Introduction
Colorectal cancer is one of the most common clinical
gastrointestinal cancers that poses a serious threat to human
health. The GLOBOCAN 2008 estimates stated that colorectal cancer
is the third most commonly diagnosed cancer in males and the second
in females, with over 1.2 million new cancer cases and 608,700
fatalities occurring every year (1). Effective treatments for colorectal
cancer include surgery, chemotherapy and targeted therapy.
Chemotherapy and targeted therapy are the final strategies
implemented to extend patient survival, particularly with advanced
metastatic colorectal cancer (2,3).
However, present evidence-based medicine indicates that colorectal
cancer patients hardly benefit from chemotherapy due to chemical
toxicity and self-resistance. Therefore, targeted therapy has a
greater potential and is currently being investigated further, with
a greater research emphasis on cancer therapeutics. Throughout the
past decade, targeted treatment of cancer has mainly focused on the
epidermal growth factor (EGF) pathway (4,5).
The epidermal growth factor receptor (EGFR) is a key
member of the ErbB family, which consists of four members: EGFR
(ErbB1), Her2 (ErbB2), Her3 (ErbB3) and ErbB4. In cancer cells, the
extracellular domain of the EGFR binds to the EGF and the EGF
pathway is activated; signaling is initiated to regulate the
differentiation, survival, proliferation and migration of cancer
cells (6). However, activation of
the EGFR is required for signaling initiation; the ligand-induced
conformational change in the receptor ectodomains results in the
association of the cytoplasmic tyrosine kinase domains of two
receptor molecules (7). The
activation of the pathway depends not only on EGF as the ligand
binding to the EGFR ectodomains, but also on the activation of
homodimerized or heterodimerized cytoplasmic domains of EGFRs
(8,9). Bill et al have identified
cytohesins as conformational activators of the cytoplasmic dimer,
which play an important role in lung cancer ErbB signaling
(10).
The cytohesin family includes four highly homologous
members: Cytohesin-1, -2 (ARNO), -3 (Grp1) and -4 (11). Cytohesins are guanine nucleotide
exchange factors (GEFs) for ADP ribosylation factors (ARFs) that
belong to the family of small Ras-like GTPases. As with the case of
other small GTPases, ARF function critically depends on activation
by GEFs (12). Therefore,
cytohesins are important regulators of cytoskeletal dynamics, cell
migration, vesicular traffic and signaling (10,11,13).
Bill et al demonstrated that cytohesin
overexpression increases EGFR activation and signaling. Moreover,
siRNA and chemical inhibition of cytohesins produced consistent
results both in vivo and in vitro in human lung
adenocarcinomas. Therefore, the authors concluded that cytohesins
were conformational activators of the ErbB receptor in lung cancer
(10). In the present study, we
demonstrated that EGFR signaling was reduced when cytohesins were
inhibited in the HT-29 cell line. Subsequently, whether cytohesins
have the potential to act as a target for colorectal cancer therapy
was preliminarily investigated.
Materials and methods
Reagents
Cell culture media included RPMI-1640, McCoy’s 5A
and L-15, which were purchased from Genom (Shanghai, China). The
following mouse anti-human antibodies were used: Cytohesin-2 (cat.
no. ab56510; Abcam, Hong Kong, China); p-EGFR (pY1068, cat. no.
1138-1; Epitomics, Burlingame, CA, USA); p-ERK1/2 (T202/Y204, cat.
no. BS5016; Bioworld Technology, Inc., St. Louis Park, MN, USA);
EGFR (cat. no. 3197; Cell Signaling Technology, Inc., Danvers, MA,
USA); GAPDH (cat. no. AP0063; Bioworld Technology, Inc.);
phycoerythrin (PE)-conjugated rabbit anti-mouse IgG and fluorescein
isothiocyanate (FITC)-conjugated goat anti- rabbit IgG (cat. no.
GAM007; Multisciences, China). TRIzol RNA Isolation and M-MLV RTase
kits were purchased from Promega Corporation (Madison, WI, USA),
and the Real-Time PCR kit was purchased from Fermentas (USA).
SecinH3 (cat. no. 565725/sc-203260) was purchased from Merck and
siRNA oligo was purchased from Shanghai Gene Pharma (China). The
following reagents, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT; cat. no. m5655), dimethyl sulfoxide
(DMSO; cat. no. D5879) and 0.25% trypsin, were purchased from Sigma
(St. Louis, MO, USA). Human EGF (cat. no. AF-100-15) was purchased
from Peprotech, Inc. (Rocky Hill, NJ, USA) and fetal bovine serum
(FBS) was purchased from Gibco (Carlsbad, CA, USA).
Cell lines and cultivation
Human colorectal cancer cell lines including HT-29,
SW620, SW480, LOVO and HCT-116, were obtained from the Key
Laboratory of Cancer Prevention and Intervention, Cancer Institute,
Second Affiliated Hospital, School of Medicine, Zhejiang
University, China. The HT-29 cell line was cultured in RPMI-1640
(with 10% FBS and 1% streptomycin/penicillin); SW620, SW480 and
LOVO cell lines were cultured in L-15 (with 10% FBS and 1%
streptomycin/penicillin); HCT-116 cell line was cultured in McCoy’s
5A (with 10% FBS and 1% streptomycin/penicillin). All cell lines
were cultured at 37°C and 5% CO2 in an incubator, and
passaged with 0.25% trypsin (Sigma) in 0.2 mol/l phosphate-buffered
saline (PBS; Sigma). The study was approved by the ethics committee
of the Cancer Institute, The Second Affliated Hospital, Zhejiang
University School of Medicine, Hangzhou, China.
RT-PCR
Primers were designed according to the Genbank
sequences and were synthesized by Shanghai Sangon (Shanghai,
China). The primer sequences were as follows: Cytohesin-1,
5′-AGTGCATTAAAGCAGCCATCAG-3′ and 5′-TCAGTGTCGCTTCGTGGAG-3′;
cytohesin-2 (ARNO), 5′-GAAACCGAACTGCTTTGAACT-3′ and
5′-CAGCCGCCTGATGGACT-3′; cytohesin-3 (Grp1), 5′-ATG
AAATCCATCAAAGCCAGTA-3′ and 5′-CAATCCTT CGTTTCCTCGTT-3′;
cytohesin-4, 5′-GTCCATCCGAGCC AGCAT-3′ and
5′-GGTAACGGGGAACAGCAAT-3′; GAPDH (human housekeeper gene),
5′-AATGTGTCCGTCGT GGATCTG-3′ and 5′-CAACCTGGTCCTCAGTGTAGC-3′. Total
RNA was extracted using the TRIzol RNA isolation kit and cDNA was
synthesized using the M-MLV RTase kit, according to the
manufacturer’s instructions. For this reaction, GAPDH acted as an
inner control and was amplified in each reaction system. The
reaction conditions were 95°C for 3 min, 40 cycles of 95°C for 10
sec, 62°C for 35 sec and 72°C for 60 sec.
Immunofluorescence
Aseptic slides were placed in 24-well plates and
after prewarming at 37°C for 24 h, 104 cells/well from
the HT-29 cell line were incubated in the plates. Cells were
cultured with RPMI-1640 culture medium at 37°C and 5%
CO2 in an incubator until cell growth covered 60–80% of
the slides. Then, the culture medium was removed and cells were
fixed in 4% paraformaldehyde for 15 min. After washing three times
with PBS and 0.25% Triton X-100/TBS for 10–15 min at room
temperature, mouse anti-cytohesins IgG were incubated overnight at
4°C. Following repeated washing with PBS, slides were incubated
with PE-conjugated rabbit anti-mouse IgG and FITC-conjugated goat
anti-rabbit IgG as secondary antibodies for 1 h at 37°C, then
washed with PBS and coverslipped. Subsequently, ARNO and EGFR
expression was observed using a Zeiss LSM-710 fluorescent
microscope with a Spot digital camera (Carl Zeiss, Germany). For
comparable analysis of the intensity levels of ARNO and EGFR
expression, the same exposure conditions were maintained throughout
the experiment.
Western blot analysis
Cells were collected and extracted by the eukaryotic
cell lysis buffer according to the manufacturer’s instructions
(Total protein extraction kit 2140, Merck Millipore, Billerica, MA,
USA). Then, proteins were separated by 12% SDS-PAGE and blotted to
a nitrocellulose membrane by a wet transfer device (Bio-Rad,
Hercules, CA, USA). Blotted membranes were blocked by 10% skimmed
milk in PBS Tween-20 (PBST) for 1 h. After washing three times with
Tris-buffered saline Tween-20 (TBST), membranes were incubated with
primary antibody diluted 1:1,000 at room temperature for 1 h, then
incubated in HRP-labeled secondary antibody diluted 1:10,000 at
room temperature for 1 h. After rinsing, visualization was
conducted using the enhanced ehemiluminescence (ECL) western
blotting detection system (Amersham Biosciences, Little Chalfont,
UK) and cells were exposed to X-ray film (Kodak, USA). GAPDH
protein was used as an inner control.
siRNA selection
Three pairs of ARNO siRNAs were designed and
synthesized by Genepharma Company (China). The siRNA sequence pairs
were as follows: siRNA-1, 5′-GUUCU UGGUGGAGAAUGAATT-3′ and
5′-UUCAUUCUCCACC AAGAACTT-3′; siRNA-2, 5′-AGGCCCUCAGGCAGUUU CUTT-3′
and 5′-AGAAACUGCCUGAGGGCCUTT-3′; siRNA-3,
5′-GCUGGUUUAUCCUCACAGATT-3′ and 5′-UCUGUGAGGAUAAACCAGCTT-3′. Each
pair of siRNA sequences was identified in the HT-29 cell line;
cells were transfected with 100 pmol of each siRNA in 5 μl
Lipofectamine 2000/105 cells/ml, and then cultured in
serum- free medium. After 24 h, cells were collected for western
blot analysis.
MTT
HT-29 cells were plated in 96-well plates with a
density of 3,000 cells/well. Cells were cultured with 1% FBS and
inhibitors (20 μmol/l SecinH3 or 50 nmol/l per 5 pmol
ARNO/negative siRNA in 0.25 μl Lipofectamine 2000) for 24,
48 and 72 h, at 37°C and 5% CO2. Then 5 mg/ml MTT (20
μl) was added to each well and incubated for 4 h. Then, 200
μl DMSO was added to resolve the MTT substrate and
absorbance was measured at 570 nm using a SpectraMax Microplate
Reader (Bio-Rad).
Statistics
Results are presented as the mean ± standard error
of the mean (SEM). The Statistical Package for the Social Sciences
(SPSS) 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. Paired comparisons were performed using a
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference between means.
Results
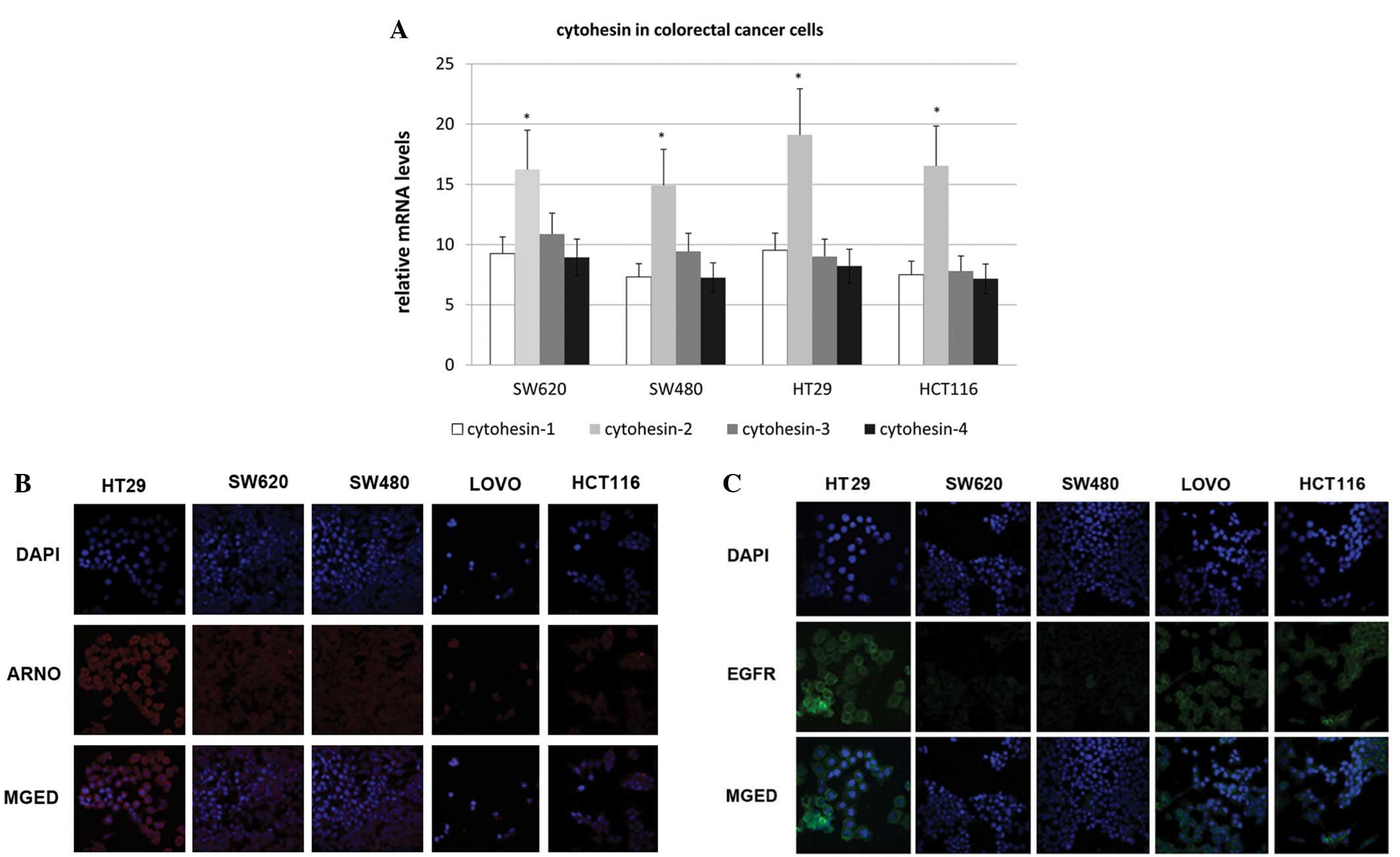
All four cytohesins were transcribed and
ARNO was expressed in colorectal cancer cells
RT-PCR was employed to detect the transcription of
the cytohesin family. Cytohesin-1, -2 (ARNO), -3 (Grp1) and -4 were
transcribed in all four cell lines, which included HT-29, SW620,
SW480 and HCT-116. We found that mRNA of the four cytohesins was
transcribed in all four cell lines, and ARNO mRNA had the highest
expression level (Fig. 1A).
Additionally, by an immunofluorescence assay, we demonstrated that
ARNO was highly expressed in HT-29 cells and was located in the
cytoplasm, near to the membrane (Fig.
1B). Therefore, the expression of EGFR in colorectal cells was
detected by immunofluorescence (Fig.
1C). The expression of EGFR in HT-29 cells was higher than that
of the other cell lines. Therefore, the HT-29 cell line was
selected for EGF pathway research in the following study.
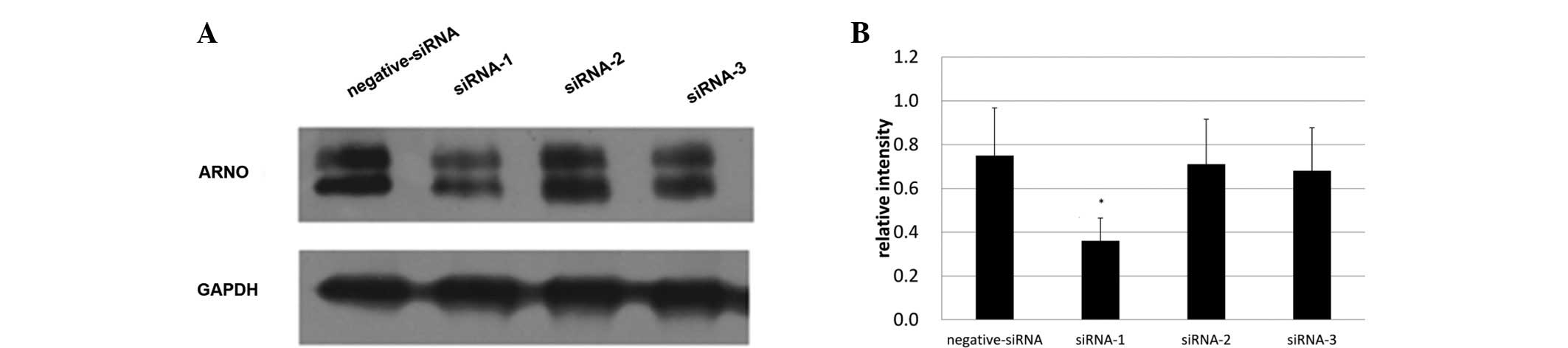
siRNA-1 with the strongest inhibitory
effects was selected for ARNO blocking
To select the most effective siRNA for ARNO, three
siRNAs (siRNA-1, -2 and -3) were designed to inhibit the expression
of ARNO. Expression of ARNO was then detected under the inhibition
of these three siRNAs. The greatest inhbitory effect was produced
by siRNA-1; the maximum inhibition rate was 49.271%. Therefore,
siRNA-1 was selected to be the ARNO siRNA inhibitor that was used
in the present study (Fig. 2). The
selected ARNO siRNA sequence pair was:
5′-AGTGCATTAAAGCAGCCATCAG-3′, and 5′-TCAGTGTCGCTTCGTGGAG-3′.
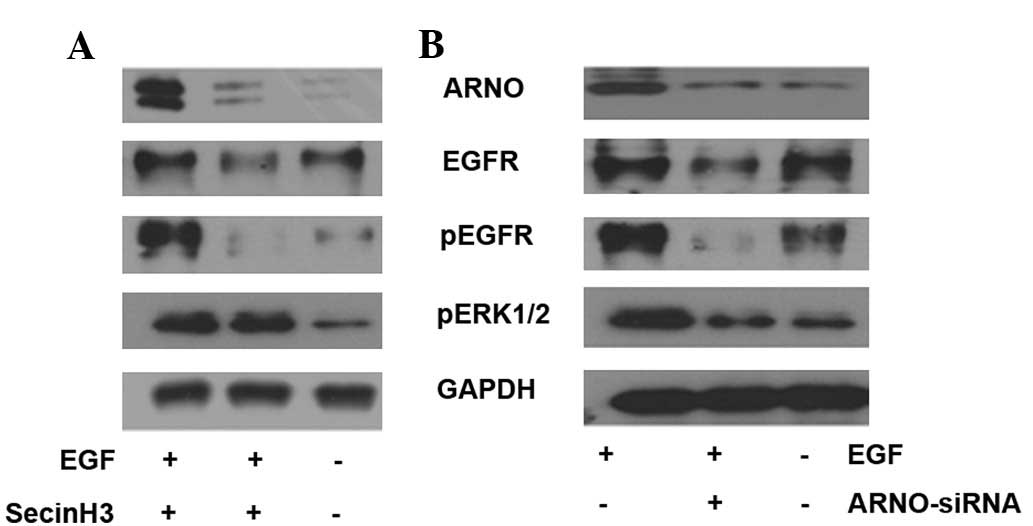
Inhibition of cytohesins reduces EGF
pathway signaling in HT-29 cells
To detect the function of cytohesins in the EGF
pathway, cytohesins were inhibited by SecinH3 and ARNO siRNA in
HT-29 cells. In the assay, HT-29 cells were cultured in 35 mm
glass-bottom dishes, marked as group A, B or C. All cells were
cultured with 1% FBS culture medium. SecinH3 (or a mixture of 100
pmol ARNO siRNA in 5 μl Lipofectamine 2000) was added to
dishes from group B when cells had spread to cover 70% of the
dishes for 10 h. Simultaneously, 0.2% DMSO (or 5 μl
Lipofectamine 2000) was added to dishes from groups A and C as a
control; then 50 ng/ml EGF (Peprotech, Inc.) was added to dishes
from groups A and B for 5 min. Western blot analysis was employed
to test the expression of the EGF pathway-associated molecules,
which included ARNO, EGFR, p-EGFR and p-ERK1/2. The results
indicated that when cytohesins were blocked by SecinH3 or inhibited
by ARNO siRNA, ARNO expression was reduced in HT-29 cells.
Additionally, phosphorylated molecules of the EGF pathway,
including p-EGFR and p-ERK1/2, were downregulated in HT-29 cells
(Fig. 3).
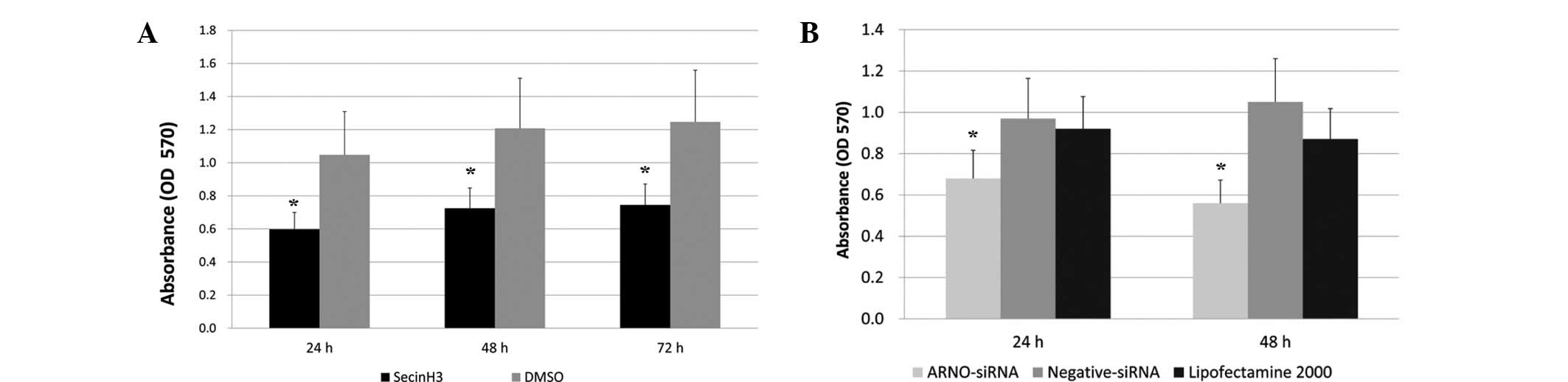
Blocking cytohesins inhibits the
proliferation of HT-29 cells
To detect whether cytohesins are involved in the
proliferation of HT-29 cells, we used the specific cytohesin
antagonist SecinH3 and the EGFR-expressing human colorectal
adenocarcinoma-derived HT-29 cells. HT-29 cells were treated with
SecinH3 and then proliferation was detected by an MTT assay. DMSO
was added to the cell culture medium in the control group. After
culture for 24, 48 and 72 h, the inhibition rates of SecinH3
compared with the control group were 56.77, 58.72 and 57.22%,
respectively (n=3, Fig. 4A).
The ARNO siRNA described previously was used as an
inhibitor to identify whether ARNO downregulation is capable of
reducing the proliferation of HT-29 cells. The MTT assay results
demonstrated that the growth and proliferation of tumor cells were
significantly inhibited by ARNO siRNA at 24 and 48 h, while the
inhibition rates were 68.63 and 58.95%, respectively, compared with
the Lipofectamine 2000 group (n=3, Fig.
4B).
Discussion
Growth and survival of cancer cells is critically
dependent on specific signaling molecules (14). The EGF pathway is considered to be
the most prominent signaling pathway in colorectal cancer, as it
regulates the differentiation, survival, proliferation and
migration of cancer cells. Recently, certain individuals with
wild-type Kras gene colorectal cancer have benefited from therapies
targeting the EGFR. However, resistance to the EGFR blockade
inevitably occurs due to a mutation in the gene encoding EGFR that
impairs the binding of cetuximab to EGFR (15–17).
Therefore, it is necessary to select new targets in this pathway to
overcome the resistance acquired due to mutations.
Recently, Yonesaka et al identified acquired
resistance to EGFR target therapy via increased signaling through
Her2 (ErbB2; also a member of the ErbB family). Notably, the
authors demonstrated that either amplification of ErbB2 or
increased levels of the ErbB3/ErbB4 ligand heregulin led to de
novo or acquired cetuximab resistance (18), and Ruan et al achieved
similar results in a breast cancer study (19). Cytohesins, family members of
GTPases, have been researched for their regulation of the
reassembly of the cytoskeleton and the activation of integrin or
the integrin signaling system, which is critically associated with
cell adhesion and migration (11,20,21). A
further study identified that cytohesins as EGFR activators may
form a layer of positive regulation by facilitating the structural
rearrangements required to convert the receptor dimer into its
active conformation in lung cancer (10).
Studies by Kolanus (11) and Ogasawara et al(22) concerning the expression of
cytohesins demonstrated that cytohesin-2 (ARNO) and -3 (Grp1) were
ubiquitously expressed, whereas cytohesins-1 and -4 were primarily
leukocyte-specific. Cytohesin-1 is a key regulator of neutrophil
adhesion to endothelial cells and to components of the
extracellular matrix, which may influence cell emigration through
its dual opposing effect on β1 and β2 integrin activation (23). Additionally, ARNO behaves as a
bistable switch, as it has an absolute requirement for activation
by an Arf protein but, once triggered, it becomes highly active
through the positive feedback effect of Arf1-GTP. This property of
ARNO may provide an explanation for its function in signaling
pathways that, once triggered, must move forward decisively
(24). Additionally, in the present
study, we detected the presence of cytohesins in the cytoplasm
(near the membrane) by immunofluorescence, and ARNO was the most
highly expressed cytohesin family member. Therefore, we employed
molecular ARNO and the HT-29 cell line as subjects for the other
sections of our study. Whether the strong expression of ARNO in
colorectal cancer cells, potentially by enhanced EGFR signaling,
contributes to tumor differentiation, survival, proliferation and
migration, is yet to be determined. However, this has been
identified in other types of cancer cells (25,26).
In the cell proliferation section of the present
study, HT-29 cells were stimulated by human EGF in the presence of
SecinH3 or treated with ARNO siRNA. As a result, both SecinH3- and
ARNO siRNA-treated cells demonstrated a 56.77-68.63% inhibition
rate compared with solvent-treated samples. Therefore, we
hypothesize that inhibiting cytohesins contributed to the reduction
in EGFR signaling. To identify the mechanism of this inhibition in
HT-29 cells, i.e. whether the enhancement of EGFR activation by
cytohesins was due to the effect of cytohesins on EGFR, we
investigated the activation of certain EGFR pathway molecules
(p-EGFR and p-ERK1/2). Our results gave support to this mechanism
of inhibition.
In conclusion, ARNO, an important isoform of the
cytohesin family, is highly expressed in colorectal cancer cells
and enhances EGFR signaling, which contributes to tumor
differentiation, survival and proliferation.
Acknowledgements
This research was supported by the
National High Technology Research and Development Program of China
(No. 2012AA02A506), the NSFC (No. 30901741), Zhejiang the
Provincial Key Scientific and Technological Research Projects of
International Cooperation (No. 2009C14010) and the Zhejiang
Provincial Natural Science Foundation of China (No. R209.353). The
authors would like to thank Dr Zhang Jiawei, Dr Fu Xianhua and Dr
Wang Zhanhuai for their cell lines (HT-29, SW620, SW480, LOVO and
HCT-116), support and enthusiasm.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, van Ballegooijen M, et al: Annual report to the nation on
the status of cancer, 1975–2006, featuring colorectal cancer trends
and impact of interventions (risk factors, screening, and
treatment) to reduce future rates. Cancer. 116:544–573. 2010.
|
|
3.
|
Mitry E, Bouvier AM, Esteve J and Faivre
J: Benefit of operative mortality reduction on colorectal cancer
survival. Br J Surg. 89:1557–1562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mayer RJ: Targeted therapy for advanced
colorectal cancer - more is not always better. N Engl J Med.
360:623–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Siena S, Sartore-Bianchi A, Di
Nicolantonio F, Balfour J and Bardelli A: Biomarkers predicting
clinical outcome of epidermal growth factor receptor-targeted
therapy in metastatic colorectal cancer. J Natl Cancer Inst.
101:1308–1324. 2009. View Article : Google Scholar
|
|
6.
|
Bublil EM and Yarden Y: The EGF receptor
family: spearheading a merger of signaling and therapeutics. Curr
Opin Cell Biol. 19:124–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bose R and Zhang X: The ErbB kinase
domain: structural perspectives into kinase activation and
inhibition. Exp Cell Res. 315:649–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Jura N, Endres NF, Engel K, Deindl S, Das
R, Lamers MH, Wemmer DE, Zhang X and Kuriyan J: Mechanism for
activation of the EGF receptor catalytic domain by the
juxtamembrane segment. Cell. 137:1293–1307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Red Brewer M, Choi SH, Alvarado D,
Moravcevic K, Pozzi A, Lemmon MA and Carpenter G: The juxtamembrane
region of the EGF receptor functions as an activation domain. Mol
Cell. 34:641–651. 2009.PubMed/NCBI
|
|
10.
|
Bill A, Schmitz A, Albertoni B, Song JN,
Heukamp LC, Walrafen D, Thorwirth F, Verveer PJ, Zimmer S, Meffert
L, Schreiber A, et al: Cytohesins are cytoplasmic ErbB receptor
activators. Cell. 143:201–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kolanus W: Guanine nucleotide exchange
factors of the cytohesin family and their roles in signal
transduction. Immunol Rev. 218:102–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bos JL, Rehmann H and Wittinghofer A: GEFs
and GAPs: critical elements in the control of small G proteins.
Cell. 129:865–877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Casanova JE: Regulation of Arf activation:
the Sec7 family of guanine nucleotide exchange factors. Traffic.
8:1476–1485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Weinstein IB: Cancer. Addiction to
oncogenes - the Achilles heal of cancer. Science. 297:63–64. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bardelli A and Jänne PA: The road to
resistance: EGFR mutation and cetuximab. Nat Med. 18:199–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Montagut C, Dalmases A, Bellosillo B,
Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S,
Tsai SP, Minoche A, et al: Identification of a mutation in the
extracellular domain of the Epidermal Growth Factor Receptor
conferring cetuximab resistance in colorectal cancer. Nat Med.
18:221–223. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Vlacich G and Coffey RJ: Resistance to
EGFR-targeted therapy: a family affair. Cancer Cell. 20:423–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yonesaka K, Zejnullahu K, Okamoto I, Satoh
T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda
M, Fujisaka Y, et al: Activation of ERBB2 signaling causes
resistance to the EGFR-directed therapeutic antibody cetuximab. Sci
Transl Med. 3:99ra862011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ruan SQ, Wang SW, Wang ZH and Zhang SZ:
Regulation of HRG-β1-induced proliferation, migration and invasion
of MCF-7 cells by upregulation of GPR30 expression. Mol Med Report.
6:131–138. 2012.
|
|
20.
|
El Azreg MA, Garceau V and Bourgoin SG:
Cytohesin-1 regulates fMLF-mediated activation and functions of the
β2 integrin Mac-1 in human neutrophils. J Leukoc Biol. 89:823–836.
2011.
|
|
21.
|
Oh SJ and Santy LC: Differential effects
of cytohesins 2 and 3 on β1 integrin recycling. J Biol Chem.
285:14610–14616. 2010.
|
|
22.
|
Ogasawara M, Kim SC, Adamik R, Togawa A,
Ferrans VJ, Takeda K, Kirby M, Moss J and Vaughan M: Similarities
in function and gene structure of cytohesin-4 and cytohesin-1,
guanine nucleotide-exchange proteins for ADP-ribosylation factors.
J Biol Chem. 275:3221–3230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
El Azreg MA and Bourgoin SG: Cytohesin-1
regulates human blood neutrophil adhesion to endothelial cells
through β2 integrin activation. Mol Immunol. 48:1408–1416.
2011.PubMed/NCBI
|
|
24.
|
Stalder D, Barelli H, Gautier R, Macia E,
Jackson CL and Antonny B: Kinetic studies of the Arf activator Arno
on model membranes in the presence of Arf effectors suggest control
by a positive feedback loop. J Biol Chem. 286:3873–3883. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Chardin P, Paris S, Antonny B, Robineau S,
Béraud-Dufour S, Jackson CL and Chabre M: A human exchange factor
for ARF contains Sec7- and pleckstrin-homology domains. Nature.
384:481–484. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
26.
|
D’Souza-Schorey C and Chavrier P: ARF
proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell
Biol. 7:347–358. 2006.
|