Introduction
Malignant glioma is the most commonly occurring
primary malignant brain tumor with a poor prognosis (1). Although conventional methods,
including surgery, radiotherapy, brachytherapy and chemotherapy,
are used to treat glioma, numerous deficiencies remain.
Non-thyroid cancers are able to take in radioiodine
following transfection with the human sodium/iodide symporter
(hNIS) gene. Tumor promoters, such as the glial fibrillary acidic
protein (GFAP) and human telomerase reverse transcriptase (hTERT)
promoters, limit the expression of the hNIS gene in specific cells,
achieving the selective expression of targeted radioiodine therapy.
In our previous study, we used hTERT promoter-modulated expression
of the hNIS gene in an experimental model of radioiodine-based
malignant glioma treatment. This study demonstrated the significant
131I-iodide-induced killing of U251 and U87 human glioma
cells transfected with the hNIS gene and that the hNIS gene was
only expressed in telomerase-positive U251 and U87 tumor cell lines
(2). However, telomerase is highly
active in >85% of human cancers, not only in glioma (3). Therefore, a glioma-specific promoter
is required to accomplish the radioiodine-based treatment of
malignant glioma.
GFAP is an intermediate-filament protein expressed
abundantly and almost exclusively in the astrocytes of the CNS
(4). Due to its specificity and
abundance, GFAP has become the most commonly used marker for
astrocytes and a target of anti-glioma therapy (5). The promoter region of the GFAP gene
has already been cloned. The GFAP promoter (the fragment −2,163 to
+47 bp relative to the transcriptional start site), consisting of
2.2 kb of 5′-flanking DNA of the human GFAP (hGFAP) gene, has been
revealed to drive astrocyte-specific expression in cultured cells
(6). Studies have successfully
demonstrated gene therapy using a GFAP promoter-driven expression
vector of the therapeutic gene, including transforming growth
factor (TGF)-β1 (7), brain-derived
neurotrophic factor (BDNF) (8), BAX
(9) and platelet-derived growth
factor β polypeptide (PDGFB) (10).
The hNIS gene belongs to the sodium/solute symporter
family and mediates the Na+/K+ ATPase
dependent active transport of iodide across the membranes of
thyroid follicular cells (11).
Using targeted transfection with the hNIS gene, undifferentiated
thyroid cancer, as well as non-thyroid cancers, become able to take
up iodide, potentially allowing treatment with radioiodine. The
hNIS expression in non-thyroidal cells results in rapid
internalization but no organification of the radioiodide.
Radioiodine therefore rapidly exits hNIS-transfected non-thyroidal
cells. This is the major limitation of hNIS-mediated radioiodine
therapy for cancer.
In the present study, we developed and evaluated the
potential functional and therapeutic effectiveness of an
adenovector incorporating the hNIS gene and the GFAP promoter in
glioma cell lines. The GFAP promoter restricts the expression of
the transfected hNIS gene to glioma cells and thus maximizes the
glioma-specific uptake, while minimizing the non-specific uptake of
radioiodine.
Materials and methods
Cell lines
U251 and U87 human glioma cells were cultured in
Dulbecco’s modified Eagle medium (DMEM; Gibco BRL, Darmstadt,
Germany) with 10% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin. MRC-5 human lung fibroblasts cells were
cultured in MEM Eagle’s with Earle’s Balanced Salts (MEM-EBSS;
Gibco BRL) with 10% FBS. All cells were grown at 37°C under 5%
CO2 in air.
Western blot analysis
An EPS 2A200 electrophoresis system (Amersham
Biosciences, Piscataway, NJ, USA) was used for western blot
analysis. The protein extracts were centrifuged at 4°C for 10 min
and electrophoresed in bis-Tris HCl-buffered 10% sodium dodecyl
sulfate polyacrylamide gels (Invitrogen, Carlsbad, CA, USA). After
gel electrophoresis at 140 V for 1 h, the proteins were transferred
to polyvinylidene fluoride (PVDF) membranes using electroblotting.
Membranes were blocked with 5% non-fat milk overnight at 4°C and
then incubated separately with goat polyclonal NIS (sc-48055; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), mouse monoclonal
β-actin (TA-09; Novus Biologicals, Littleton, CO, USA) and goat
polyclonal GFAP antibodies (sc-6171; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. The membranes were then
incubated with secondary antibodies at room temperature for a
further 2 h and covered with Pierce ECL Western Blotting Substrate
(Thermo Fisher Scientific, Waltham, MA, USA) at room temperature
for 1 min and exposed to Fuji X-ray film in a darkroom. Prestained
protein molecular weight standards (Spectra Multicolor Broad Range
Protein Ladder, SM1841; Fermentas, Sankt Leon-Rot, Germany) were
run in the same gels to compare molecular weights and estimate
transfer efficiency (12).
Construction of recombinant plasmids
The GFAP promoter region was amplified from human
genomic DNA by PCR using Platinum® Taq DNA
Polymerase High Fidelity (Invitrogen). The GFAP promoter introduced
both MluI and NheI restriction sites at the 5′ and 3′
ends and occupied the region from −2,163 to +47 bp relative to the
transcriptional start site (GenBank M67446), which contained the
core promoter and two E-boxes (6).
The GFAP promoter PCR product was ligated with
PGL3-basic vector (Promega, Madison, WI, USA; creating PGL3-GFAP)
and ligated with PGL3-hNIS vector (previously constructed). This
was digested by MluI and NheI (creating
PGL3-GFAP-hNIS).
Luciferase assay
The expression of the luciferase gene by the GFAP
promoter in tumor cells was determined using the Dual-Glo
Luciferase Assay System (Promega) according to the manufacturer’s
instructions. Briefly, cells seeded in 24-well plates were exposed
by transfection with recombinant luciferase reporter plasmids
PGL3-GFAP and background control plasmid vector pRL-TK (Promega)
for 6 h at 37°C. The cells were harvested 24 h after the
transfection. Then Luciferase assays were performed using a Safire2
microplate reader (TECAN, Seestrasse, Switzerland). The
PGl3-control vector, containing the SV40 promoter was used as a
positive control and PGL3-basic without the promoter was used as a
negative control. All experiments were performed in triplicate.
Construction of recombinant
adenovirus
The hNIS gene was PCR cloned from the PGL3-GFAP-hNIS
vector (previously constructed) with HindIII and SalI
restriction sites at the 5′ and 3′ ends and inserted into the
pDC311 plasmid (Microbix Biosystems, Mississauga, ON, Canada) and
named pDC311-N. The fragment which carried the GFAP promoter was
then PCR cloned from the PGL3-GFAP-hNIS, with EcoRI and
HindIII restriction sites at the 5′ and 3′ ends, and
inserted into the pDC311-N vector, creating pDC311-GN. Ad-GFAP-hNIS
was produced according to the instructions provided by the
manufacturer of the AdMax™ Adenoviral Vector System (Microbix
Biosystems). Ad-cytomegalovirus (CMV)-hNIS (previously constructed)
was amplified and purified. Ad-CMV-enhanced green fluorescent
protein (EGFP) was a control vector unrelated to iodine uptake and
metabolism. The recombinant virus was stored at −80°C until
use.
Transfection of U251 and U87 cell lines
by adenoviral infection in vitro
The cells were seeded in 6-well plates to obtain
1×106 cells per well at the time of infection and
infected with the adenovirus in 1,000 μl of serum-free
medium for 6 h, followed by addition of 10% FBS new medium. The
transfected cells were incubated for 24 h. Ad-CMV-hNIS,
Ad-GFAP-hNIS and Ad-CMV-EGFP were used to infect U87, U251 and
MRC-5 cells, respectively.
Iodide uptake and efflux assays
Cells were seeded in 6-well plates and infected with
the recombinant adenovirus for 6 h, then placed in fresh DMEM with
10% FBS and incubated for an additional 24 h. The radioactivity was
measured with a γ counter (LKB Gamma 1261; LKB Instruments, Mt
Waverly, Australia). To measure the 125I uptake,
1×106 cells per well were cultured with 1 ml 10%
FBS-DMEM (containing 0.5 μCi Na125I) for 0, 10,
20, 30 and 40 min. The 125I-containing medium was then
decanted, cells were washed twice with PBS, lysed with 0.3 mol/l
sodium hydroxide and counted. To measure the 125I
efflux, 1×106 cells per well were cultured for 1 h with
1 ml 10% FBS-DMEM (containing 0.5 μCi Na125I),
then the 125I-containing medium was decanted. After the
cells were washed twice with PBS, fresh non-radioactive medium was
added to the 6-well plates. The cells were then cultured again for
0, 5, 10, 15 and 20 min. Subsequently, the cells were washed, lysed
and counted. All experiments were performed in triplicate (11).
Cell killing with 131I and
clonogenic assay in vitro
Cells were seeded in 6-well plates and infected with
the adenovirus for 6 h and then placed in fresh DMEM with 10% FBS
for an additional 24 h, washed twice with PBS and incubated with 1
ml of DMEM with 10% FBS, containing 500 μCi 131I.
After a 12-h incubation, cells were washed twice with PBS. For each
condition [Ad-GFAP-hNIS, Ad-CMV-hNIS and Ad-CMV-EGFR (the control
adenovirus) infected], cells were plated in 24-well plates at
densities of 100 cells/well and incubated for 1 week at 37°C. The
cells were then washed twice with PBS, fixed with 0.5 ml Carnoy’s
solution (a freshly prepared 3:1 mixture of methanol and acetic
acid) and stained with a crystal violet solution (for 250 ml, 0.5 g
crystal violet, 25 ml 40% formaldehyde, 50 ml ethanol and 175 ml
water). Colonies of >20 cells were counted. All experiments were
performed in triplicate (13,14).
Animal model
Experiments involving animals were performed with
the approval of the Beijing Experimental Animal Center of Peking
Union Medical, China. The generation of subcutaneous tumors was
performed as follows (11):
5×106 U87 tumor cells were transplanted subcutaneously
into the right shoulder of 4-week-old BALB/c female nude mice
weighing 190–210 g. When the tumors had reached a minimum
size of 10 mm in diameter (∼3 weeks after cell injection), the
recombinant adenovirus was injected into the tumor
(5×109 PFU in 50 μl of PBS). U251 and U87 are to
glioma cell lines and the data for the U87 and U251 cells were
similar to the in vitro clonogenic assay, so U87 cells were
selected as representative for animal testing.
Radioiodine therapy study in vivo
The 5×109 PFU in 50 μl of PBS
recombinant adenovirus was injected into the tumor when the tumor
grew to a minimum of 10 mm in diameter and 1 day later, 2 mCi
131I was intraperitoneally injected. The tumor size was
monitored prior to the administration of radio-iodine and every 7
days thereafter by measuring 3 diameters with a sliding caliper and
converting them to volume using the formula V=4πabc/3. For the
therapeutic experiments, all rats were divided into 2 groups in
terms of Ad-GFAP-hNIS and Ad-CMV-EGFP (the control adenovirus).
Each group was then divided into 2 subgroups in terms of
131I administration (3).
Tumor imaging
For imaging studies, only animals bearing tumors
with a minimum size of 10 mm in diameter were accepted. The
Ad-GFAP-hNIS (5×109 PFU in 50 μl of PBS) was
injected into the tumor, then 1 mCi of
99mTcO4 was intraperitoneally injected 1 day
later and 20 min after that, images were obtained using the Gamma
Camera (Discovery VH; GE Healthcare, Piscataway, NJ, USA) (15).
Statistical analysis
All experiments were performed in triplicate unless
otherwise indicated. Statistical analysis was performed using SPSS
software (SPSS 13.0; Tianjin, China). The results are presented as
the mean ± SD. Statistical significance was tested using the
student t-test procedure. P<0.05 was considered to indicate a
statistically significant result.
Results
Western blot analysis
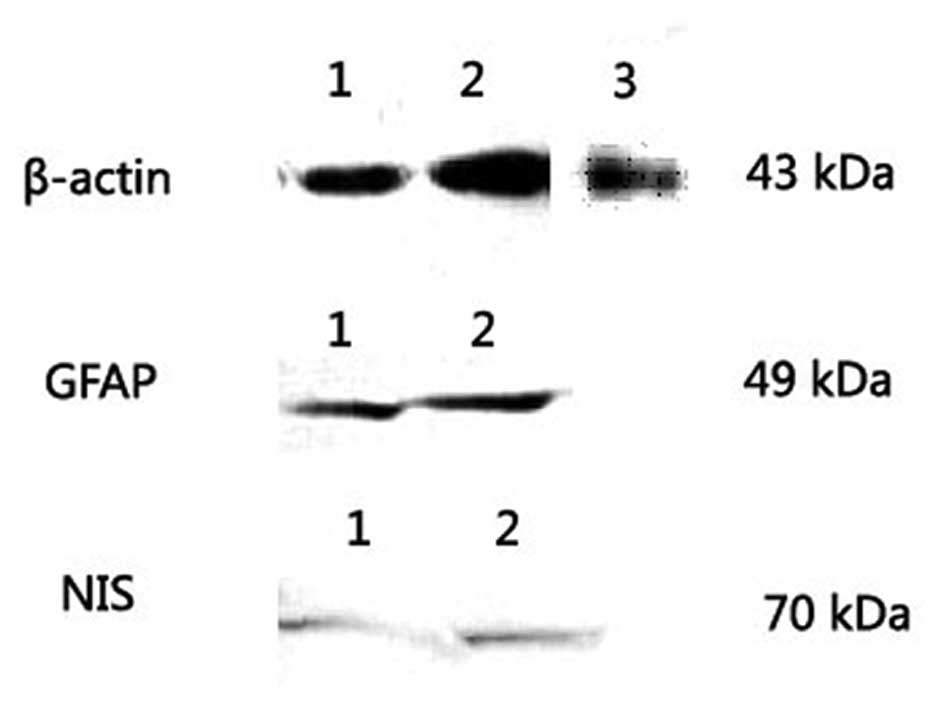
The GFAP and hNIS protein levels were analyzed by
western blotting. In U87, U251 and MRC-5 cells, the western
blotting showed that the GFAP protein was expressed in the
GFAP-positive U251 and U87 tumor cell lines as a major band of 49
kDa but not in the GFAP-negative MRC-5 cells, since MRC-5 was a
normal cell line. The β-actin protein was used as a positive
control and expressed in the U251, U87 and MRC-5 cells as a major
band corresponding to a molecular weight of 43 kDa (Fig. 1). Following transfection with
Ad-GFAP-hNIS, the protein level of the hNIS gene was analyzed by
western blotting in the U251 and U87 tumor cells and MRC-5 normal
cells. The hNIS protein was detected as a major band corresponding
to a molecular weight of 70 kDa in U251 and U87 tumor cell lines
but was not expressed in the GFAP-negative MRC-5 cell line
(Fig. 1).
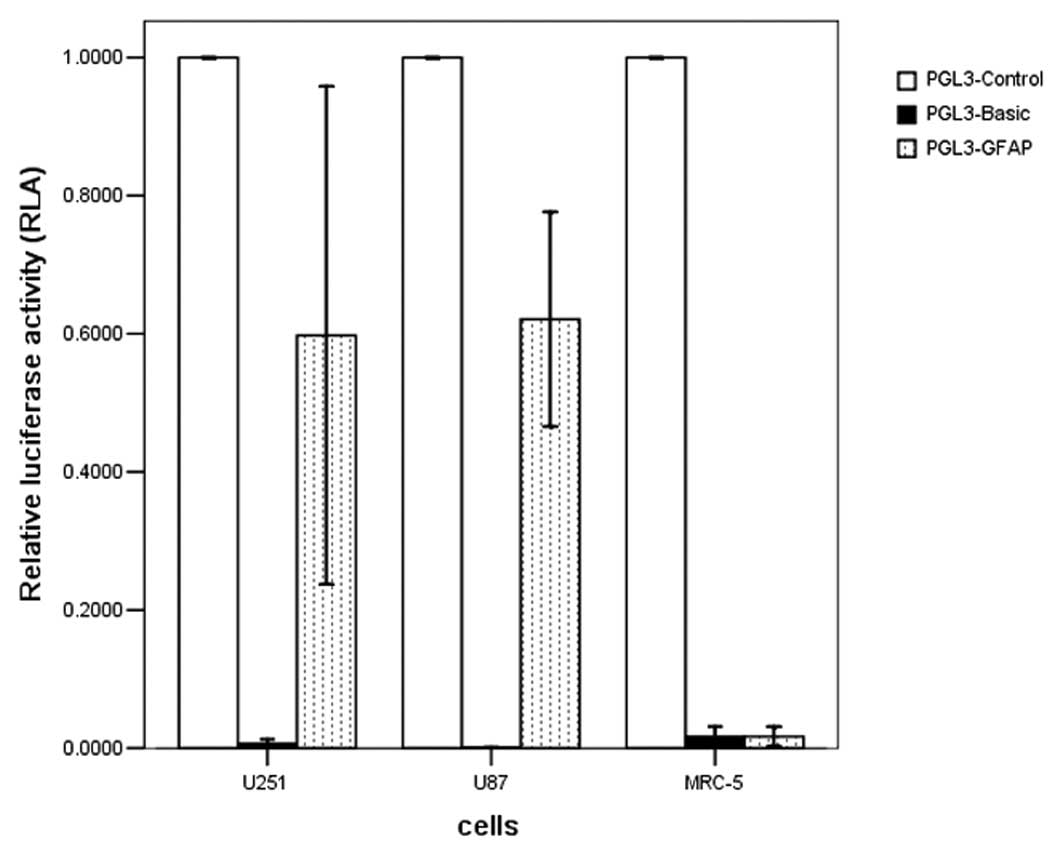
Luciferase assay
To assess the cell-specific transcriptional activity
of the GFAP promoter, a reporter gene assay using a luciferase
assay was performed in transiently transfected cells. The transient
transfection showed that the GFAP promoter is able to cause
luciferase gene expression in GFAP-positive U251 and U87 tumor cell
lines, without expression in the GFAP-negative MRC-5 cells. The
transcriptional activity of the GFAP promoter (PGL3-GFAP) reached
59.75±0.34 and 62.1±0.6% of that in the positive-control cells with
the SV40 promoter (PGL3-control) in the U251 and U87 cells
(Fig. 2).
Iodide uptake and efflux assays
To determine the iodide uptake and efflux,
125I-timed activity measurements were performed in U251
and U87 human glioma cells. Radioiodide uptake in the
adenovirus-transfected U251 and U87 cells was rapid (with the
exception of Ad-CMV-EGFP), reaching maximal levels within 30 min
and the Ad-CMV-hNIS-transfected cells had the highest rate
(Fig. 3A and B). To determine the
iodide efflux, the iodide uptake was permitted to proceed for 1 h,
so the steady-state level of accumulation was achieved. Following
replacement of the 125I-containing medium with
nonradioactive medium, the intracellular iodide was continuously
released into the medium and a rapid efflux of radioiodine from
cells was evident (Fig. 3C and D).
125I has a long physical half-life of 60 days, so the
effect of decay over the 20- to 50-min duration of the experiments
was ignored.
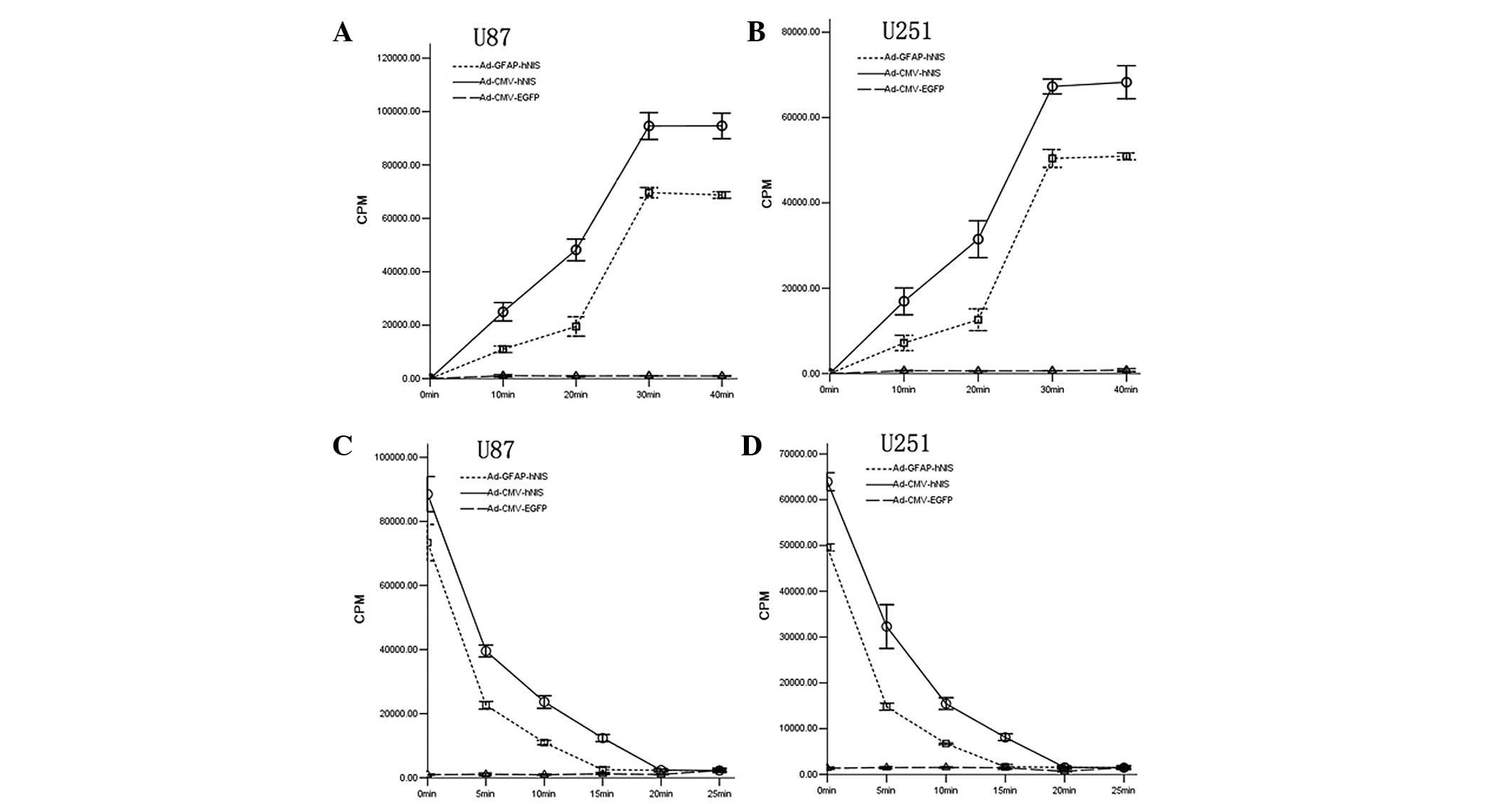 | Figure 3.Iodide uptake and efflux assays in U87
and U251 cell lines. (A) Iodide uptake assays in U87 cell lines.
Maximal 125I iodide uptake of U87 transfected with
Ad-CMV-hNIS and Ad-GFAP-hNIS was 95.1- and 69.5-fold higher,
respectively, than the control cells (transfected with
Ad-CMV-EGFP). All four experimental cell lines reached the maximum
within 30 min when radioiodine was present in the medium. (B)
Iodide uptake assays in U251 cell lines. Maximal 125I
iodide uptake of U251 transfected with Ad-CMV-hNIS, and
Ad-GFAP-hNIS was 94.7- and 79.8-fold higher, respectively, than the
control cells. (C) Iodide efflux assays in U87 cells. The efflux of
radioiodide from U87 cells was rapid and the biological half-times
were short. The biological times of iodide in U87 cells transfected
with Ad-CMV-hNIS, with Ad-GFAP-hNIS were 17.8 and 10.9 min,
respectively. The biological half-life of the iodide efflux assays
in this study was based on the cubic or inverse model on SPSS 13.0.
(D) Iodide efflux assays in U251 cells. The efflux of radioiodide
from the U251 cells was also rapid. The biological times of iodide
in U251 cells transfected with Ad-CMV-hNIS and Ad-GFAP-hNIS were
16.3 and 10.3 min, respectively. CMV, cytomegalovirus; hNIS, human
sodium/iodide symporter; GFAP, glial fibrillary acidic protein;
EGFP, enhanced green fluorescent protein. |
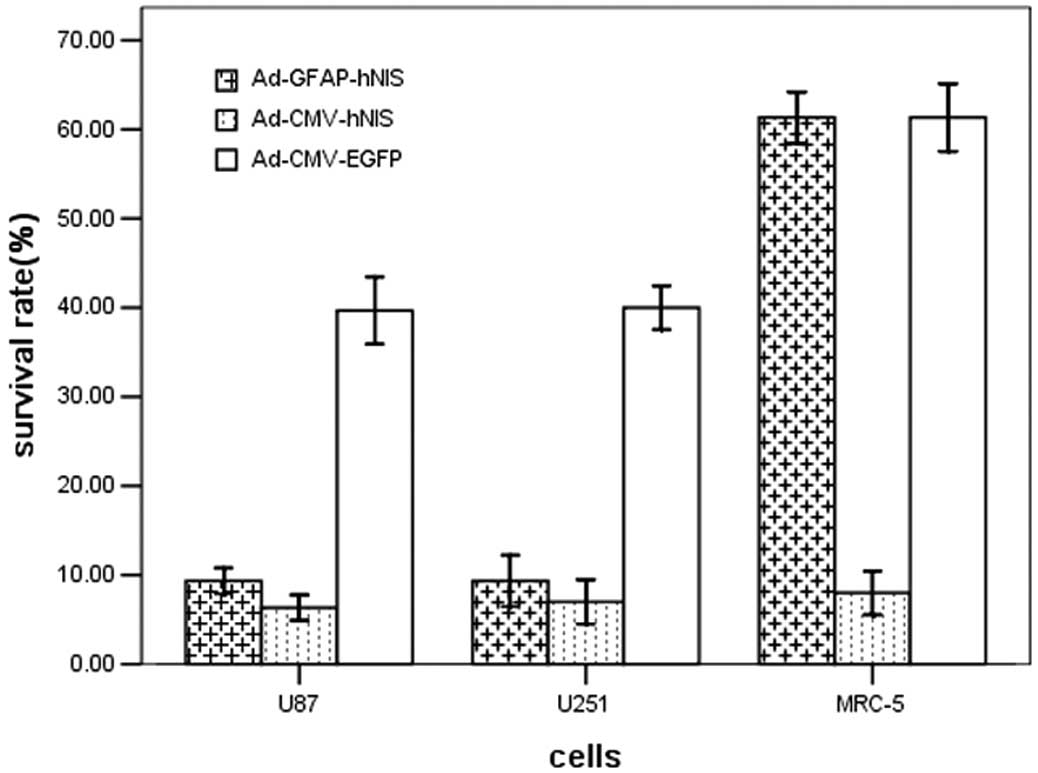
Clonogenic assay in vitro
The clonogenic assay investigated whether
131I showed selective cytotoxic activity in the hNIS
gene-expressing cells. The survival rates of transfected U251, U87
and MRC-5 cells not incubated with 131I were ∼90%. The
survival rates of the CMV promoter-transfected U251 and U87 tumor
cells and MRC-5 cells were all lower compared with the GFAP
promoter-transfected cells. Since the GFAP promoter was unable to
express the hNIS gene in normal MRC-5 cells, the survival rates of
the cells transfected with the GFAP promoter adenovirus and
transfected with Ad-CMV-EGFR (control empty adenovirus) were
similar. Transfection with the hNIS, whether with the GFAP or CMV
promoter, resulted in more 131I-induced cell killing
than without hNIS gene transfection (Fig. 4).
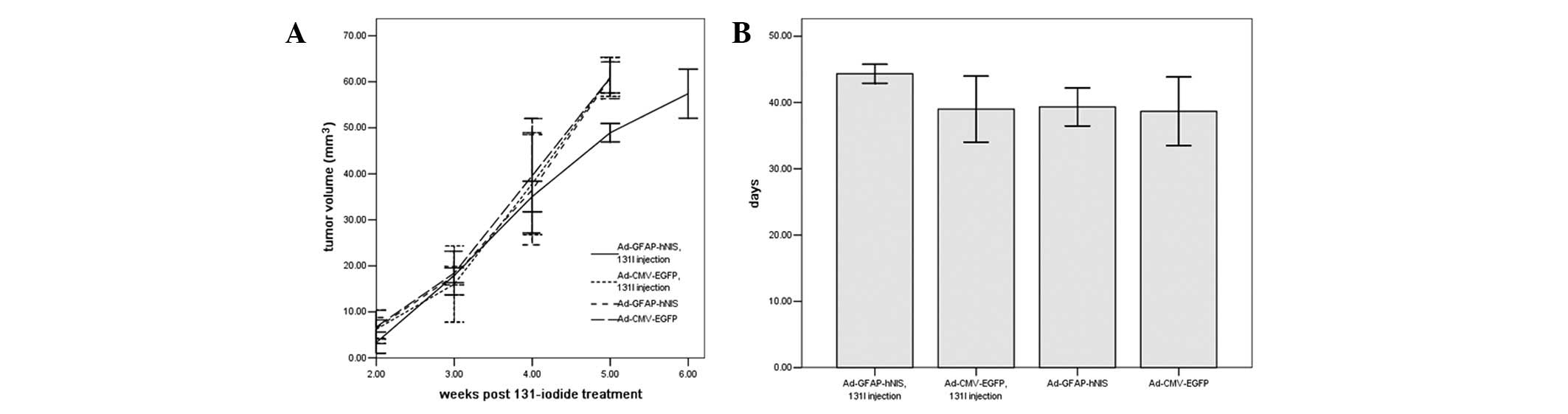
Radioiodine therapy study in vivo
After the U87 xenografts were injected with
adenovirus, the group injected with Ad-GFAP-hNIS (5×109
PFU in 50 μl of PBS) and 500 μCi 131I
survived the longest of the three groups, demonstrating that the
131I therapy was capable of increasing the U87 xenograft
nude mice survival. The three groups (Ad-CMV-EGFP and
131I, Ad-CMV-EGFP and Ad-GFAP-hNIS injection) survived
for similar periods, showing that the adenovirus injection and 500
μCi 131I injection had no effect on the nude
mice’s life span (Fig. 5).
Moreover, after the U87 xenografts were injected with 2 mCi
131I, the 131I therapy retarded the
Ad-GFAP-hNIS transfected-tumor growth, whereas the U87 tumors grew
significantly after transfection with Ad-CMV-EGFP since they were
unable to take up 131I; Fig.
5). Since the data for the U87 and U251 cells were similar in
the in vitro clonogenic assay, only the U87 cells were
selected to use in the the radio-iodine therapy study in
vivo.
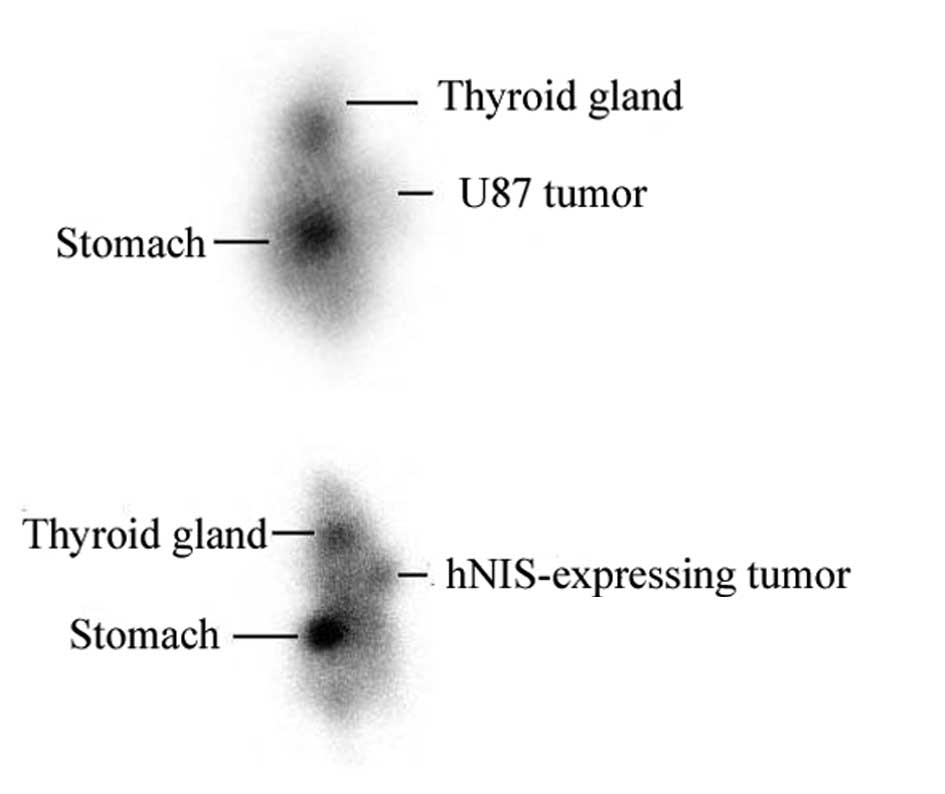
Tumor imaging
The hNIS-expressing tumor tissue accumulated
99mTcO4 rapidly when
99mTcO4 was intraperitoneally injected 20 min
later, whereas the control tumor injected with the control virus
(Ad-CMV-EGFP) was not visualized (Fig.
6). Normal NIS-expressing tissues, including those of the
thyroid gland and stomach, were also clearly visible.
Discussion
Radioiodine therapy for differentiated thyroid
carcinoma has been used for a number of years. Through targeted
transfection and expression of the hNIS gene, non-thyroid cancers
become able to take in iodine and so respond to radioiodine therapy
in the same manner as thyroid cancer. Moreover, due to the
crossfire effect of radiation therapy, radioiodine may kill not
only the NIS-expressing cells but also adjacent tumor cells.
However, if the hNIS gene was expressed in all transfected cells,
the therapeutic gene would affect tumor and normal cells. Using a
tumor-specific promoter system is likely to solve this problem and
the tumor-specific transfection of the hNIS gene has been applied
to a variety of tumors, including thyroid, prostate, colon, breast,
liver and lung cancer (11,13,15,16).
Studies have demonstrated that the α-fetoprotein (AFP) (15), carcinoembryonic antigen (CEA)
(17), ubiquitin C (UbC) (18), murine albumin (mAlb) (11), prostate-specific antigen (PSA)
(12) and the glucose transporter
gene 1 (GTI-1.3) promoters (19)
all lead to tumor-specific hNIS expression. However, the
tumor-specific promoters are often useful only for the particular
types of cancer from which they were derived and generally less
effective than non-specific or constitutive promoters such as the
CMV and simian virus 40 (SV40) promoters.
In the present study, we constructed and evaluated
the potential functional and therapeutic effectiveness of a
recombinant adenovirus Ad-GFAP-hNIS carrying the hNIS gene
controlled by the GFAP promoter in U251 and U87 glioma cell lines.
The cells transfected with the hNIS gene under the control of the
tumor-specific GFAP promoter, took in radioiodine and had lower
survival rates for 131I-treated U251 and U87 cells
compared with the control cells (transfected with Ad-CMV-EGFP)
in vitro. In vivo, the radioiodine therapy study showed that
the U87 xenografts injected with Ad-GFAP-hNIS and 2 mCi
131I survived the longest of the three groups
(Ad-CMV-EGFP and 131I, Ad-CMV-EGFP and Ad-GFAP-hNIS
injection). 131I injection had similar data to the other
three groups (not shown in Fig. 6),
so the adenovirus and 131I injection had no effect on
the life spans of the nude mice and was able to accumulate
99mTcO4 successfully in the
99mTcO4 scans. In the studies of
tumor-specific promoters, the GFAP promoter exhibited
glioma-specific hNIS expression in the U87 and U251 cells and did
not cause hNIS gene expression in MRC-5 normal cells.
The data showed that glioma-specific radioiodine
intake was caused by transfection with the hNIS gene under the
control of the GFAP promoter, but the GFAP promoter may also have
been activated by normal astrocytes in the CNS. Identifying how to
increase the selectivity of the GFAP promoter using dual promoters
to restrict the gene expression may be a way to solve this problem.
Furthermore, Doloff et al constructed an adenovirus that
used DF3/Muc1 and hTERT tumor-specific promoters to drive separate
E1A expression and exhibited improved oncolysis in numerous cancer
cell lines (20). Löw et al
developed a dual promoter lentiviral vector in which the EGFP gene
was expressed from the CMV-enhanced chicken β-actin (CAG) promoter
and copGFP was expressed from the elongation factor-1α (EF-1α)
promoter (21). These dual-promoter
studies drove the expression of same or different genes,
respectively.
In conclusion, hNIS gene expression mediated by the
GFAP promoter was restricted to only GFAP-positive cells. Nude mice
harboring U87 xenografts transfected with Ad-GFAP-hNIS and injected
with 131I survived the longest of the three groups and
were able to accumulate 99mTcO4 successfully
in the 99mTcO4 scans.
Acknowledgements
The present study was supported by
grants from the National Natural Science Foundation of China (to
Jian Tan; No. 81171372) and Tianjin Research Program of Application
Foundation and Advanced Technology (to Jian Tan; No.
12JCZDJC26000).
References
|
1.
|
Vredenburgh JJ, Desjardins A, Reardon DA
and Friedman HS: Experience with irinotecan for the treatment of
malignant glioma. Neuro Oncol. 11:80–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Li W, Tan J, Wang P and Wu P:
Cotransfected sodium iodide symporter and human tyroperoxidase
genes following human telomerase reverse transcriptase promoter for
targeted radio-iodine therapy of malignant glioma cells. Cancer
Biother Radiopharm. 26:443–451. 2011. View Article : Google Scholar
|
|
3.
|
Gu J, Kagawa S, Takakura M, Kyo S, Inoue
M, Roth JA and Fang B: Tumor-specific transgene expression from the
human telomerase reverse transcriptase promoter enables targeting
of the therapeutic effects of the Bax gene to cancers. Cancer Res.
60:5359–5364. 2000.
|
|
4.
|
Brenner M, Kisseberth WC, Su Y, Besnard F
and Messing A: GFAP promoter directs astrocyte-specific expression
in transgenic mice. J Neurosci. 14:1030–1037. 1994.PubMed/NCBI
|
|
5.
|
Nolte C, Matyash M, Pivneva T, Schipke CG,
Ohlemeyer C, Hanisch UK, Kirchhoff F and Kettenmann H: GFAP
promoter-controlled EGFP-expressing transgenic mice: a tool to
visualize astrocytes and astrogliosis in living brain tissue. Glia.
33:72–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Besnard F, Brenner M, Nakatani Y, Chao R,
Purohit HJ and Freese E: Multiple interacting sites regulate
astrocyte-specific transcription of the human gene for glial
fibrillary acidic protein. J Biol Chem. 266:18877–18883. 1991.
|
|
7.
|
Yang N and Mahato RI: GFAP promoter-driven
RNA interference on TGF-β1 to treat liver fibrosis. Pharm Res.
28:752–761. 2011.PubMed/NCBI
|
|
8.
|
Giralt A, Friedman HC, Caneda-Ferrón B,
Urban N, Moreno E, Rubio N, Blanco J, Peterson A, Canals JM and
Alberch J: BDNF regulation under GFAP promoter provides engineered
astrocytes as a new approach for long-term protection in
Huntington’s disease. Gene Ther. 17:1294–1308. 2010.PubMed/NCBI
|
|
9.
|
Huang J, Gao J, Lv X, Li G, Hao D, Yao X,
Zhou L, Liu D and Wang R: Target gene therapy of glioma:
overexpression of BAX gene under the control of both
tissue-specific promoter and hypoxia-inducible element. Acta
Biochim Biophys Sin (Shanghai). 42:274–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hede SM, Hansson I, Afink GB, Eriksson A,
Nazarenko I, Andrae J, Genove G, Westermark B and Nistér M: GFAP
promoter driven transgenic expression of PDGFB in the mouse brain
leads to glioblastoma in a Trp53 null background. Glia.
57:1143–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Chen L, Altmann A, Mier W, Eskerski H,
Leotta K, Guo L, Zhu R and Haberkorn U: Radioiodine therapy of
hepatoma using targeted transfer of the human sodium/iodide
symporter gene. J Nucl Med. 47:854–862. 2006.PubMed/NCBI
|
|
12.
|
Spitzweg C, Zhang S, Bergert ER, Castro
MR, McIver B, Heufelder AE, Tindall DJ, Young CY and Morris JC:
Prostate-specific antigen (PSA) promoter-driven androgen-inducible
expression of sodium iodide symporter in prostate cancer cell
lines. Cancer Res. 59:2136–2141. 1999.PubMed/NCBI
|
|
13.
|
Boland A, Ricard M, Opolon P, Bidart J,
Yeh P, Filetti S, Schlumberger M and Perricaudet M:
Adenovirus-mediated transfer of the thyroid sodium/iodide symporter
gene into tumors for a targeted radiotherapy. Cancer Res.
60:3484–3492. 2000.PubMed/NCBI
|
|
14.
|
Schipper M, Weber A, Béhé M, Göke R, Joba
W, Schmidt H, Bert T, Simon B, Arnold R, Heufelder A and Behr T:
Radioiodide treatment after sodium iodide symporter gene transfer
is a highly effective therapy in neuroendocrine tumor cells. Cancer
Res. 63:1333–1338. 2003.PubMed/NCBI
|
|
15.
|
Ma XJ, Huang R and Kuang AR: AFP promoter
enhancer increased specific expression of the human sodium iodide
symporter (hNIS) for targeted radioiodine therapy of
hepato-cellular carcinoma. Cancer Invest. 27:673–681. 2009.
View Article : Google Scholar
|
|
16.
|
Scholz IV, Cengic N, Baker CH, Harrington
KJ, Maletz K, Bergert ER, Vile R, Göke B, Morris JC and Spitzweg C:
Radioiodine therapy of colon cancer following tissue-specific
sodium iodide symporter gene transfer. Gene Ther. 12:272–280. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Spitzweg C, Baker CH, Bergert ER, O’Connor
MK and Morris JC: Image-guided radioiodide therapy of medullary
thyroid cancer after carcinoembryonic antigen promoter-targeted
sodium iodide symporter gene expression. Hum Gene Ther. 18:916–924.
2007. View Article : Google Scholar
|
|
18.
|
Kim HJ, Jeon YH, Kang JH, Lee YJ, Kim KI,
Chung HK, Jeong JM, Lee DS, Lee MC and Chung JK: In vivo long-term
imaging and radioiodine therapy by sodium-iodide symporter gene
expression using a lentiviral system containing ubiquitin C
promoter. Cancer Biol Ther. 6:1130–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sieger S, Jiang S, Schönsiegel F, Eskerski
H, Kübler W, Altmann A and Haberkorn U: Tumour-specific activation
of the sodium/iodide symporter gene under control of the glucose
transporter gene 1 promoter (GTI-1.3). Eur J Nucl Med Mol Imaging.
30:748–756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Doloff JC, Jounaidi Y and Waxman DJ: Dual
E1A oncolytic adenovirus: targeting tumor heterogeneity with two
independent cancer-specific promoter elements, DF3/MUC1 and hTERT.
Cancer Gene Ther. 18:153–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Löw K, Blesch A, Herrmann J and Tuszynski
MH: A dual promoter lentiviral vector for the in vivo evaluation of
gene therapeutic approaches to axon regeneration after spinal cord
injury. Gene Ther. 17:577–591. 2010.PubMed/NCBI
|