Introduction
Gastrointestinal stromal tumors (GISTs) are a type
of cancer that develops in supportive or connective tissues of the
digestive system (1). The disease
generally affects adults aged 50–70 years, but gender predilection
is unclear. The most frequent site of occurrence is the stomach
(60% of cases), followed by the small bowel (35%) and other sites
(colon, rectum and esophagus; <5%) (2). They primarily arise from mesenchymal
tumors of the gastrointestinal tract. Previous evidence
demonstrated that most GISTs originate from Cajal pacemaker cells;
however, the presence of receptors in omental, mesentery and
uterine tumors has raised doubts about the exclusivity of their
origin from pacemaker cells (3–5). GISTs
express the cell surface transmembrane receptor KIT, which leads to
uncontrolled cell proliferation and resistance to apoptosis upon
activation (6–9). Tumor resection is one option for
treating the localized disease, but recurrence is common. Tyrosine
kinase inhibitors (TKIs) such as imatinib and sunitinib are the
standard therapy for metastatic or unresectable GISTs (10,11).
Usually, Response Evaluation Criteria in Solid Tumors (RECIST)
combined with imaging data (CT scan and PET) are used to assess
tumor response to treatment (12,13).
Case report
An 80-year-old female underwent several examinations
in August 2009 for a gall stone. A CT scan disclosed a gastric
mass. The patient underwent a partial gastric resection in
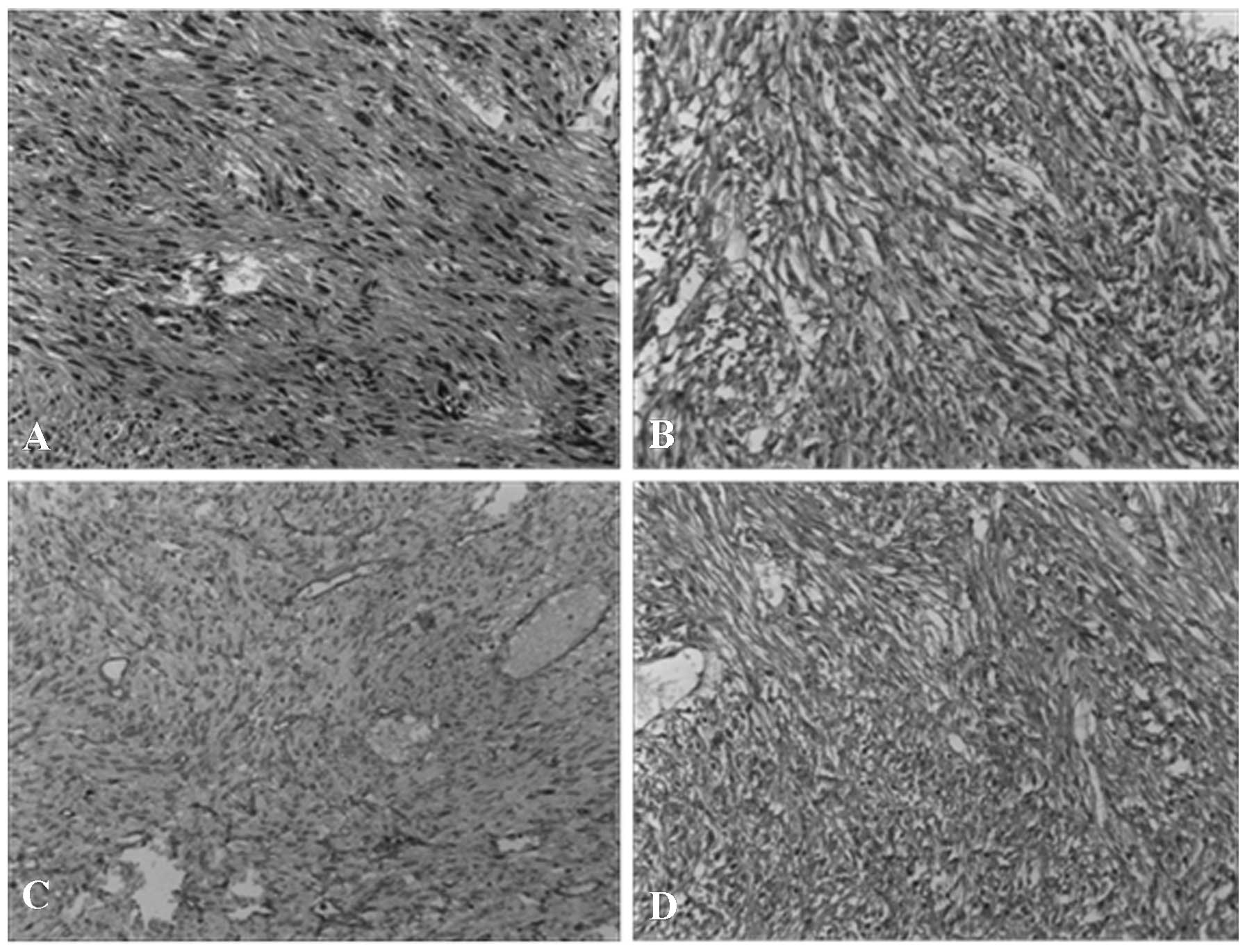
September 2009 (Fig. 1A). The tumor
size was 7.5×5 cm, and the immunohistochemical analysis revealed
the tumor was positive for CD117 (Fig.
1B), CD34 (Fig. 1C) and DOG-1
(Fig. 1D), but negative for S100.
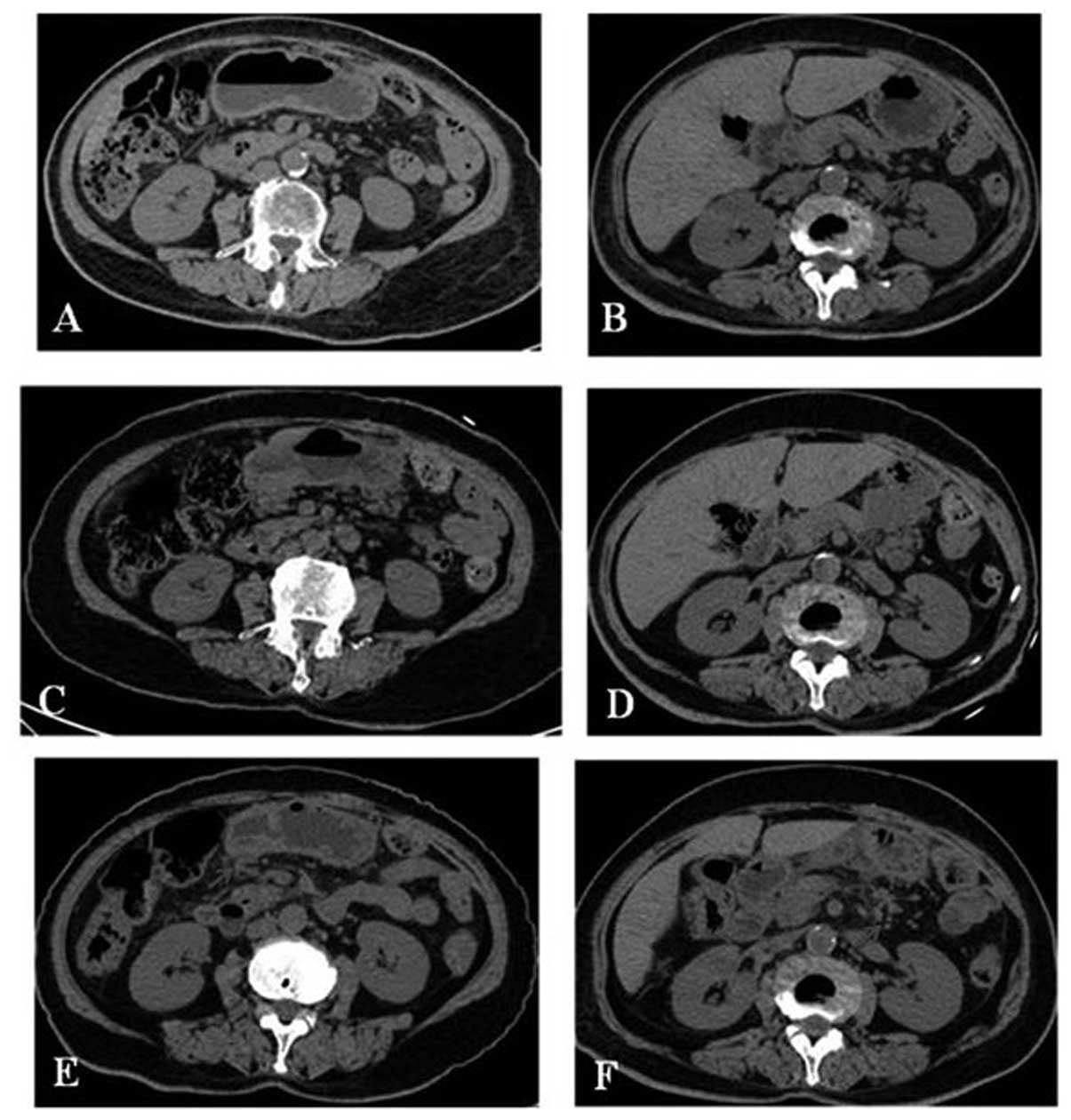
The patient started imatinib treatment at 400 mg/day and was
examined every three months (Fig. 2A
and B). She remained well, and stopped imatinib treatment in
March 2011. In June 2011, when the patient was referred to
Zhengzhou People’s Hospital, recurrence was documented in the
gastric remnant (Fig. 2C and D).
Beginning in July 2011, she was treated with sunitinib (37.5
mg/day), but demonstrated poor tolerance. She experienced frequent
lack of hunger, fatigue, somnolence, nausea and vomiting. In August
2011, she was hospitalized for fatigue. A CT scan presented
reductions in the size of the gastric mass and enlarged lymph nodes
(Fig. 2E and F). In August 2011,
the patient began to exhibit hematemesis and was hospitalized.
Later, she presented with digestive tract hemorrhage, and following
this, melena and bloody stool occurred. On September 4, 2011, the
patient’s hemoglobin concentration was 102 g/l. By September 6,
2011, the hemoglobin concentration was down to 76 g/l. Therefore,
conservative medical management was adopted. Hemorrhage stopped
gradually. Although the patient experienced gastrointestinal
bleeding complications, her treatment was effective. Thus, we
suggested continuing sunitinib treatment at a reduced dose or
participating in clinical trials of new drugs. The patient rejected
these suggestions. She is currently receiving best supportive care
(BSC), and follow-up is in progress. Written informed consent was
obtained from the patient for publication of this case report and
accompanying images.
Discussion
Pathogenetic mechanisms of GISTs are poorly
understood. KIT and PDGFRA mutations drive mesenchymal tumors,
including GISTs (gastrointestinal tract sarcomas). Histologically,
GISTs vary from spindled to epitheloid and mixed cell tumors. The
pathological features are different according to different sites.
Gastric GISTs appear as spindle cells and epitheloid cells, but
most small intestinal GISTs are spindle cells. Mutations in KIT or
PDGFRA lead to increased cellular proliferation and decreased
apoptosis. Approximately 85% of GISTs have mutations in KIT or
PDGFRA (14–17). Tumors with kinase mutations in exon
11 or 9 have a higher overall response to therapy with receptor
tyrosine kinase; therefore, these patients have a significantly
longer overall survival.
GISTs are often presented with related symptoms such
as anemia or mucosal ulcerations. The diagnostic evaluation is
determined by pathological examination. KIT (CD117) is a
transmembrane receptor which is a part of the tyrosine kinase
receptor complex. GISTs are typically immunoreactive for KIT, thus
the presence of CD117 confirms GIST diagnosis by
immunohistochemistry. Approximately 90–100% of GISTs express CD117,
and 70–80% are positive for CD34, which is the hematopoietic
progenitor cell antigen (3,18,19).
GISTs are not sensitive to conventional
chemotherapy. The response rate to chemotherapy is <10%.
However, targeted therapy has shown some promising results.
Imatinib mesylate (a TKI) is considered to be the standard
first-line agent in the treatment of unresectable or metastatic
GISTs (20). Imatinib, formerly
known as STI-571, has been shown to decrease the density of tumor
cells without causing inflammation or necrosis (21–23).
Sunitinib is an oral multi-targeted tyrosine kinase inhibitor with
activity against KIT, PDGFRs, VEGFRs, glial cell line-derived
neurotrophic factor receptor, colony-stimulating factor 1 receptor
(CSF-1R) and FMS-like tyrosine kinase-3 receptor (FLT3) (24–29).
Sunitinib appears to be an effective treatment for patients with
imatinib-resistant/intolerant GISTs (7).
TKI-associated side effects mainly include
nonhematological and hematological toxicities (30). TKI-associated side-effects affect
the curative effect. Thus, the appropriate management of
TKI-associated side-effects is important. However, systematic
research on the management of TKI-related toxicities remains
scarce.
In conclusion, the complication of digestive tract
hemorrhage in patients treated with sunitinib is rare. However,
this case demonstrates that it does occur. Thus, we should be
watchful of this complication in the clinic with sunitinib
treatment. Its mechanism remains unclear, therefore data on
molecular background, risk factors, treatment response and
prognostic significance should be collected in a larger patient
population and be further defined.
References
|
1.
|
Hauber AB, Gonzalez JM, Coombs J, et al:
Patient preferences for reducing toxicities of treatments for
gastrointestinal stromal tumor (GIST). Patient Prefer Adherence.
5:307–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Di Scioscio V, Greco L, Pallotti MC, et
al: Three cases of bone metastases in patients with
gastrointestinal stromal tumors. Rare Tumors. 3:e172011.
|
|
3.
|
Afuwape OO, Irabor DO and Ladipo JK:
Gastrointestinal stromal tumour in Ibadan, Nigeria: a case report
and review of current treatment. Afr Health Sci. 11:134–138.
2011.PubMed/NCBI
|
|
4.
|
Issar P, Dwivedi MK, Issar SK, Pal RK and
Dewanagan L: Malignant gastrointestinal stromal tumours. Indian J
Radiol Imaging. 16:65–67. 2006. View Article : Google Scholar
|
|
5.
|
Wingen CB, Pauwels PA, Debiec-Rychter M,
van Gemert WG and Vos MC: Uterine gastrointestinal stromal tumours
(GIST). Gynecol Oncol. 97:970–972. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
DeMatteo RP, Lewis JJ, Leung D, et al: Two
hundred gastrointestinal stromal tumors: recurrence patterns and
prognostic factors for survival. Ann Surg. 231:51–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chen YY, Yeh CN, Cheng CT, et al:
Sunitinib for Taiwanese patients with gastrointestinal stromal
tumor after imatinib treatment failure or intolerance. World J
Gastroenterol. 17:2113–2119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kindblom LG, Remotti HE, Aldenborg F, et
al: Gastrointestinal pacemaker cell tumor (GIPACT):
gastrointestinal stromal tumors show phenotypic characteristics of
the interstitial cells of Cajal. Am J Pathol. 152:1259–1269.
1998.
|
|
9.
|
Hirota S, Isozaki K, Moriyama Y, et al:
Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Demetri GD, von Mehren M, Blanke CD, et
al: Efficacy and safety of imatinib mesylate in advanced
gastronitestinal stromal tumors. N Engl J Med. 347:472–480. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Demetri GD, van Oosterom AT, Garrett CR,
et al: Efficacy and safety of sunitinib in patients with advanced
gastrointestinal stromal tumour after failure of imatinib: a
randomised controlled trial. Lancet. 368:1329–1338. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Choi H, Charnsangavej C, Faria SC, et al:
Correlation of computed tomography and positron emission tomography
in patients with metastatic gastrointestinal stromal tumor treated
at a single institution with imatinib mesylate: proposal of new
computed tomography response criteria. J Clin Oncol. 25:1753–1759.
2007. View Article : Google Scholar
|
|
14.
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: review on morphology, molecular pathology,
prognosis, and differential diagnosis. Arch Pathol Lab Med.
130:1466–1478. 2006.PubMed/NCBI
|
|
15.
|
Daniels M, Lurkin I, Pauli R, Erbstösser
E, et al: Spectrum of KIT/PDGFRA/BRAF mutations and
Phosphatidylinositol-3-Kinase pathway gene alterations in
gastrointestinal stromal tumors (GIST). Cancer Lett. 312:43–54.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: pathology and prognosis at different sites. Semin
Diagn Pathol. 23:70–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Corless CL, Fletcher JA, Heinrich MC, et
al: Biology of gastrointestinal stromal tumors. J Clin Oncol.
22:3813–3825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Bucher P, Villiger P, Egger JF, et al:
Management of gastrointestinal stromal tumours: from diagnosis to
treatment. Swiss Med Wkly. 134:145–153. 2004.PubMed/NCBI
|
|
19.
|
Annaberdyev S, Gibbons J and Hardacre JM:
Dramatic response of a gastrointestinal stromal tumour to
neoadjuvant imatinib therapy. World J Surg Oncol. 7:302009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dematteo RP, Ballman KV, Antonescu CR, et
al: Adjuvant imatinib mesylate after resection of localised,
primary gastrointestinal stromal tumour: a randomised,
double-blind, placebo-controlled trial. Lancet. 373:1097–104. 2009.
View Article : Google Scholar
|
|
21.
|
Ibrahim HH, Ahmad MS, Eskaf WA, et al:
Malignant gastrointestinal stromal tumor of the tongue: case report
and review of the literature. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 111:e24–e29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Nowain A, Bhakta H, Pais S, et al:
Gastrointestinal stromal tumors: clinical profile,
pathogenesis,treatment strategies and prognosis. J Gastroenterol
Hepatol. 20:818–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Arru JM and Richardson JD:
Gastrointestinal stromal tumors: pathogenesis and current
treatment. J Ky Med Assoc. 103:211–215. 2005.PubMed/NCBI
|
|
24.
|
Osusky KL, Hallahan DE, Fu A, et al: The
receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell
migration, tubule formation, and blood vessel formation in vivo,
but has little effect on existing tumor vessels. Angiogenesis.
7:225–233. 2004. View Article : Google Scholar
|
|
25.
|
Abrams TJ, Lee LB, Murray LJ, et al:
SU11248 inhibits KIT and platelet-derived growth factor receptor
beta in preclinical models of human small cell lung cancer. Mol
Cancer Ther. 2:471–478. 2003.PubMed/NCBI
|
|
26.
|
Mendel DB, Laird AD, Xin X, et al: In vivo
antitumor activity of SU11248, a novel tyrosine kinase inhibitor
targeting vascular endothelial growth factor and platelet-derived
growth factor receptors: determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.
|
|
27.
|
Murray LJ, Abrams TJ, Long KR, et al:
SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an
experimental breast cancer bone metastasis model. Clin Exp
Metastasis. 20:757–766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
O’Farrell AM, Abrams TJ, Yuen HA, et al:
SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent
activity in vitro and in vivo. Blood. 101:3597–3605.
2003.PubMed/NCBI
|
|
29.
|
Schueneman AJ, Himmelfarb E, Geng L, et
al: SU11248 maintenance therapy prevents tumor regrowth after
fractionated irradiation of murine tumor models. Cancer Res.
63:4009–4016. 2003.PubMed/NCBI
|
|
30.
|
Joensuu H, Trent JC and Reichardt P:
Practical management of tyrosine kinase inhibitor-associated side
effects in GIST. Cancer Treat Rev Feb. 37:75–88. 2011. View Article : Google Scholar : PubMed/NCBI
|