Introduction
Cervical cancer is the third most common cancer in
females. In 2008, 529,800 women were diagnosed with cervical
cancer, accounting for 9% of all types of cancer diagnosed in
females worldwide. Furthermore, cervical cancer is the fourth
leading cause of mortality in females, accounting for 8% (275,100)
of total cancer-related mortality rates in females worldwide in
2008 (1,2). Thus, developing an effective drug is
of utmost importance.
Abnormal signaling pathways play crucial roles in
the pathogenesis and progression of cancer (3). Mammalian target of rapamycin (mTOR) is
a serine/threonine protein kinase, which forms two distinct
multiprotein complexes, mTORC1 and mTORC2. mTORC1 phosphorylates
downstream proteins p70S6K (S6K), which are involved in protein
translation, while mTORC2 phosphorylates Akt, increasing its
enzymatic activity by 5- to 10-fold (4,5).
Furthermore, mTOR functions as a sensor of mitogen, energy and
nutrient levels, and is a central controller of cell growth and a
negative regulator of autophagy (6). AZD8055 is a first-in-class orally
available, potent and specific inhibitor of mTOR kinase activity,
and shows a promise for suppressing tumor growth (7). Currently, AZD8055 is in phase I
clinical development. Understanding the molecular mechanisms of
AZD8055 on human cervical cancer HeLa cells may facilitate the
development of strategies for the therapy of cervical cancer.
Energy metabolism is considered to be a promising
target for cancer therapy. In multiple types of malignant tumors,
energy metabolism is aberrantly upregulated. Cancer cells
preferentially metabolize glucose through glycolysis even under
adequate levels of oxygen, which is known as the Warburg
effect.
miRNAs are small endogenous and non-coding RNAs that
inhibit gene expression via interaction with target sites in the
3’-untranslated region (UTRs) of mRNAs. miRNAs play important roles
in regulating multiple biological processes, including the
pathogenesis of a variety of human types of cancer (8). Lee et al(4) reported that miRNA deregulation may
play a key role in the malignant transformation of cervical
squamous cells. Accumulating evidence has shown that the expression
of miR-143 is decreased in multiple types of cancer, and has been
shown to be associated with lower survival rates and poor
prognostic features (5,9).
The aim of the present study was to elucidate the
role of AZD8055 in cervical cancer cells. We treated the cervical
cancer cell line HeLa with AZD8055 for different durations to
dynamically observe the effect of AZD8055 on cell proliferation,
glycolysis and apoptosis. We also explored the effect of AZD8055 on
the expression of miR-143 as well as several important enzymes
involved in growth control and energy metabolism.
Materials and methods
Materials and reagents
The human cervical cancer cell line HeLa was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). AZD8055 was obtained from Selleck (Houston, TX
USA). Fetal bovine serum (FBS) was from Invitrogen (Carlsbad, CA,
USA). Dulbecco’s modified Eagle’s medium (DMEM) and MTT were
purchased from Sigma (St. Louis, MO, USA). The Annexin V-FITC
Apoptosis Detection kit was purchased from Biovision (Mountain
View, CA, USA). All antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
The HeLa cells were cultured in DMEM containing 10%
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin (Life
Technologies, Carlsbad, CA, USA). The cells were cultured at 37°C
in a humidified atmosphere with 5% CO2.
MTT assays
Exponentially growing cells were seeded at
1×104 cells/well in 96-well plates and incubated with
AZD8055 at 10 nM for 24, 48 and 72 h, respectively. MTT (5 mg/ml)
was added to each well and the plates were incubated for 4 h at
37°C. The formazan product was dissolved by adding 100 μl
DMSO to each well. The MTT absorbance value was detected at 490 nm
with a micro-plate reader (Bio-Rad, Hercules, CA, USA).
Flow cytometric analysis
For the detection of the percentage of apoptotic
cells with Annexin V/propidium iodide (PI), following incubation
with AZD8055 for 24-72 h, the HeLa cells were harvested and washed
with cold PBS twice and then resuspended in 200 μl binding
buffer at a concentration of 1×106 cells/ml. The cells
were incubated with 10 μl Annexin V-fluorescein isothio
cyanate (FITC) and 5 μl PI in the dark for 15 min. In total,
300 μl binding buffer was then added in each tube prior to
being analyzed with the flow cytometer.
Measurement of glycolytic activity
The cellular glycolysis level was determined by
measuring the cellular glucose uptake and lactate production. To
measure glucose uptake, cells were washed with glucose-free medium
and incubated in fresh glucose-free RPMI-1640 medium for 3 h, and
then incubated with 0.2 Ci/ml 3H-2-deoxyglucose for 1 h.
The glucose uptake represented by 3H radioactivity was
determined by liquid scintillation counting and normalized by cell
number. Lactate concentration in the culture medium was measured
using the Lactate Acid Assay kit (Biovison). The protein expression
of HK2, a key time-limiting enzyme in the activation of glucose,
was also measured.
Western blotting
Cells were lysed in cold RIPA lysis buffer. The
protein concentrations were measured by a bicinchoninic acid (BCA)
protein assay kit (Pierce Biotechnology, Rockford, IL, USA). The
samples (20 μg) were loaded and separated by SDS-PAGE and
then electrophoretically transferred to PVDF membranes (Pall, New
York, NY, USA). The membranes were probed with various antibodies
according to the manufacturer’s instructions and visualized with
peroxidase and an enhanced chemiluminescence system (Pierce
Biotechnology).
Statistical analysis
Data are expressed as the mean ± SEM. The
statistical significance of the differences was determined by ANOVA
followed by Student-Newman-Keuls (S-N-K) test or unpaired
two-tailed t-tests. P<0.05 was considered to indicate
statistically significant differences. Statistical analysis was
performed using SPSS 17.0 statistical software.
Results
AZD8055 inhibits proliferation of human
cervical cancer HeLa cells
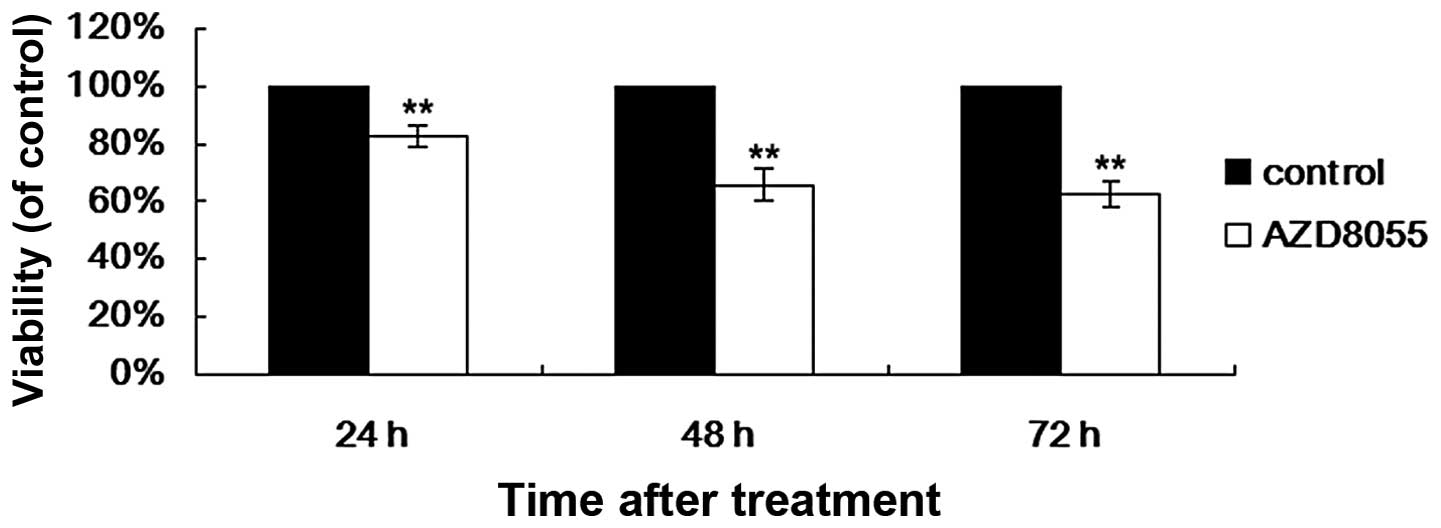
We first examined the effect of AZD8055 on the
proliferation of HeLa cells. An MTT assay was applied to determine
the relative proliferation rate of HeLa cells treated with AZD8055
for different durations (Fig. 1).
Following AZD8055 treatment for 24, 48 and 72 h, the average cell
proliferation rate was 82.97±3.74, 65.93±5.89 and 62.42±4.10%,
respectively. These data demonstrate that AZD8055 time-dependently
inhibited the proliferation of HeLa cells compared with controls
(P<0.01 compared with the control).
AZD8055 induces apoptosis of human
cervical cancer HeLa cells
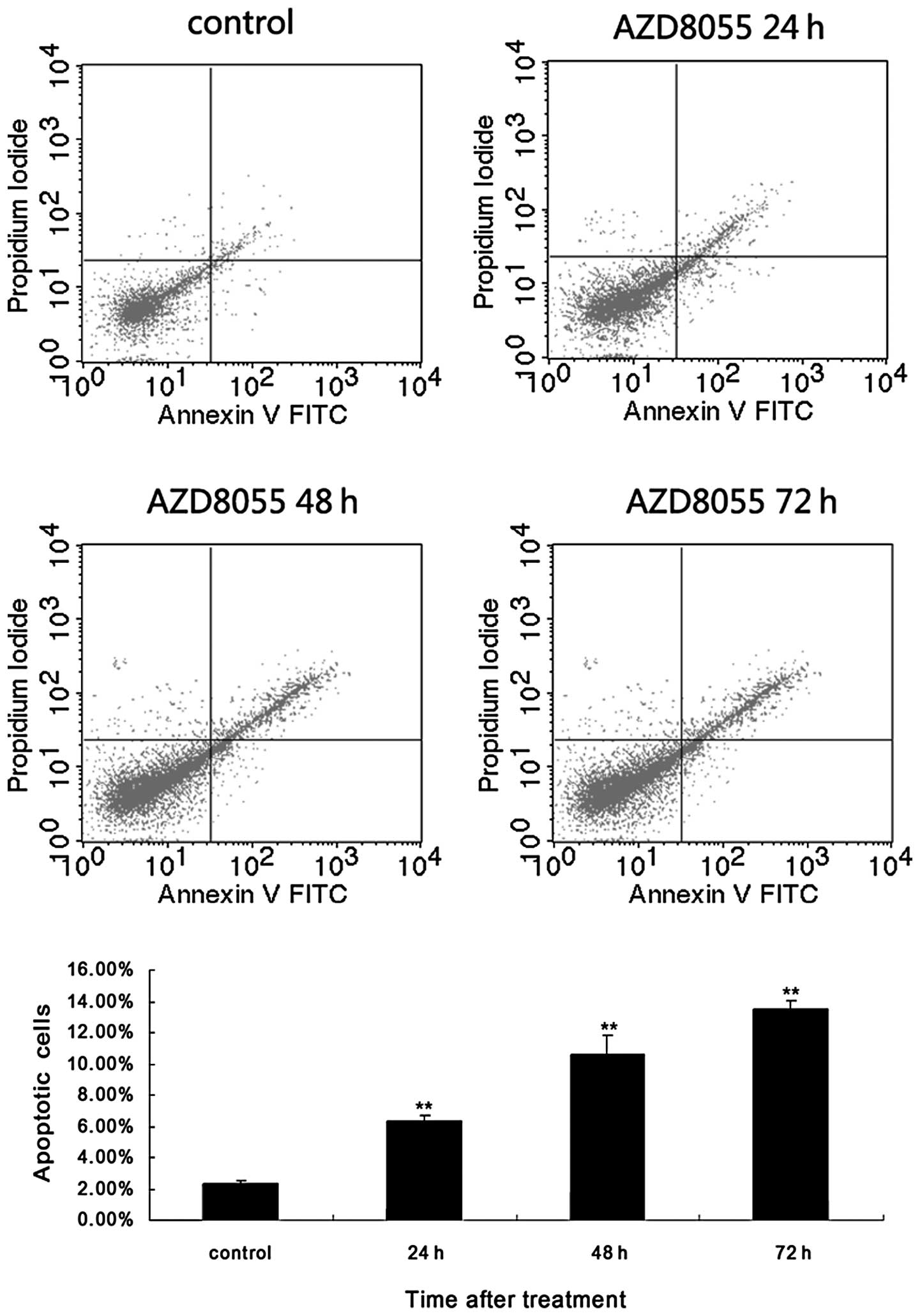
The inhibition of cell proliferation by AZD8055 in
HeLa cells may also be due to the induction of apoptosis. We
therefore examined whether the antiproliferative effect of AZD8055
was accompanied by the induced apoptosis. HeLa cells treated with
AZD8055 for different durations were analyzed using Annexin V/PI
double-staining and flow cytometry analysis. As shown in Fig. 2, incubation with 10 nM AZD8055
increased the Annexin-positive cells from 2.31±0.17 to 6.37±0.37,
10.58±1.25 and 13.47±0.59% at 24, 48 and 72 h, respectively. Thus,
AZD8055 time-dependently decreased the viability of HeLa cells.
Moreover, AZD8055 significantly increased the early- and late-stage
apoptotic fraction in cervical cancer cells, suggesting that
suppression of cell proliferation by AZD8055 was partly due to the
induction of apoptosis.
AZD8055 inhibits glycolysis of human
cervical cancer HeLa cells
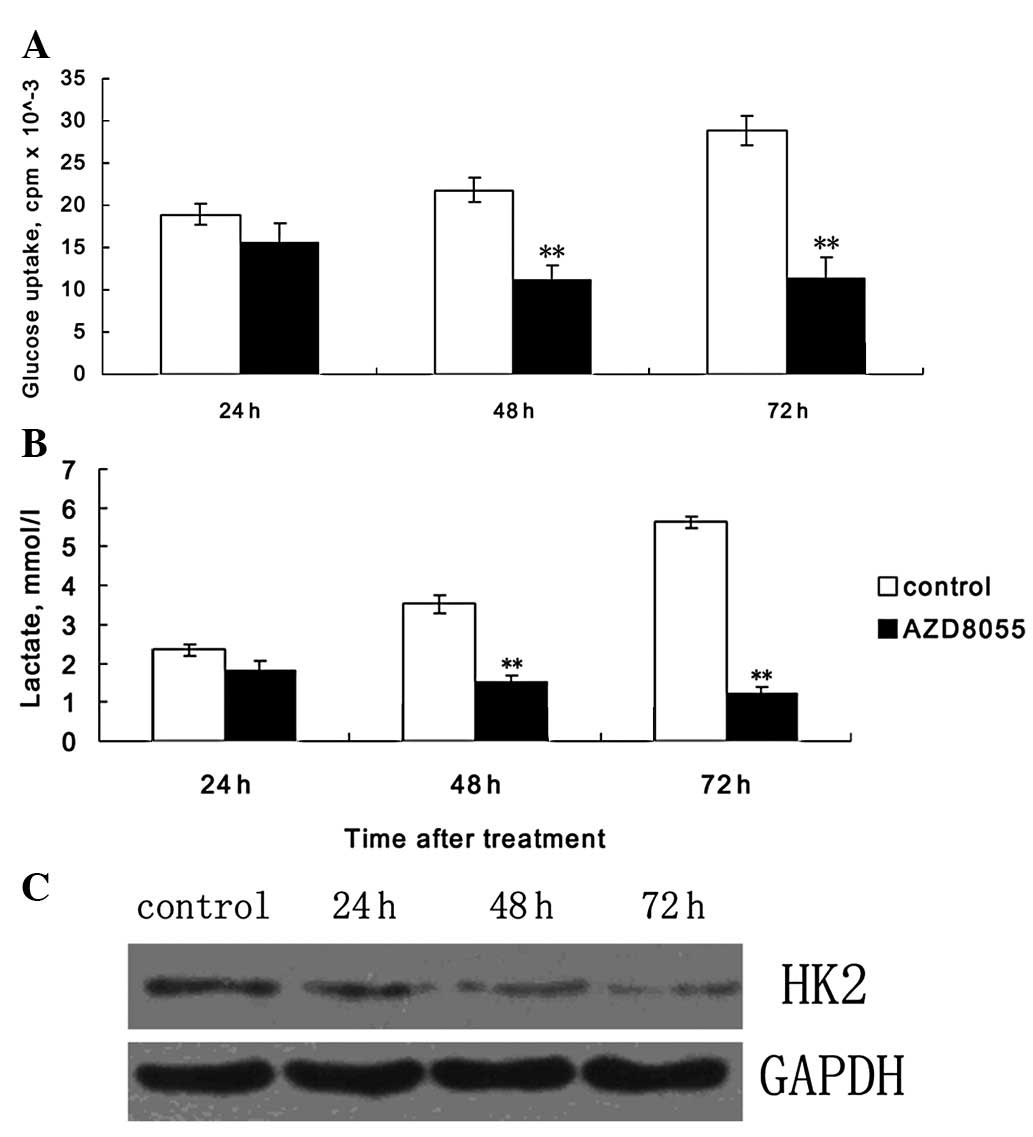
The level of glycolysis is always aberrantly
upregulated in cancer, and is needed for the rapid proliferation of
cancer cells. Thus, we further examined whether AZD8055 inhibited
glycolysis in HeLa cells. We measured the cellular glucose uptake
and lactate production in different groups. As shown in Fig. 3A and B, HeLa cells treated with
AZD8055 for different durations showed decreased glucose uptake and
lactate production, compared with controls. HK2 is a key
time-limiting enzyme in glycolysis. Thus, we further examined the
expression of HK2 by western blotting. As shown in Fig. 3C, during the treatment of HeLa cells
with AZD8055, the expression of HK2 was time-dependently decreased,
suggesting that AZD8055 may inhibit HeLa cell proliferation through
inhibiting glycolysis at least partially by downregulating the
expression of HK2.
AZD8055 inhibits the activity of
mTOR
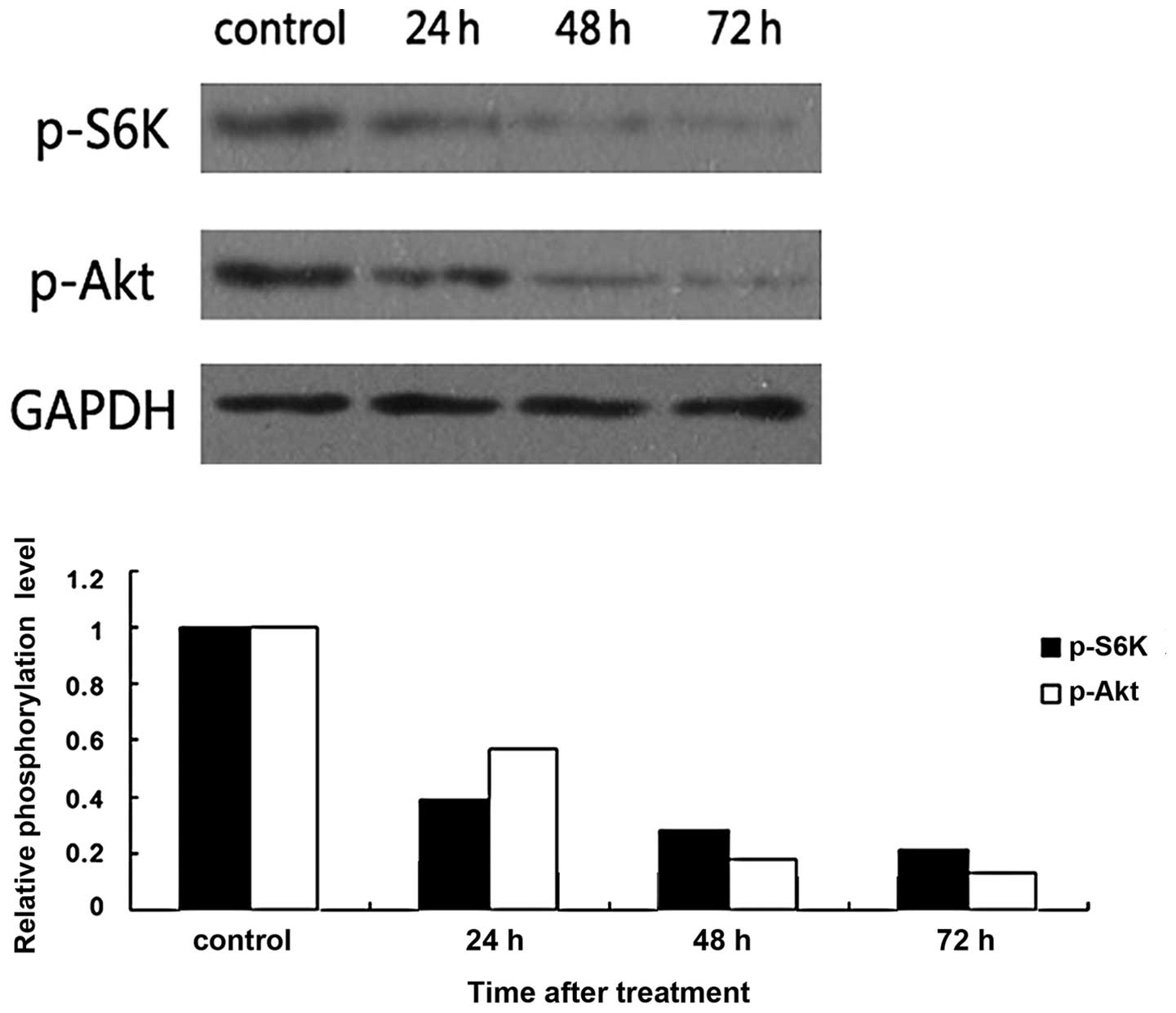
To investigate the molecular mechanism of AZD8055 on
HeLa cells, we examined the effect of AZD8055 on the activity of
mTOR, an important component of the PI3K/Akt/mTOR signaling pathway
that plays a crucial role in the pathogenesis of cervical cancer
(10). Our data showed that the
phosphorylation of S6K and Akt were significantly reduced by
AZD8055 in a time-dependent manner in HeLa cells (Fig. 4), which are the best characterized
targets of the mTOR complex cascade. These findings suggest that
AZD8055 may inhibit cervical cancer cell proliferation by
repressing the mTOR pathway.
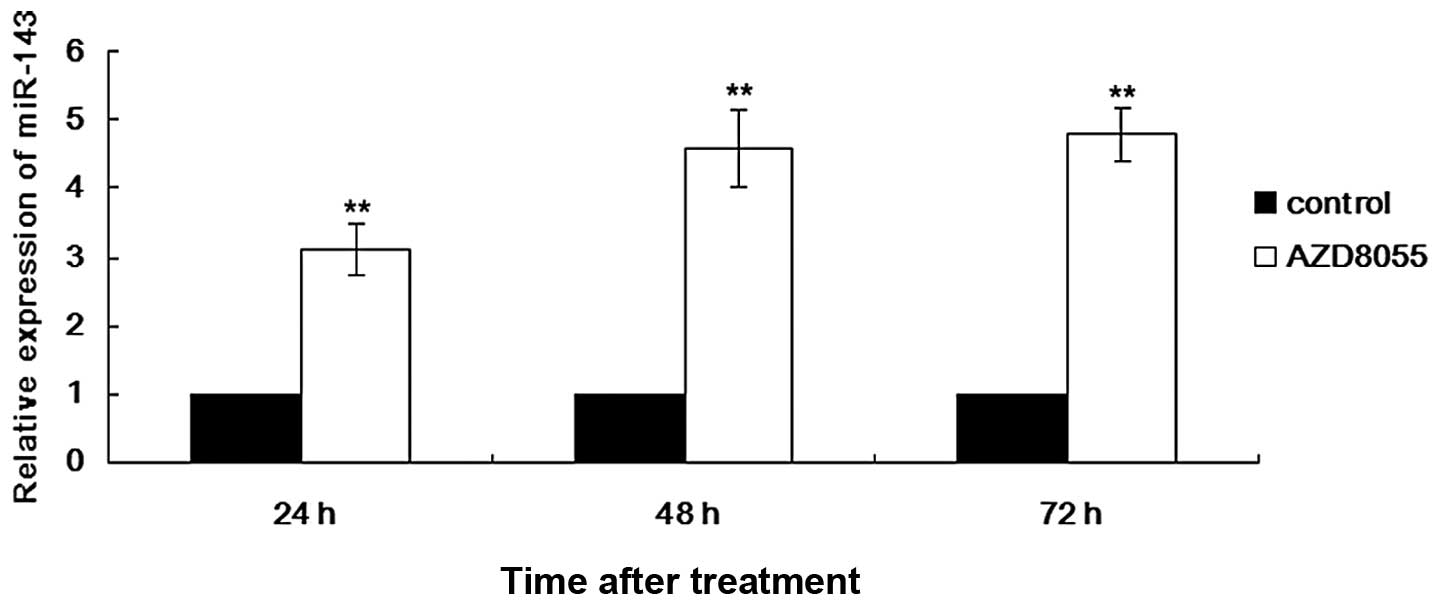
AZD8055 upregulates the expression of
miR-143
miRNAs play critical roles in cell biology,
including regulating cell proliferation and apoptosis (11). In recent years, an increasing number
of studies has reported that miRNAs are involved in the progression
of cancer (12). mTOR was recently
demonstrated to regulate the expression of miR-143 in lung
adenocarcinoma cell lines (13).
However, whether this regulating mechanism exists in cervical
cancer remains unclear. Based on these findings, we further
determined the expression of miR-143 in HeLa cells treated with
AZD8055 for 24-72 h by qRT-PCR. As shown in Fig. 5, the upregulation of miR-143 by
AZD8055 is time-dependent with the maximal increase (3.5-fold)
achieved at 24 h of treatment. These results reveal a novel
molecular mechanism of AZD8055/mTOR-mediated growth inhibition of
cervical cancer cells.
Discussion
The present study described AZD8055 as a potent mTOR
inhibitor involved in multiple cellular responses, including
inhibiting proliferation and inducing apoptosis in human cervical
cancer HeLa cells. Notably, this is the first study to demonstrate
that AZD8055 is able to reduce glycolysis levels in cancer cells.
Our study suggested that AZD8055-mediated cervical cancer cell
proliferation inhibition may occur through deregulating mTOR
activity and upregulating the expression of miR-143, whose targets
include HK2, a key time-limiting enzyme in the process of glucose
activation.
mTOR is a protein kinase involved in the PI3K/Akt
signaling pathway with a central role in the control of cell
growth, proliferation, metabolism, survival and angiogenesis
(14,15). It has been well established that the
PI3K/Akt/mTOR signaling pathway plays a crucial role in cancer
development. Multiple studies have reported that this signaling
pathway is aberrantly activated in multiple types of cancer
(16,17). As a result, the frequent
dysregulation of this signaling pathway in cancer make it an
important target in the treatment of multiple types of malignant
tumors (18,19). However, only limited published data
are available on new mTOR inhibitors and the detailed mechanism of
AZD8055 in treating cervical cancer has yet to be fully understood.
The present study showed that AZD8055 inhibits the phosphorylation
of the mTORC1 substrates p70S6K and phosphorylation of the mTORC2
substrate Akt, suggesting that the activity of mTOR signaling was
deregulated. These results indicated that the deregulation of the
mTOR signaling pathway may be involved in the AZD8055-induced
antiproliferative effects and apoptosis in cervical cancer HeLa
cells.
The Warburg effect is a type of aberrant glucose
metabolism common in cancer, which makes cancer cells less
dependent on oxygen and promotes their survival under hypoxic
conditions (3). Thus, glycolysis in
cancer may become a promising target for the treatment of malignant
tumors. In this study, AZD8055 time-dependently inhibited
glycolysis in HeLa cells, indicated by the decreased LDH activity
and lactate production. Additionally, the high glycolysis level in
cancer is often associated with the aberrant overexpression of some
key enzymes in glucose metabolism. Mathupala et al(20) reported that HK2 is involved in the
maintenance of the malignant state of cancer. In fact, HK2 was able
to promote the first step of glycolysis, thus, its overexpression
would provide cancer cells with adequate glycolytic flux and
further the shift towards aerobic glycolysis (21). Our data demonstrated that AZD8055
significantly deregulated the protein expression of HK2, providing
a potential mechanism by which AZD8055 inhibited glycolysis in HeLa
cells.
miRNAs are small endogenous non-coding RNAs,
involved in the post-transcriptional regulation of gene expression
by binding to the 3’-UTR of target mRNAs, which eventually leads to
translational repression or mRNA degradation (22). miR-143 has been reported to play a
crucial role in cancer development (23–26).
The downregulation or loss of miR-143 may contribute to
tumorigenesis, while forced expression of miR-143 may effectively
inhibit the proliferation of cancer cells. Recently, Fang et
al found that mTOR was able to inhibit the expression of
miR-143, which further regulates cancer glycolysis via targeting
HK2 in lung adenocarcinoma cell lines (12). In fact, HK2 has recently been
demonstrated to be a direct target of miR-143 (27,28).
In this study, the molecular experiments showed that AZD8055
downregulated the activity of mTOR, upregulated the expression of
miR-143 and inhibited the expression of HK2 in HeLa cells. Based on
these data, we suggested that AZD8055 inhibited the proliferation
and glycolysis of HeLa cells partially through upregulating the
expression of miR-143, which further deregulated the expression of
HK2.
In conclusion, the present study provides a novel
antitumor mechanism of AZD8055 in human cervical cancer cells. Our
findings suggest that miR-143 may be an important potential target
for cervical cancer treatment and AZD8055 may be a useful
therapeutic agent for the treatment of human cervical cancer.
References
|
1.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3.
|
Toschi A, Lee E, Thompson S, et al:
Phospholipase D-mTOR requirement for the Warburg effect in human
cancer cells. Cancer Lett. 299:72–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lee JW, Choi CH, Choi JJ, et al: Altered
MicroRNA expression in cervical carcinomas. Clin Cancer Res.
14:2535–2542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Liu H, Zhang SZ, Cai SR, Peng JP and Zheng
S: Effect of microRNA143 expression on cell proliferation in
colonic carcinoma. Zhonghua Zhong Liu Za Zhi. 30:498–501. 2008.(in
Chinese).
|
|
6.
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar
|
|
7.
|
Chresta CM, Davies BR, Hickson I, et al:
AZD8055 is a potent, selective, and orally bioavailable
ATP-competitive mammalian target of rapamycin kinase inhibitor with
in vitro and in vivo antitumor activity. Cancer Res. 70:288–298.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rottiers V and Naar AM: MicroRNAs in
metabolism and metabolic disorders. Nat Rev Mol Cell Biol.
13:239–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Osaki M, Takeshita F, Sugimoto Y, et al:
MicroRNA-143 regulates human osteosarcoma metastasis by regulating
matrix metalloprotease-13 expression. Mol Ther. 19:1123–1130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kang S, Dong SM, Kim BR, et al:
Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR
pathway in cervical and endometrial cancer cells. Apoptosis.
17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Osman A: MicroRNAs in health and disease -
basic science and clinical applications. Clin Lab. 58:393–402.
2012.PubMed/NCBI
|
|
12.
|
Hudson RS, Yi M, Esposito D, et al:
MicroRNA-106b-25 cluster expression is associated with early
disease recurrence and targets caspase-7 and focal adhesion in
human prostate cancer. Oncogene. Sep 17–2012.(Epub ahead of print).
View Article : Google Scholar
|
|
13.
|
Fang R, Xiao T, Fang Z, et al:
MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting
hexokinase 2 gene. J Biol Chem. 287:23227–23235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fasolo A and Sessa C: Targeting mTOR
pathways in human malignancies. Curr Pharm Des. 18:2766–2777. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/Akt/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Grzybowska-Izydorczyk O and Smolewski P:
mTOR kinase inhibitors as a treatment strategy in hematological
malignancies. Future Med Chem. 4:487–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Diaz-Padilla I, Duran I, Clarke BA and Oza
AM: Biologic rationale and clinical activity of mTOR inhibitors in
gyneco-logical cancer. Cancer Treat Rev. 38:767–775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Barnett CM: Everolimus: targeted therapy
on the horizon for the treatment of breast cancer. Pharmacotherapy.
32:383–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hill EK and Dizon DS: Medical therapy of
endometrial cancer: current status and promising novel treatments.
Drugs. 72:705–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase II: cancer’s double-edged sword acting as both
facilitator and gatekeeper of malignancy when bound to
mitochondria. Oncogene. 25:4777–4786. 2006.
|
|
21.
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009.PubMed/NCBI
|
|
22.
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Li X, Zhang G, Luo F, et al:
Identification of aberrantly expressed miRNAs in rectal cancer.
Oncol Rep. 28:77–84. 2012.PubMed/NCBI
|
|
24.
|
White NM, Youssef YM, Fendler A, Stephan
C, Jung K and Yousef GM: The miRNA-kallikrein axis of interaction:
a new dimension in the pathogenesis of prostate cancer. Biol Chem.
393:379–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ng EK, Tsang WP, Ng SS, et al:
MicroRNA-143 targets DNA methyltransferases 3A in colorectal
cancer. Br J Cancer. 101:699–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Michael MZ, SM OC, van Holst Pellekaan NG,
Young GP and James RJ: Reduced accumulation of specific microRNAs
in colorectal neoplasia. Mol Cancer Res. 1:882–891. 2003.PubMed/NCBI
|
|
27.
|
Jiang S, Zhang LF, Zhang HW, et al: A
novel miR-155/miR-143 cascade controls glycolysis by regulating
hexokinase 2 in breast cancer cells. EMBO J. 31:1985–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Gregersen LH, Jacobsen A, Frankel LB, Wen
J, Krogh A and Lund AH: microRNA-143 down-regulates Hexokinase 2 in
colon cancer cells. BMC Cancer. 12:2322012. View Article : Google Scholar : PubMed/NCBI
|