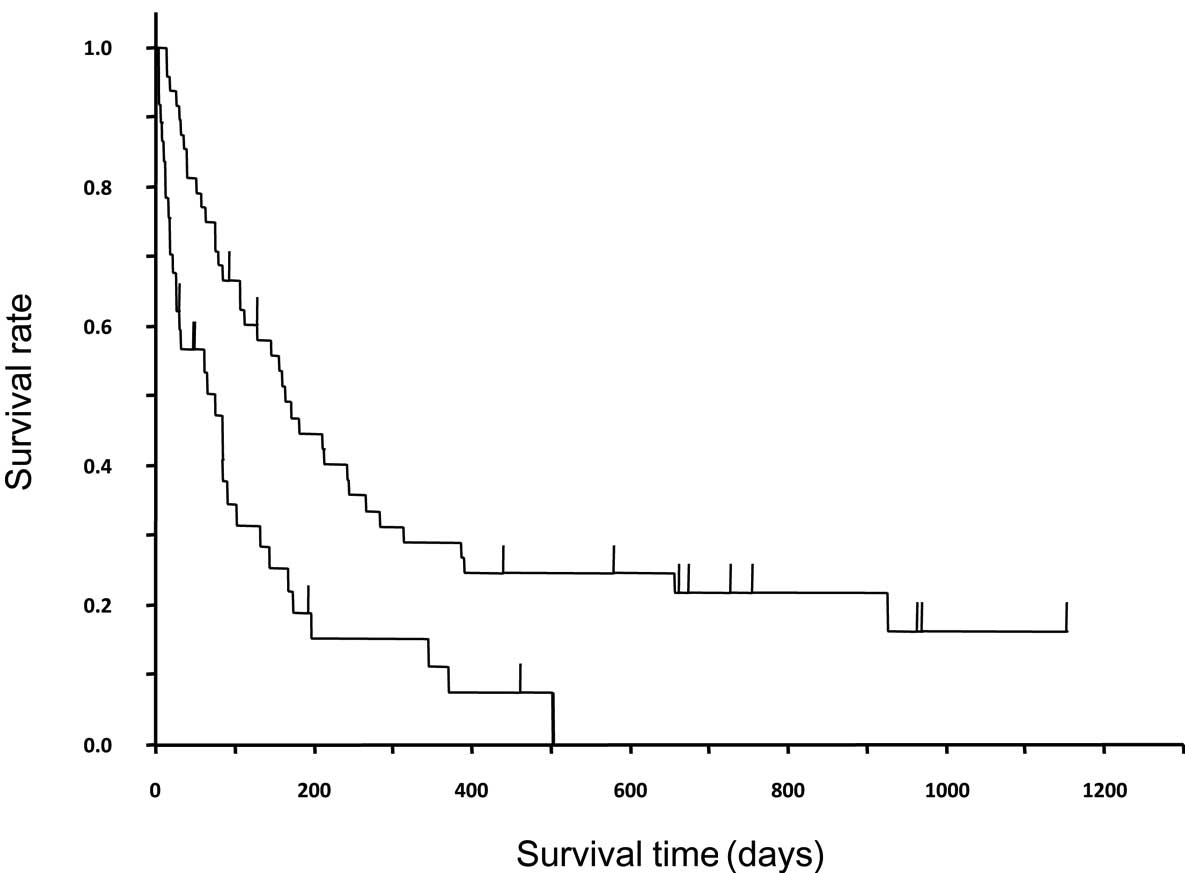
Fig. 3 shows the
survival curves of EGFR mutation-positive and -negative patients.
In EGFR mutation-positive and -negative patients, the median TTFs
were 3.9 months (95% CI, 63–183 days) and 1.0 month (21–43 days),
while the median survival times were 13.8 months (247–607 days) and
3.5 months (75–163 days), respectively; significant differences
were observed in both of these characteristics (P<0.0001 and
P=0.0002, respectively). In the EGFR mutation-positive patients, an
earlier line of treatment typically resulted in a longer TTF; the
TTFs were 721, 302, 142 and 73 days in the first-, second-, third-
and fourth-line or subsequent treatments, respectively. However, in
the EGFR mutation-negative patients, no significant differences
were identified in TTF between the treatment lines; the TTFs were
34.5, 27, 40.5 and 29 days in the first-, second-, third- and
fourth-line or subsequent treatments, respectively. In patients
with and without a history of smoking, the median TTFs were 1.5
months (95% CI, 37–56 days) and 1.8 months (37–66 days), and the
median survival times were 4.8 months (113–180 days) and 6.0 months
(134–276 days), respectively. A significant difference was observed
between survival times (P=0.0159). Fig.
4 shows the survival curves of patients with or without a skin
rash as a complication of treatment with erlotinib. In patients
with and without a rash, the median TTFs were 2.2 months (95% CI,
58–78 days) and 0.9 months (22–33 days), and the median survival
times were 8.2 months (181–88 days) and 2.8 months (60–114 days),
respectively. Significant differences were observed for both TTF
and survival time between patients with and without a rash
(P<0.0001 for both comparisons). The univariate analysis of the
correlations between TTF and variables, including gender, age,
treatment line, rash development and tumor response, showed that
smoking history, PS, histological type, rash development and
response to treatment were significant prognostic factors (Table II). The multivariate analysis of the
correlation between survival and the aforementioned six factors
showed that PS 0–1, rash development, at least a PR and at least SD
were independent prognostic factors (Table III).
 | Table II.Univariate analysis for survival. |
Table II.
Univariate analysis for survival.
| Characteristic | 1 | 2 | P-valuea | Hazard ratio | 95% CI |
|---|
| Smoking history | Yes | No | 0.0147 | 0.717 | 0.544–0.937 |
| PS | ≥2 | 0/1 | <0.0001 | 0.341 | 0.259–0.450 |
| Histological
type |
Non-adenocarcinoma | Adenocarcinoma | <0.0001 | 0.548 | 0.414–0.733 |
| Rash | No | Yes | <0.0001 | 0.459 | 0.355–0.595 |
| RR | SD/PD/NE | CR/PR | <0.0001 | 0.199 | 0.109–0.331 |
| DCR | PD/NE | CR/PR/SD | <0.0001 | 0.346 | 0.266–0.450 |
 | Table III.Multivariate analysis for
survival. |
Table III.
Multivariate analysis for
survival.
| Characteristic | 1 | 2 | P-valuea | Hazard ratio | 95% CI |
|---|
| PS | ≥2 | 0/1 | <0.0001 | 0.394 | 0.295–0.528 |
| Rash | No | Yes | 0.0002 | 0.596 | 0.455–0.782 |
| RR | SD/PD/NE | CR/PR | <0.0001 | 0.328 | 0.176–0.565 |
| DCR | PD/NE | CR/PR/SD | <0.0001 | 0.437 | 0.329–0.577 |
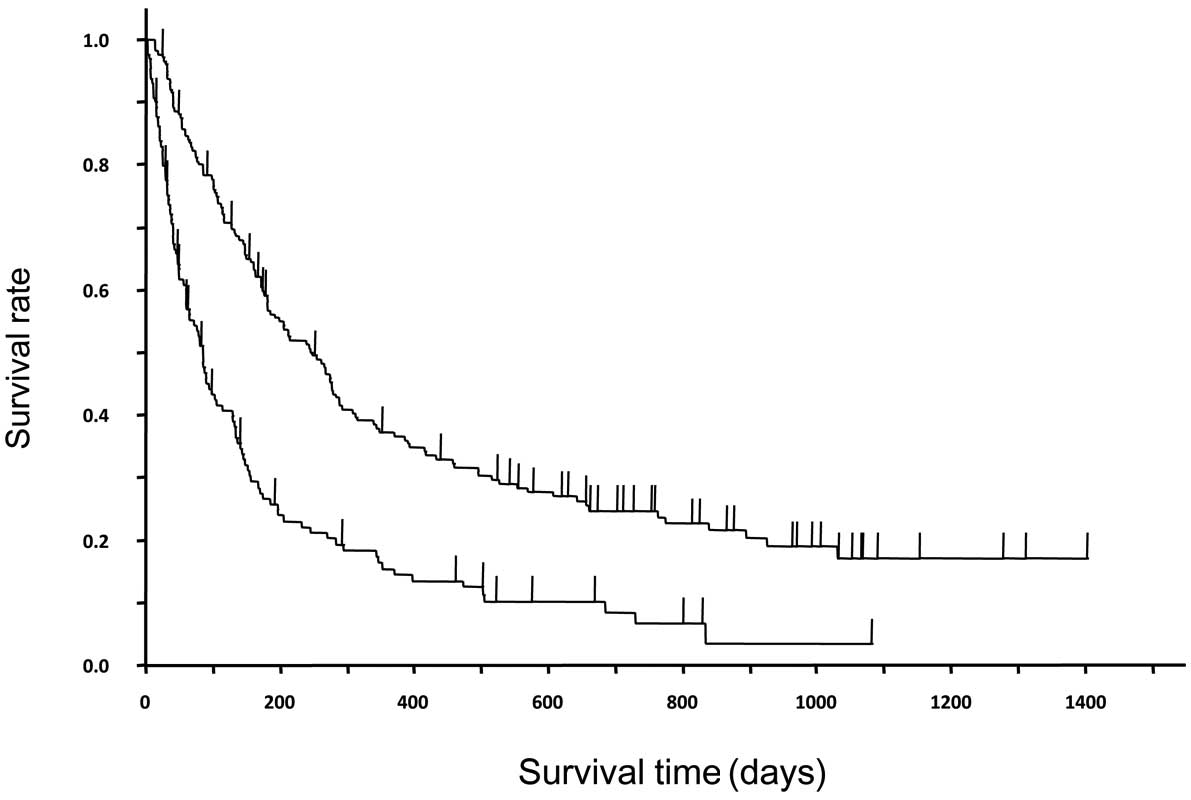
In the EGFR mutation-negative patients, there were
significant differences in TTF (P=0.0003) and survival (P=0.0009)
between those with and without a rash; the median values were 1.5
months (95% CI, 31–62 days) and 5.4 months (106–245 days) in the
former, and 0.7 months (12–26 days) and 2.5 months (25–102 days) in
the latter, respectively (Fig.
5).
Discussion
In a phase III study (BR.21) that compared erlotinib
with a placebo as the second- or third-line treatment of NSCLC
patients who were not responding to standard chemotherapy, patients
with a median age of 62 years receiving erlotinib had a median
survival of 6.67 months and a median PFS of 2.2 months. In a phase
III study (TITAN) that compared the efficacy of erlotinib with that
of docetaxel or pemetrexed in the second-line treatment of NSCLC
patients, the erlotinib group had a median overall survival of 5.3
months and a median PFS of 1.4 months (12). In the TITAN study, the median age of
the erlotinib group (59 years) was lower than that of the patients
in the present study. Additionally, only PS 0–2 patients were able
to enrol, and 81% of the patients in the erlotinib group were PS
0–1 patients. In the present study, the median age of the patients
was 67 years, the median survival time was of 5.3 months and the
median TTF was 1.6 months; however, 45.6% of patients received
erlotinib as a fourth-line or subsequent treatment. These results
were similar to those of clinical studies where eligibility
criteria for enrollment were established. As the present study has
demonstrated a difference in the effect of erlotinib depending on
the presence of a rash, we suggest that erlotinib may be more
effective in patients with a rash than in those without a rash.
Moreover, similar results were obtained in the EGFR
mutation-negative patients, suggesting that erlotinib may be
effective in EGFR mutation-negative patients, in whom EGFR-TKIs are
considered to be less effective, on developing a rash. The
incidence of adverse events in the present study was similar to
that of post-marketing surveillance study of erlotinib in Japan;
the rates of rash, liver disorder and diarrhea of grade 3 or more
were 7.5, 1.6 and 0.7% in the former and 7.8, 1.8 and 1.23% in the
latter, respectively. In contrast to the clinical studies that only
included patients with a relatively good PS, the present study
included all patients treated with erlotinib in clinical practice.
We consider it important to investigate the efficacy and
tolerability of erlotinib in this way.
References
|
1.
|
Thatcher N, Chang A, Parikh P, et al:
Gefitinib plus best supportive care in previously treated patients
with refractory advanced non-small cell lung cancer: results from a
randomised, placebo controlled, multicentre study (Iressa Survival
Evaluating in Lung Cancer). Lancet. 366:1527–1537. 2005. View Article : Google Scholar
|
|
2.
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small cell lung
cancer (The IDEAL 1 Trial). J Clin Oncol. 21:2237–2246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Inoue A, Kobayashi K, Usui K, et al:
First-line gefitinib for patients with advanced non-small-cell lung
cancer harboring epidermal growth factor receptor mutations without
indication for chemotherapy. J Clin Oncol. 27:1394–1400. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kobayashi K, Inoue A, Maemondo M, et al:
First line gefitinib versus first line chemotherapy by CBDCA plus
paclitaxel in NSCLC patients with EGFR mutations: a Phase III study
(002) by North East Japan (NEJ) Gefitinib study group. J Clin
Oncol. 27(Suppl): 15s2009.(abstr 8016).
|
|
5.
|
Mok T, Wu YL, Thongprasert S, et al: Phase
III, randomized, open-label, first-line study of gefitinib (G) vs
carboplatin/paclitaxel (C/P) in clinically selected patients (pts)
with advanced non-small-cell lung cancer (NSCLC) (IPASS). Ann
Oncol. 19(Suppl 8): abst LBA4. 2008.
|
|
6.
|
Bezjak A, Tu D, Seymour L, et al National
Cancer Institute of Canada Clinical Trials Group Study BR.21:
Symptom improvement in lung cancer patients treated with erlotinib:
quality of life analysis of the National Cancer Institute of Canada
Clinical Trials Group Study BR.21. J Clin Oncol. 24:3831–3837.
2006. View Article : Google Scholar
|
|
7.
|
Kubota K, Nishiwaki Y, Tamura T, et al:
Efficacy and safety of erlotinib as monotherapy for Japanese
patients with advanced non-small-cell lung cancer: a phase II
study. J Thoracic Oncol. 3:1439–1445. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pirker R, Su W, Rooneem R, et al: Clinical
outcome with erlotinib in relation to biomarker status: analysis
from the open-label TRUST study in advanced non-small-cell lung
cancer (NSCLC). Ann Oncol. 19(Suppl 8): 265P2008.
|
|
9.
|
Massuti B, Moran T, Porta R, et al:
Multicenter prospective trial of customized erlotinib for advanced
non-small-cell lung cancer (NSCLC) patients with epidermal growth
factor receptor (EGFR) mutations: Final results of the Spanish Lung
Cancer Group (SLCG) trial. J Clin Oncol. 27(Suppl): 15s2009.(abstr
8023).
|
|
10.
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al:
SATURN: A double-blind, randomized, phase III study of maintenance
erlotinib versus placebo following nonprogression with first-line
platinum-based chemotherapy in patients with advanced NSCLC. J Clin
Oncol. 27(Suppl): 15s2009.(abstr 8001).
|
|
11.
|
Hayashibara K, Satoh H, Shinohara Y, et
al: A population study of gefitinib in patients with non-small-cell
lung cancer. Med Oncol. 26:222–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ciuleanu T, Stelmakh L, Cicenas S, et al:
Efficacy and safety of erlotinib versus chemotherapy in second-line
treatment of patients with advanced, non-small-cell lung cancer
with poor prognosis (TITAN): a randomised multicentre, open-label,
phase 3 study. Lancet Oncol. 13:300–308. 2012. View Article : Google Scholar : PubMed/NCBI
|