Introduction
Breast cancer is the most frequent malignant tumor
and a leading cause of cancer mortality among females in the
majority of Asian countries, including China (1). Despite significant progress, 40% of
patients diagnosed with breast cancer succumb to the disease. The
high mortality rate may be attributed in part to a lack of
diagnostic methods allowing early detection. Another major cause of
mortality among breast cancer patients continues to be the presence
of the metastatic disease with ∼5% of patients exhibiting
clinically detectable metastases at the time of the initial
diagnosis and a further 30–40% of patients with no clinically
detectable disease harboring occult metastases. Although mammograms
are the most effective tool for detecting breast cancer, the US
Food and Drug Administration reports that mammography is able to
identify only ∼80% of breast cancers in females (2). Hence, there is a requirement for
further understanding of tumor biology and host response mechanisms
so that new diagnostic and therapeutic tools may be developed.
Early diagnosis is essential for the optimal management of breast
cancer. Thus, extensive studies are being conducted to identify and
validate new biomarkers to add to current markers and increase the
sensitivity and specificity of breast cancer detection.
Numerous studies have demonstrated that cancer sera
contain antibodies that react with a unique group of autologous
cellular antigens called tumor-associated antigens (TAAs) (3,4). The
types of cellular proteins that induce these autoantibody responses
are varied and include tumor suppressors, such as p53 (5) and p16 (6), oncogene products, such as c-myc
(7) and HER-2/neu (8), and other cancer-related proteins, such
as Imp2/p62 (9), CRD-BP (10), CIP2A/p90 (11), survivin (12,13)
and LEDGF (14). The various
factors leading to the increased production of such autoantibodies
are not completely understood. However, the available data show
that a number of the target antigens are cellular proteins, such as
p53, whose aberrant regulation or overexpression is capable of
leading to tumorigenesis (5,15,16).
The immune systems of certain cancer patients are able to sense
these aberrant tumor-associated proteins as unknown antigens and
have the capability to respond by producing autoantibodies
(17). Thus, cancer-associated
autoantibodies may be regarded as reporters identifying aberrant
de novo or disregulated cellular mechanisms in tumorigenesis
(3,4). The potential utility of
TAA-autoantibody systems as early cancer biomarker tools to monitor
therapeutic outcomes or as indicators of disease prognosis has been
investigated. The present study evaluated whether a mini-array of
multiple TAAs would enhance autoantibody detection and be an
effective tool in the immunodiagnosis of breast cancer.
Materials and methods
Serum samples and antibodies
In the present study, sera from 41 patients with
breast cancer and 82 normal individuals who had no clear evidence
of malignancy were provided by our collaborator in China. Based on
clinical information, all cancer sera were collected at the first
time of diagnosis and patients did not receive any treatment with
chemotherapy or radiotherapy. Normal control sera were collected
during annual health examinations. The present study was approved
by the Institutional Review Boards of the University of Texas at El
Paso (UTEP) and collaborating academic institutions.
Recombinant TAAs
All TAAs used in the present study, including Imp1,
p62, Koc, p53, p16, c-myc, survivin, cyclin B1, cyclin D1, cyclin E
and CDK2, were derived from our previous studies. The reactivities
of these selected TAAs were determined with either polyclonal or
monoclonal antibodies against the respective proteins.
Enzyme-linked immunosorbent assay
(ELISA)
Purified recombinant TAAs were individually diluted
in PBS to a final concentration of 0.5 μg/ml and 200
μl were pipetted into each well to coat Immulon 2 microtiter
plates (Fisher Scientific, Houston, TX, USA) overnight at 4°C. The
human serum samples were diluted at 1:200, incubated with the
antigen-coated wells at 37°C for 90 min followed by washing with
PBS containing 0.05% Tween-20. The samples were then incubated with
horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Caltag
Laboratories, Burlingame, CA, USA) as a secondary antibody diluted
1:2,000 for 90 min followed by washing with PBS containing 0.05%
Tween-20. A solution of 3,3’,5,5’-tetramethyl benzidine
(TMB)-H2O2-urea was used as the detecting
agent. The OD of each well was read at 450 nm. Each sample was
tested in duplicate. The cut-off value for determining a positive
reaction was designated as the mean absorbance of the 82 normal
human sera (NHS) plus 2 standard deviations (mean + 2SD). Since
several hundred test sera were analyzed at various time periods,
each run of the ELISA included at least 8 NHS samples and 2
positive control samples. These 8 NHS samples, representing a range
of 2SD above and below the mean of the 82 NHS, were used in each
experiment and the average value of the 8 NHS samples was used in
each run to normalize all absorbance values to the mean of the
entire 82 normal samples. In addition, all positive sera were
confirmed with repeat testing, as were certain negative sera. The
detailed protocol of the ELISA has been described previously
(9,18).
Western blotting and slot blot
analysis
Western blot analysis was used to confirm that the
bands observed in SDS-PAGE were reactive with the reference
antibodies. In brief, the purified TAAs were electrophoresed by
SDS-PAGE and subsequently transferred to a nitrocellulose membrane.
The individual strips were pre-blocked in PBS containing 0.05%
Tween-20 (PBST) with 5% non-fat milk for 30 min at room
temperature, then incubated for 90 min with patient sera diluted
1:100 and finally incubated with HRP-conjugated goat anti-human IgG
diluted 1:3,000 for 90 min, followed by washing with PBST solution.
The positive signals were recorded by autoradiography. Slot blot
analysis was used to confirm the positive sera samples detected by
ELISA. The method for the slot blotting was identical to that of
the western blotting with the exception that the purified
recombinant protein (100 ng/well) was applied directly to the
nitrocellulose membrane using a vacuum source. Membranes were not
cut into strips and therefore, the detection of autoantibodies to
all the TAAs in an individual patient’s serum was performed in one
blot simultaneously.
Statistical analysis
To determine whether the frequency of autoantibodies
binding to selected TAAs in the cancer sera was significantly
higher than that in NHS, the data were analyzed using the
χ2 tests with Yates’ correction. Two levels of
statistical significance (0.05 and 0.01) were used and P<0.05
was considered to indicate statistically significant differences.
The comprehensive evaluations of the testing results for each
anti-TAA antibody, including the methods for calculating the
sensitivity, specificity, Youden’s index (YI), positive and
negative likelihood ratio, positive (PPV) and negative predictive
value (NPV), agreement rate and κ-value, were based on the
methodology provided in the Epidemiology textbook (19).
Results
Prevalence of antibodies in a mini-array
of multiple TAAs in breast cancer
In order to evaluate whether the combination of
antibodies to multiple TAAs yields higher sensitivity for the
diagnosis of breast cancer, the present study tested breast cancer
sera for the presence of anti-TAA antibodies with a panel of ten
selected recombinant TAAs using an ELISA and revealed that the
combined the antibody frequency was 61.0% (25/41), significantly
higher than the frequency (13.4%) in the sera from normal
individuals (11/82). As shown in Table
I, antibody frequency for any individual TAA in breast cancer
was variable, ranging between 7.3 and 22.0%. The highest
frequencies were against c-myc (22.0%), survivin (22.0%), cyclin B1
(17.1%) and cyclin D1 (17.1%), followed by p62 (12.2%), p53
(12.2%), p16 (12.2%), Imp1 (12.2%), CDK2 (9.8%) and Koc (7.3%). It
was observed that, with the successive addition of TAAs to a total
of eight antigens (c-myc, survivin, cyclin B1, cyclin D1, p62, p53,
p12 and CDK2), there was a stepwise increase in the sensitivity, up
to 61.0%, and the specificity was 89.0%. If additional antigens
(Imp1 and Koc) were added to the panel, there was no further
increase in the sensitivity (see Table
II). These results indicate that an array of eight TAAs is able
to serologically distinguish breast cancer patients from normal
individuals at a sensitivity of 61.0%. However, it should be
determined whether this TAA combination distinguishes breast cancer
from other types of cancer. Positive results were also confirmed by
slot blotting. Slot blot analysis of four representative breast
cancer sera is shown in Fig. 1.
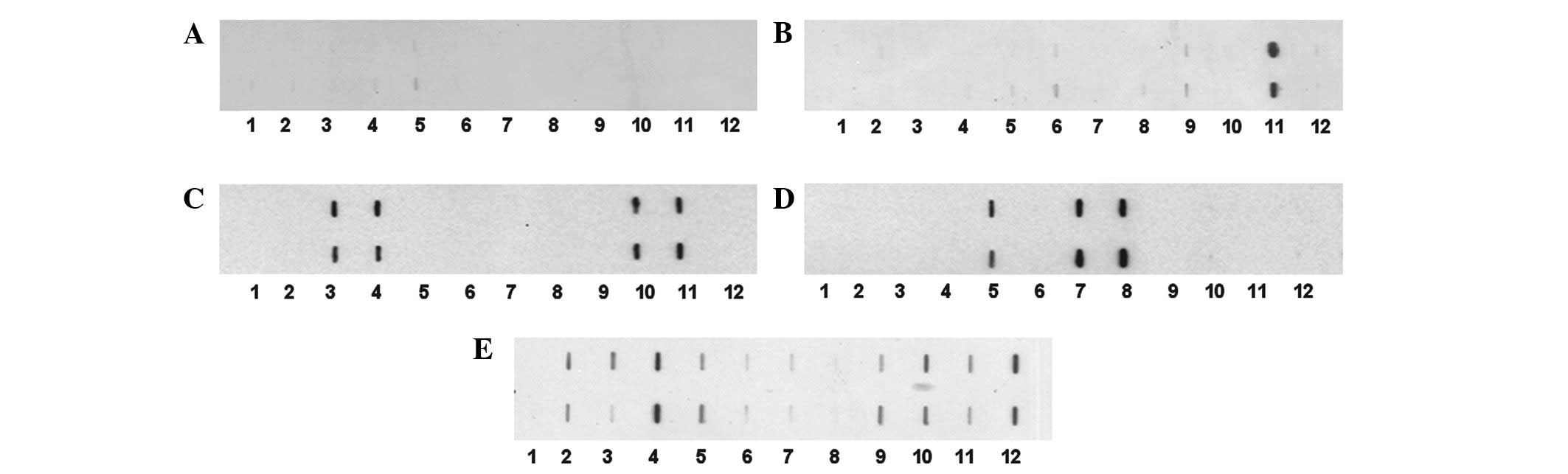 | Figure 1.Mini-array of multiple TAAs with four
representative breast cancer sera using slot blot analysis. Each
blot represents a duplicate test for autoanti-bodies against a
panel of eleven recombinant TAAs, with PBS as a negative control.
Purified recombinant protein (100 ng per well) was applied directly
to the nitrocellulose membrane using a vacuum device. Membranes
were used for the simultaneous detection of autoantibodies in an
individual patient’s serum to any of the eleven TAAs, following
standard immunoblotting procedures. 1, PBS; 2, survivin; 3, p53; 4,
p16; 5, cyclin B1; 6, cyclin D1; 7, cyclin E; 8, Koc; 9, Imp1; 10,
p62; 11, CDK2; 12, c-myc. (A) Normal human serum showing no
reactivity to any of the eleven TAAs. (B–E) Four representative
breast cancer sera showing different antibody profiles with the 11
TAAs. TAA, tumor-associated antigen; PBS, phosphate-buffered
saline. |
 | Table I.Frequency of antibodies for ten TAAs
in breast cancer. |
Table I.
Frequency of antibodies for ten TAAs
in breast cancer.
| No. (%) of
autoantibodies in:
|
|---|
| Autoantibodies
to: | BC (41) | NHS (82) |
|---|
| c-myc | 9 (22.0)b | 0 (0) |
| survivin | 9 (22.0)b | 1 (1.2) |
| cyclin B1 | 7 (17.1)b | 1 (1.2) |
| cyclin D1 | 7 (17.1)a | 2 (2.4) |
| p62 | 5 (12.2)a | 1 (1.2) |
| p53 | 5 (12.2)a | 2 (2.4) |
| p16 | 5 (12.2)a | 2 (2.4) |
| Imp1 | 5 (12.2)a | 2 (2.4) |
| CDK2 | 4 (9.8)a | 1 (1.2) |
| Koc | 3 (7.3) | 1 (1.2) |
| Cumulative to ten
antigens | 61.0 (25/41)b | 11 (13.4) |
 | Table II.Sequential addition of antigen into
the panel of ten TAAs in breast cancer. |
Table II.
Sequential addition of antigen into
the panel of ten TAAs in breast cancer.
| No. (%) of
autoantibodies in:
|
|---|
| Antigen | BC (41) | NHS (82) |
|---|
| c-myc | 9 (22.0)a | 0 (0) |
| c-myc and
survivin | 14 (34.1)a | 1 (1.2) |
| c-myc, surviving and
cyclin B1 | 16 (39.0)a | 2 (2.4) |
| c-myc, survivin,
cyclin B1 and cyclin D1 | 18 (43.9)a | 4 (4.9) |
| c-myc, survivin,
cyclin B1, cyclin D1 and p62 | 20 (48.8)a | 5 (6.1) |
| c-myc, survivin,
cyclin B1, cyclin D1, p62 and p53 | 21 (51.2)a | 7 (8.5) |
| c-myc, survivin,
cyclin B1, cyclin D1, p62, p53 and p16 | 23 (56.1)a | 9 (11.0) |
| c-myc, survivin,
cyclin B1, cyclin D1, p62, p53, p16 and CDK2 | 25 (61.0)a | 9 (11.0) |
| c-myc, survivin,
cyclin B1, cyclin D1, p62, p53, p16, CDK2 and Imp1 | 25 (61.0)a | 11 (13.4) |
| c-myc, survivin,
cyclin B1, cyclin D1, p62, p53, p16, Imp1, CDK2 and Koc | 25 (61.0)a | 11 (13.4) |
Evaluation of diagnostic values of a
mini-array of multiple TAAs in immunodiagnosis of breast
cancer
The validity of a test is defined as its ability to
distinguish between individuals who have a disease and those who do
not. In order to address the question of how valuable the approach
of antibody detection to a mini-array of multiple TAAs is in
separating individuals with and without cancer, a group of
parameters, including the sensitivity/specificity, YI and PPV/NPV,
were calculated and are shown in Tables
III and IV. Table III shows the comprehensive
evaluation of antibodies to a panel of ten TAAs. With the
successive addition of TAAs to a total of eight antigens, there was
a stepwise increase in positive antibody reactions, up to 61.0%, as
well as a slight decrease of specificity from 100% with one TAA to
89.0% with a panel of eight. If additional antigens (Imp1 and Koc)
were added to the panel, there was no further increase in the
sensitivity but a slight decrease of specificity from 89.0 to
86.6%. The sensitivity and specificity are consistent with the
results of other two parameters (PPV/NPV). The PPV/NPVs were also
variable in the various combinations of TAAs. In the panel with a
total of eight TAAs, the PPV was 73.5% and the NPV was 82.0%. The
YI was also increased from 0.220 with one TAA to 0.500 with eight
TAAs. The positive and negative likelihood ratios were 5.545 and
0.438, respectively, indicating that the clinical diagnostic value
of a parallel assay of five TAAs was high. This also suggests that
a parallel assay of eight TAAs is able to raise the diagnostic
accuracy significantly. The agreement rate and κ-value were 79.7%
and 0.52, respectively, indicating that the observed value of this
assay had a middle range coincidence with the actual value. Taken
together, these data show the usefulness of the multiple antigen
array in increasing the clinical diagnostic quality and value for
cancer.
 | Table III.Evaluation of antibodies for ten TAAs
selected in the detection of breast cancer. |
Table III.
Evaluation of antibodies for ten TAAs
selected in the detection of breast cancer.
| Positive % (No.)
|
|---|
| Panel of TAAs | BC (41) | NHS (82) | Sensitivity | Specificity | YI | PPV | NPV |
|---|
| c-myc | 22.0 (9/41)a | 0 (0/82) | 22.0 | 100.0 | 0.220 | 100.0 | 71.9 |
| c-myc+survivin | 34.1 (14/41)a | 1.2 (1/82) | 34.1 | 98.8 | 0.329 | 93.3 | 75.0 |
| c-myc+survivin+cyclin
B1 | 39.0 (16/41)a | 2.4 (2/82) | 39.0 | 97.6 | 0.366 | 88.9 | 76.2 |
|
c-myc+survivin+cyclin B1+cyclin D1 | 43.9
(18/41)a | 4.9 (4/82) | 43.9 | 95.1 | 0.390 | 81.8 | 77.2 |
|
c-myc+survivin+cyclin B1+cyclin D1
+p62 | 48.8
(20/41)a | 6.1 (5/82) | 48.8 | 93.9 | 0.427 | 80.0 | 78.6 |
|
c-myc+survivin+cyclin B1+cyclin D1
+p62+p53 | 51.2
(21/41)a | 8.5 (7/82) | 51.2 | 91.5 | 0.427 | 75.0 | 78.9 |
|
c-myc+survivin+cyclin B1+cyclin D1
+p62+p53+p16 | 56.1
(23/41)a | 11.0 (9/82) | 56.1 | 89.0 | 0.451 | 71.9 | 80.2 |
|
c-myc+survivin+cyclin B1+cyclin D1
+p62+p53+p16+CDK2 | 61.0
(25/41)a | 11.0 (9/82) | 61.0 | 89.0 | 0.500 | 73.5 | 82.0 |
|
c-myc+survivin+cyclin B1+cyclin D1
+p62+p53+p16+CDK2+Imp1 | 61.0
(25/41)a | 13.4 (11/82) | 61.0 | 86.6 | 0.476 | 69.4 | 81.6 |
|
c-myc+survivin+cyclin B1+cyclin D1
+p62+p53+p16+Imp1+CDK2+koc | 61.0
(25/41)a | 13.4 (11/82) | 61.0 | 86.6 | 0.476 | 69.4 | 81.6 |
 | Table IV.Summary of the diagnostic value of
antibodies for a panel of eight TAAs in breast cancer. |
Table IV.
Summary of the diagnostic value of
antibodies for a panel of eight TAAs in breast cancer.
| Serum | Any TAA
positive | All TAA
negative | Total |
|---|
| BC | 25 (A) | 16 (C) | 41 (R1) |
| NHS | 9 (B) | 73 (D) | 82 (R2) |
| Total | 34 (C1) | 89 (C2) | 123 (N) |
Discussion
Interest in the use of anti-TAA antibodies as
serological markers for cancer diagnosis derives from the
recognition that these antibodies are generally absent, or present
in very low titers, in normal individuals and in non-cancer
conditions (with the exception of autoimmune conditions). The
persistence and stability of autoantibodies in the serum of cancer
patients is an advantage over other potential markers, including
the TAAs themselves, which are released by tumors but are rapidly
degraded or cleared after circulating in the serum for a limited
time (17). Furthermore, the
widespread availability of methods and reagents to detect serum
autoantibodies facilitates their characterization in cancer
patients and assay development. However, in contrast to autoimmune
diseases, where the presence of a particular autoantibody may have
diagnostic value, individually evaluated cancer-associated
autoantibodies have little diagnostic value primarily due to their
low frequency, sensitivity and specificity. This drawback may be
overcome using mini-arrays of carefully selected TAAs and different
types of cancer may require different TAA arrays to achieve the
sensitivity and specificity required to make immunodiagnosis a
feasible adjunct to tumor diagnosis (18,20–25).
In the future we aim to increase the sensitivity and
specificity of anti-TAA antibodies as diagnostic markers of cancer
by expanding the TAA array to include antigens which may be more
selectively associated with one specific type of cancer, such as
breast cancer, and not with others. We expect that our mini-array
of multiple TAAs may be used as a novel non-invasive approach to
identify cancer in the normal population and high-risk individuals.
Our concern is that the approach may be not suitable for
distinguishing one type of cancer from another. The reason is that
certain TAAs, such as p53, p16 and c-myc, which were used in the
present mini-array approach, are associated with several types of
cancer, including liver, colon, gastric, lung, ovarian and prostate
cancer (18,20,22–25).
For future studies, we propose that certain selected
antibody-antigen systems may be unique to one type of cancer and
others may not. A comprehensive analysis and evaluation of various
combinations of selected antibody-antigen systems is likely to be
useful for the development of autoantibody profiles involving
various panels or arrays of TAAs and the results may be useful for
diagnosis of certain other types of cancer. In the present study, a
mini-array of multiple TAAs were used as coating antigens in an
ELISA to detect autoantibodies against these antigens in 41 sera
from patients with breast cancer and 82 sera from normal
individuals. The antibody frequency to the individual TAAs in
breast cancer was variable and ranged between 7.3 and 22.0%. This
relatively low sensitivity using one individual anti-TAA antibody
as a diagnostic marker does not meet the requirements of clinical
early diagnosis of breast cancer. With the successive addition of
TAAs to a total of eight antigens, there was a stepwise increase in
positive antibody reactions, reaching a sensitivity of 61.0% and a
specificity of 89.0% in breast cancer. The positive and negative
likelihood ratios were 5.545 and 0.438, respectively, which showed
that the clinical diagnostic value of a parallel assay of eight
TAAs was high. The PPVs and NPVs were 73.5 and 82.0%, respectively,
indicating that the parallel assay of eight TAAs raised the
diagnostic precision significantly. The agreement rate and κ-value
were 79.7% and 0.52, respectively, which indicated that the
observed value of this assay had a middle range coincidence with
the actual value.
In conclusion, this preliminary study further
supports our hypothesis and also suggests that additional breast
cancer-specific TAAs are likely to be necessary to enhance the
frequency of anti-TAA antibody detection using an array of multiple
TAAs with potential immunodiagnostic value. Once a TAA array that
is highly specific and sensitive to breast cancer is identified, we
plan to develop a breast cancer-specific mini-array TAA chip for
automated high-throughput breast cancer screening. Given that the
presence of serum autoantibodies to TAAs may signal molecular
events associated with tumorigenesis, it would be possible to use
highly sensitive and specific TAA chips for screening populations
at a high risk of developing breast cancer, which may lead to early
preventive or therapeutic interventions aimed at suppressing or
slowing the appearance of tumors.
Acknowledgements
The authors would like to thank Dr
Xuanxian Peng at Sun Yat-sen University, China providing a number
of the serum samples to this study. This study was supported by
grants from the National Natural Science Foundation of China
(#81172086) and the NIH of the USA (#SC1CA166016).
References
|
1.
|
Shin HR, Joubert C, Boniol M, et al:
Recent trends and patterns in breast cancer incidence among Eastern
and Southeastern Asian women. Cancer Causes Control. 21:1777–1785.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
American Cancer Society: Cancer Facts
& Figures 2007. ACS Publication; Atlanta GA: 2007
|
|
3.
|
Tan EM and Zhang J: Autoantibodies to
tumor-associated antigens: reporters from the immune system.
Immunol Rev. 222:328–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zhang JY and Tan EM: Autoantibodies to
tumor-associated antigens as diagnostic biomarkers in
hepatocellular carcinoma and other solid tumors. Expert Rev Mol
Diagn. 10:321–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Soussi T: p53 Antibodies in the sera of
patients with various types of cancer: a review. Cancer Res.
60:1777–1788. 2000.PubMed/NCBI
|
|
6.
|
Looi K, Megliorino R, Shi FD, Peng XX,
Chen Y and Zhang JY: Humoral immune response to p16, a
cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep.
16:1105–1110. 2006.PubMed/NCBI
|
|
7.
|
Yamamoto A, Shimizu E, Takeuchi E, et al:
Infrequent presence of anti-c-Myc antibodies and absence of c-Myc
oncoprotein in sera from lung cancer patients. Oncology.
56:129–133. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Disis ML, Pupa SM, Gralow JR, Dittadi R,
Menard S and Cheever MA: High-titer HER-2/neu protein-specific
antibody can be detected in patients with early-stage breast
cancer. J Clin Oncol. 15:3363–3367. 1997.PubMed/NCBI
|
|
9.
|
Zhang JY, Chan EK, Peng XX and Tan EM: A
novel cytoplasmic protein with RNA-binding motifs is an autoantigen
in human hepatocellular carcinoma. J Exp Med. 189:1101–1110. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Doyle GA, Bourdeau-Heller JM, Coulthard S,
Meisner LF and Ross J: Amplification in human breast cancer of a
gene encoding a c-myc mRNA-binding protein. Cancer Res.
60:2756–2759. 2000.PubMed/NCBI
|
|
11.
|
Soo Hoo L, Zhang JY and Chan EK: Cloning
and characterization of a novel 90 kDa ‘companion’ auto-antigen of
p62 overexpressed in cancer. Oncogene. 21:5006–5015. 2002.
|
|
12.
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Megliorino R, Shi FD, Peng XX, et al:
Autoimmune response to anti-apoptotic protein survivin and its
association with antibodies to p53 and c-myc in cancer detection.
Cancer Detect Prev. 29:241–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Daniels T, Zhang J, Gutierrez I, et al:
Antinuclear autoantibodies in prostate cancer: immunity to
LEDGF/p75, a survival protein highly expressed in prostate tumors
and cleaved during apoptosis. Prostate. 62:14–26. 2005. View Article : Google Scholar
|
|
15.
|
Crawford LV, Pim DC and Bulbrook RD:
Detection of antibodies against the cellular protein p53 in sera
from patients with breast cancer. Int J Cancer. 30:403–408. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Winter SF, Minna JD, Johnson BE, Takahashi
T, Gazdar AF and Carbone DP: Development of antibodies against p53
in lung cancer patients appears to be dependent on the type of p53
mutation. Cancer Res. 52:4168–4174. 1992.PubMed/NCBI
|
|
17.
|
Anderson KS and LaBaer J: The sentinel
within: exploiting the immune system for cancer biomarkers. J
Proteome Res. 4:1123–1133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhang JY, Casiano CA, Peng XX, Koziol JA,
Chan EK and Tan EM: Enhancement of antibody detection in cancer
using panel of recombinant tumor-associated antigens. Cancer
Epidemiol Biomarkers Prev. 12:136–143. 2003.PubMed/NCBI
|
|
19.
|
Chen WQ: Assessing the validity and
reliability of screening test. Epidemiology. Li LM: Publishing
Company of the People’s Health; Beijing: pp. 289–292. 2004
|
|
20.
|
Koziol JA, Zhang JY, Casiano CA, et al:
Recursive partitioning as an approach to selection of immune
markers for tumor diagnosis. Clin Cancer Res. 9:5120–5126.
2003.PubMed/NCBI
|
|
21.
|
Zhang JY, Megliorino R, Peng XX, Tan EM,
Chen Y and Chan EK: Antibody detection using tumor-associated
antigen mini-array in immunodiagnosing human hepatocellular
carcinoma. J Hepatol. 46:107–114. 2007. View Article : Google Scholar
|
|
22.
|
Chen Y, Zhou Y, Qiu S, et al:
Autoantibodies to tumor-associated antigens combined with abnormal
alpha-fetoprotein enhance immunodiagnosis of hepatocellular
carcinoma. Cancer Lett. 289:32–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu W, Wang P, Li Z, et al: Evaluation of
tumour-associated antigen (TAA) miniarray in immunodiagnosis of
colon cancer. Scand J Immunol. 69:57–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chen Y, Lin P, Qiu S, et al:
Autoantibodies to Ca2+ binding protein Calnuc is a
potential marker in colon cancer detection. Int J Oncol.
30:1137–1144. 2007.
|
|
25.
|
Li L, Wang K, Dai L, Wang P, Peng XX and
Zhang JY: Detection of autoantibodies to multiple tumor-associated
antigens in the immunodiagnosis of ovarian cancer. Mol Med Rep.
1:589–594. 2008.PubMed/NCBI
|















