Introduction
Breast cancer continues to be a leading worldwide
cause of cancer-related mortality among females, despite
significant advances in screening techniques that lead to early
detection of the disease (1). The
incidence of breast cancer is lower in Asia than in Western
countries (2). This may be
attributable to Asian diets that are rich in flavonoid-containing
plants, which are thought to be anti-tumorigenic. Chrysin
(5,7-dihydroxyflavone, ChR), a natural flavonoid present in daily
diets, possesses the ability to inhibit growth and induce apoptosis
in a variety of cancer cells, including cervical cancer (3), leukemia (4,5), colon
carcinoma (6), esophageal
adenocarcinoma (7) and lung
adenocarcinoma (8). Poor oral
bioavailability has been a major limitation for the successful use
of dietary flavonoids as cancer chemotherapeutic agents (9,10).
In order to improve the biological activities of
ChR, we synthesized 5,7-dihydroxy-8-nitrochrysin (NOC) (11). We previously identified that NOC
inhibits proliferation of the colon cancer cell line, HT-29 and the
gastric cancer cell line, SGC-7901 to a greater extent than ChR
(11). Additionally, we
demonstrated that NOC induces breast cancer cell apoptosis by
generation of reactive oxygen species (ROS) and Akt
dephosphorylation (12). However,
the link between Akt dephosphorylation and apoptosis induction in
breast cancer cells remains to be elucidated.
Forkhead box O3a transcription factor (FOXO3a) is a
transcription factor that functions downstream in the Akt signaling
pathway and it is an important regulator of cell death (13). A number of anticancer drugs,
including doxorubicin and paclitaxel, induce apoptosis through
oxidative stress, which enhances FOXO3a activity by stimulating
FOXO3a dephosphorylation and nuclear translocation (14). This causes overexpression of
FOXO3a-responsive genes, including Bim, p27 and p21 (15). Therefore, regulation of FOXO3a
factors by the Akt pathway is receiving increasing attention in
cancer research.
In this study, we demonstrated that NOC
significantly induced apoptosis of the breast cancer cell line
MDA-MB-453 and the underlying molecular mechanisms are associated
with regulation of the Akt/FOXO3a signaling pathway.
Materials and methods
Cell line and cell culture
The human breast cancer cell line MDA-MB-453 (ER
negative, HER-2/neu-overexpressing) was grown in Dulbecco’s
modified Eagle’s medium (DMEM; Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(Invitrogen Life Technologies), 100 U/ml penicillin and 100 U/ml
streptomycin in a humidified atmosphere with 5% CO2 at
37°C.
The study was approved by the Ethics Committee of
Hunan Normal University, Changsha, China.
Medicines and chemical reagents
NOC was synthesized at the Institute of Pharmacy and
Pharmacology, University of South China, as previously described
(11). NOC has a molecular weight
of 299 kD, appears as yellow crystals and has a purity of 99.0%.
NOC was dissolved in dimethyl sulfoxide (DMSO) and was prepared as
a 10 mmol/l stock solution. The antibody against Bim was purchased
from Calbiochem (San Diego, CA, USA). Antibodies against
phospho-Akt (Ser473), Akt, FOXO3a, p-FOXO3a
(Thr32), cytochrome c, caspase-9 and caspase-3
were purchased from Cell Signaling Technology (Danvers, MA, USA).
The antibody against β-actin was purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA) and LY294002 was from
Sigma-Aldrich (St. Louis, MO, USA). Lipofectamine 2000 was
purchased from Invitrogen Life Technologies. All other chemicals
used were of analytical grade and were purchased from Fisher
Scientific (Suwanee, GA, USA) and Sigma-Aldrich.
Flow cytometry (FCM) analysis
To detect cell apoptotic rates, cells were seeded at
a density of 4×106 cells/ml in 100 ml culture flasks for
24 h and then treated for 24 h with medium containing various
concentrations of ChR or NOC and 10% fetal bovine serum. Propidium
iodide staining for DNA content analysis was performed, as
previously described (16).
Mitochondrial membrane potential (ΔΨm)
was measured by FCM using cationic lipophilic green fluorochrome
rhodamine-123 (Rh123; Molecular Probes, Eugene, OR, USA).
Disruption of ΔΨm is associated with a lack of Rh123
retention and a decrease in fluorescence. Briefly, cells were
washed twice with phosphate-buffered saline (PBS) and incubated
with 1 μg/ml Rh123 at 37°C for 30 min. Cells were then
washed twice with PBS and Rh123 intensity was determined by FCM.
Cells with reduced fluorescence (less Rh123) were counted as having
lost mitochondrial membrane potential.
Plasmids and transfections
A control non-specific siRNA
(UUCUCCGAACGUGUCACGUdTdT) was purchased from Dharmacon, Inc.
(Lafayette, CO, USA). FOXO3a siRNA (ACUCCGGGUCCAGCUCCAC) and Bim
siRNA (5′-GATCCGT TCTGAGTGTGACCGAGA-3′) were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). Transfection of
siRNA was carried out with Lipofectamine 2000 according to the
manufacturer’s instructions. Forty-eight hours after transfection,
the cells were exposed to either DMSO (control), 40 μmol/l
ChR or 10 μmol/l NOC for 24 h. The cells were then collected
and processed for western blot analysis and functional assays.
Cellular fractionation
To measure the release of cytochrome c, cells
were fractionated into cytosolic and mitochondrial fractions as
described by Reuter et al(17). In brief, cells were incubated in
buffer containing 20 mM HEPES-KOH (pH 7.2), 10 mM KCl, 1.5 mM
MgCl2, 1 mM ethylenediamime tetraacetic acid (EDTA), 0.1
mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin and 10
μg/ml aprotinin at 4°C for 10 min and then cells were
homogenized with a Dounce homogenizer for 20 strokes. After
addition of buffer containing 210 mM mannitol, 70 mM sucrose, 5 mM
ethylene glycol tetraacetic acid (EGTA) and 5 mM Tris-HCl (pH 7.5),
the homogenates were centrifuged at 1,000 × g for 10 min at 4°C.
The supernatants were further centrifuged at 15,000 × g for 30 min
at 4°C and collected as a cytosolic fraction.
Western blot analysis
Western blot analysis was carried out as previously
described (16). Cells were lysed
in lysis buffer by incubating for 20 min at 4°C. Protein
concentrations were determined using the Bio-Rad assay system
(Bio-Rad, Hercules, CA, USA). Total proteins were fractionated
using sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto a polyvinylidene fluoride membrane
(PVDF; Millipore, Bedford, MA. USA). Antibodies against phospho-Akt
(Ser473), Akt, FOXO3a, p-FOXO3a (Thr32), Bim
and β-actin were used as primary antibodies. The signals were
detected using an enhanced chemiluminescence (ECL) advanced western
blot analysis system (Amersham Pharmacia Biotech Inc., Piscataway,
NJ, USA).
Statistical analysis
A database was set up with the SPSS 15.0 software
package (SPSS Inc., Chicago, IL, USA) for analysis. Data were
represented as mean ± standard deviation (SD). The means of
multiple groups were compared with one-way analysis of variance
(ANOVA), after checking variance for equality. The comparisons
among the means were performed using the least significant
difference (LSD) method. Statistical comparison was also performed
with a two-tailed t-test when appropriate. P<0.05 was considered
to indicate a statistically significant difference.
Results
NOC induces apoptosis of MDA-MB-453 cells
through a mitochondrial death cascade
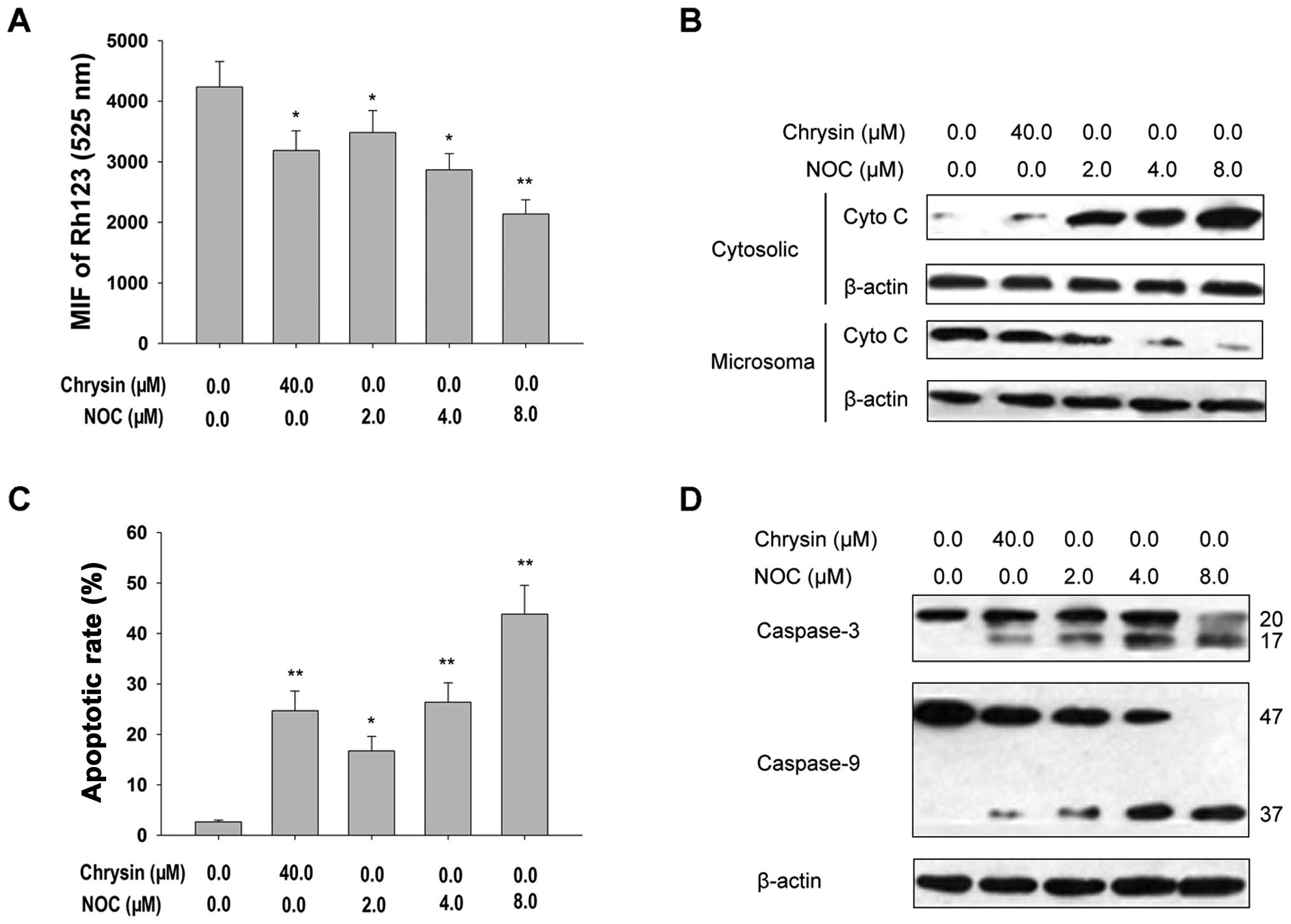
Since NOC-induced apoptosis of breast cancer cells
is associated with the production of ROS (12), we sought to determine whether NOC
activates the mitochondrial death cascade by triggering apoptosis
of the MDA-MB-453 cell line. We first examined the effects of NOC
on several important events in the mitochondrial apoptotic pathway.
Our results indicated the following: i) treatment with NOC clearly
elicited ΔΨm dissipation, as demonstrated by the
decrease in Rh123-derived fluorescence in FCM assays (Fig. 1A); ii) NOC triggered a rapid release
of cytochrome c from the mitochondria to the cytoplasm, as
documented by western blot analysis using cytosolic extracts
(Fig. 1B); and iii) treatment with
NOC caused activation of caspase-9 and -3 and increased the rate of
apoptosis (Fig. 1C and D). Similar
results were observed in MDA-MB-453 cells treated with ChR
(Fig. 1). These data demonstrate
that NOC-induced apoptosis is involved in the mitochondrial death
pathway.
NOC activates FOXO3a in MDA-MB-453
cells
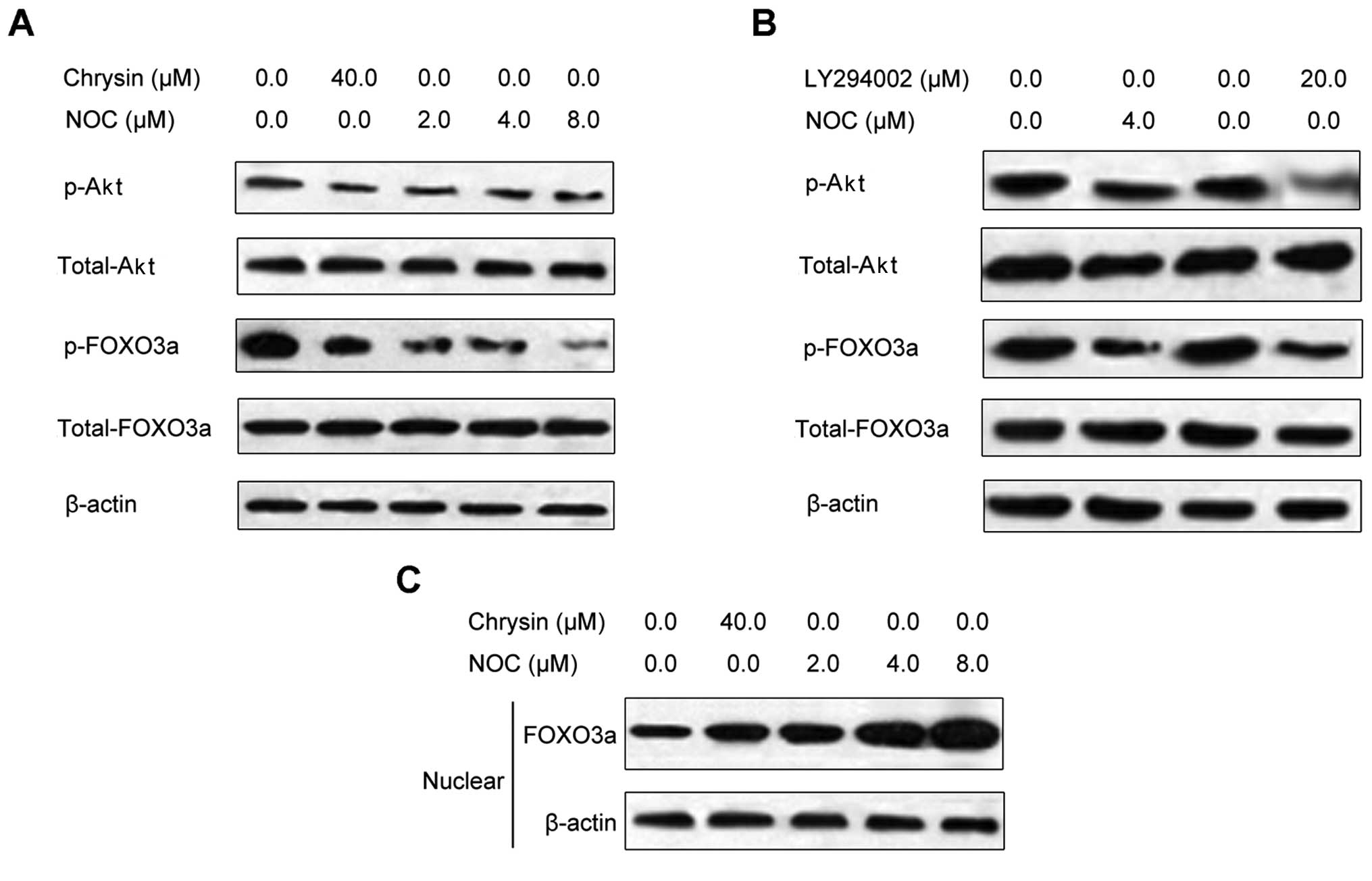
We previously reported that NOC inhibited the
phosphorylation of Akt in MDA-MB-453 cells (12). To further investigate whether NOC
affects the expression of the downstream targets of Akt, we tested
the effects of NOC on Akt and its downstream molecule FOXO3a. We
observed that NOC inhibited the phosphorylation of Akt and FOXO3a
in MDA-MB-453 cells (Fig. 2A). We
also observed that levels of FOXO3a were increased in nuclear
lysate following NOC treatment (Fig.
2B). This suggests that the increased ratio of FOXO3a to
phospho-FOXO3a in the cytoplasm and nuclei of MDA-MB-453 cells
represents retention of a greater amount of active FOXO3a in the
nuclear compartment, thereby inducing cancer cell apoptosis.
Similar results were observed in MDA-MB-453 cells treated with ChR
(Fig. 2A and B). To further confirm
the effects of NOC on FOXO3a, we conducted studies using LY294002,
a specific phosphoinositide 3-kinase (PI3K) inhibitor. We observed
that LY294002 treatment decreased phosphorylation levels of Akt and
FOXO3a (Fig. 2C), similar to the
effects of NOC. This suggests that the effect of NOC on FOXO3a is
mediated through Akt signaling.
FOXO3a activation is required for
induction of apoptosis by NOC in MDA-MB-453 cells
We next examined whether activation of FOXO3a
affects NOC-induced caspase-3 activity and apoptosis. NOC induced
caspase-3 activity and apoptosis in MDA-MB-453/control siRNA cells.
Inhibition of FOXO3a expression by specific siRNA, significantly
inhibited NOC-induced caspase-3 and-9 activity and apoptosis
(Fig. 3A and B). These data suggest
that NOC induces caspase-3 activity and apoptosis through
activation of FOXO3a, while silencing of FOXO3a inhibits activities
associated with caspase-3 and -9 and apoptosis.
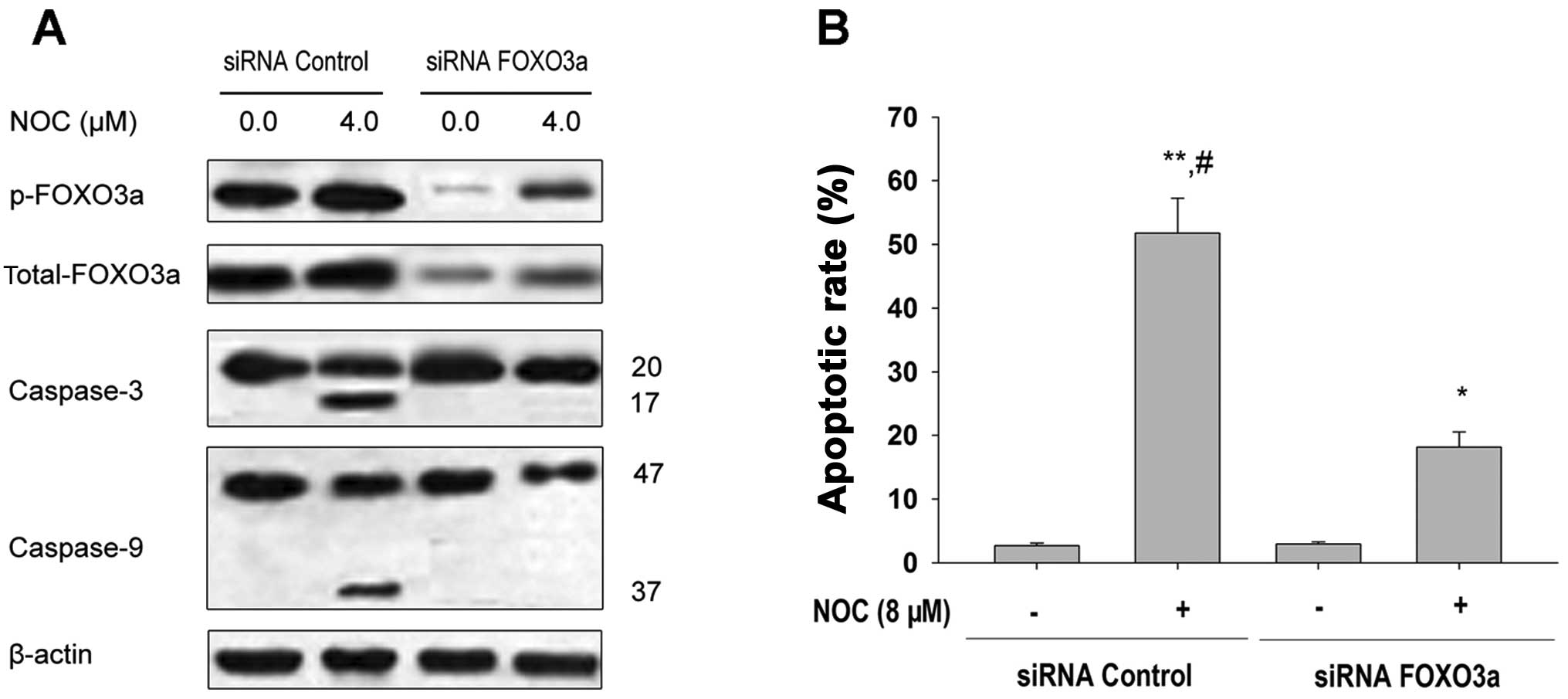 | Figure 3Effects of FOXO3a downregulation by
siRNA transfection on FOXO3a expression and apoptosis in MDA-MB-453
cells. (A) MDA-MB-453 cells were transfected with 100 nM siRNA
control or the siRNA duplexes against FOXO3a mRNA. Forty-eight
hours after transfection, the cells were treated with 4.0 μM
NOC for 24 h. Western blotting of p-FOXO3a, FOXO3a, caspase-3 and
caspase-9 was completed to confirm the downregulation of FOXO3a and
the effects of caspase-3 and -9 cleavage by siRNA transfection.
β-actin was used as the loading control. (B) MDA-MB-453 cells were
transfected with 100 nM siRNA control or the siRNA duplexes against
FOXO3a mRNA. Forty-eight hours after transfection, cells were
treated with 8.0 μM NOC for 24 h. The apoptotic rate was
analyzed by FCM using PI staining. Data shown are means ± SD (n=3).
*P<0.05; **P<0.01 vs. 0.1% DMSO;
#P<0.05 vs. the same concentration of NOC in
combination with siRNA FOXO3a transfection. FOXO3a, forkhead box
O3a; NOC, 7-dihydroxy-8-nitrochrysin; FCM, flow cytometry; PI,
propidium iodide; SD, standard deviation; DMSO, dimethyl
sulfoxide. |
FOXO3a activation regulates expression of
Bim in MDA-MB-453 cells
Mitochondrial dysfunction plays an important role in
breast cancer apoptosis. Changes in the expression of B cell
lymphoma (Bcl)-2-family proteins are involved in ChR-induced
apoptosis of cancer cells (18).
However, it is unclear whether BH3 proteins function in MDA-MB-453
cells following NOC treatment. Therefore, we investigated the
expression of Bcl-2-family proteins in MDA-MB-453 cells following
NOC treatment. Pro-apoptotic proteins, including Bcl-2-associated X
protein (Bax), p53 upregulated modulator of apoptosis (PUMA) and
Noxa, were slightly increased in MDA-MB-453 cells following NOC
treatment (Fig. 4A). Anti-apoptotic
Bcl-2 and Bcl-extra large (XL) proteins also exhibited a slight
decrease. However, in contrast to other proteins, the expression
level of Bim was markedly increased following NOC treatment,
providing evidence that Bim was involved in apoptotic cell death in
MDA-MB-453 cells. Similar results were observed in MDA-MB-453 cells
treated with ChR (Fig. 4A).
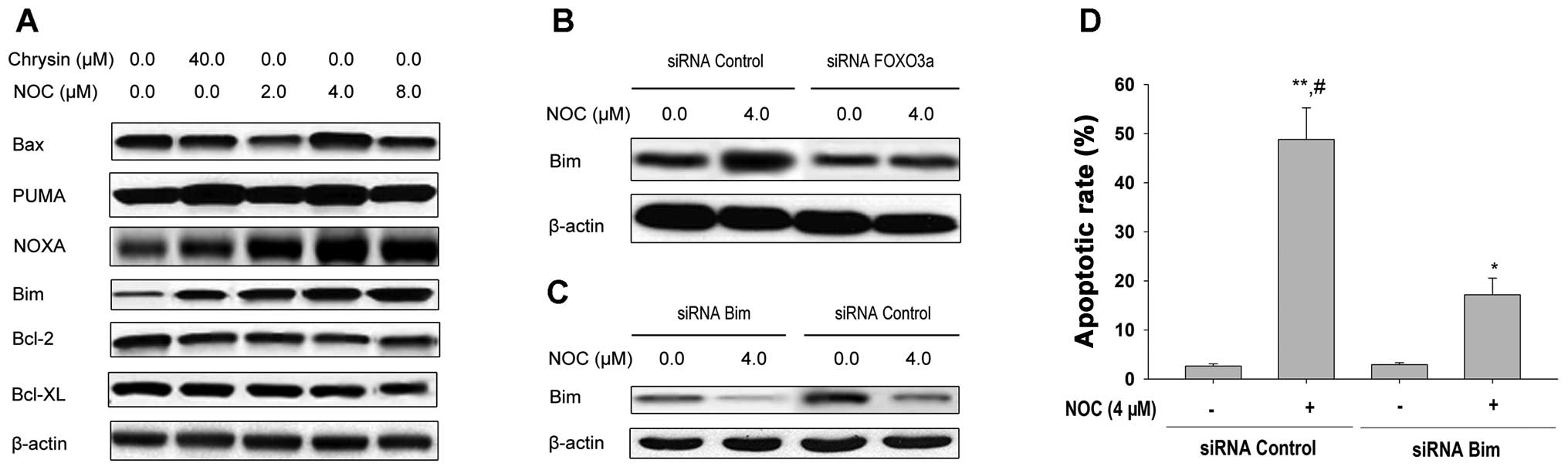 | Figure 4Effects of NOC on the expression of
Bcl-2 family proteins in MDA-MB-453 cells. (A) MDA-MB-453 cells
were treated with the indicated concentrations of NOC or chrysin
for 24 h. Expressions of Bax, PUMA, Noxa, Bim, Bcl-2 and Bcl-XL
proteins were examined by western blotting of total cell lysates
and β-actin was used as the loading control. (B) MDA-MB-453 cells
were transfected with 100 nM siRNA control or the siRNA duplexes
against FOXO3a mRNA. Forty-eight hours after transfection, cells
were treated with 4.0 μM NOC for 24 h. Expression of Bim
protein was determined by western blotting of total cell lysates
and β-actin was used as the loading control. (C) MDA-MB-453 cells
were transfected with 100 nM siRNA control or the siRNA duplexes
against Bim mRNA. Forty-eight hours after transfection, cells were
treated with 4.0 μM NOC for 24 h. Expression of Bim protein
was determined by western blotting of total cell lysates and
β-actin was used as the loading control. (D) MDA-MB-453 cells were
transfected with 100 nM siRNA control or siRNA duplexes against Bim
mRNA. Forty-eight hours after transfection, the cells were treated
with 4.0 μM NOC for 24 h. The apoptotic rate was analyzed by
FCM using PI staining. Data shown are means ± SD (n=3).
*P<0.05; **P<0.01 vs. 0.1% DMSO;
#P<0.05 vs. the same concentration of NOC in
combination with siRNA Bim transfection. NOC,
7-dihydroxy-8-nitrochrysin; Bcl-2, B cell lymphoma 2; Bax, Bcl-2
associated X protein; PUMA, p53 upregulated modulator of apoptosis;
FCM, flow cytometry; PI, propidium iodide; SD, standard deviation;
DMSO, dimethyl sulfoxide. |
We next examined the effect of FOXO3a activation on
the expression of Bim. This gene is a direct target of the FOXO3a
transcription factor. MDA-MB-453 cells were pretreated with FOXO3a
siRNA followed by treatment with NOC for 24 h and the expression of
Bim was measured by western blotting (Fig. 4B). FOXO3a siRNA attenuated the
induction of Bim expression by NOC treatment (Fig. 4B). These data suggest that NOC may
also regulate the expression of the FOXO3a transcriptional target,
Bim.
We next sought to determine whether Bim is involved
in NOC-induced apoptosis of MDA-MB-453 cells. Cells were
transiently transfected with Bim-specific siRNA following the
determination of caspase-3 activity and apoptosis by NOC treatment.
As shown in Fig. 4C and D, cells
receiving Bim siRNA displayed a reduced proportion of Bim-induced
apoptosis, suggesting that Bim upregulation mediates MDA-MB-453
cell apoptosis induced by NOC.
Discussion
Our previous study demonstrated that Akt
inactivation, ROS generation, c-Jun N-terminal kinase (JNK)
activation and caspase activation contribute to NOC-induced
apoptosis in human breast cancer MDA-MB-453 cells. However, the
precise molecular mechanisms responsible for the apoptotic effect
of NOC remain to be fully characterized. In this study, we first
identified that NOC induces apoptosis of MDA-MB-453 cells and this
is primarily mediated through the mitochondrial death pathway. This
study demonstrated that activation of the Akt/FOXO3a axis, followed
by increased Bim expression, contributes to NOC-induced apoptosis
in MDA-MB-453 cells.
Apoptosis is initiated via two alternative signaling
pathways, the death receptor-mediated extrinsic apoptotic pathway
and the mitochondrion-mediated intrinsic apoptotic pathway
(17,19). Mitochondria play critical roles in
the regulation of various apoptotic processes, including
drug-induced apoptosis (20). Our
results demonstrated that NOC induces MDA-MB-453 cell apoptosis by
activating the mitochondrion-mediated intrinsic apoptotic pathway
(Fig. 1). Bcl-2 family proteins
regulate mitochondria-dependent apoptosis, with the balance of
anti- and pro-apoptotic members arbitrating life or death
decisions. Bim, a pro-apoptotic member of the Bcl-2 family, causes
apoptosis by disrupting mitochondrial integrity. In the present
study, we identified that NOC induces Bim expression and that Bim
siRNA attenuates NOC-induced MDA-MB-453 cell apoptosis (Fig. 4). This suggests that Bim expression
is causally related to NOC-induced MDA-MB-453 cell apoptosis.
Furthermore, NOC-induced Bim expression was inhibited by FOXO3a
siRNA transfection (Fig. 4). Thus,
it is plausible that NOC activates FOXO3a, thereby causing Bim
expression and subsequent cell apoptosis.
The FOXO3a transcription factor is a tumor
suppressor that is inactivated in the majority of human cancers,
owing to over-activation of the PI3K/Akt pathway (21–23).
The FOXO3a protein regulates a variety of genes that affect cell
proliferation, survival, metabolism and responses to stress
(24). Upon the activation of
PI3K/Akt signaling, FOXO3a undergoes Akt-mediated phosphorylation,
which promotes binding to the 14-3-3 protein and nuclear export
through CRM1 (also known as XPO1 and exportin 1) and cytoplasmic
sequestration. FOXO3a proteins translocate to cell nuclei for
execution of their transcriptional functions when the PI3K/Akt
pathway is inhibited, including under stress conditions or in the
absence of growth or survival factors (21).
This study revealed a novel mechanism utilized by
NOC to induce apoptosis in MDA-MB-453 cells. Apoptotic resistance
in HER-2/neu-overexpressing breast cancer cells is mediated by a
loss of FOXO3a activity (25) and
we demonstrated that this pathway is inhibited by NOC. Upstream,
NOC activates FOXO3a by targeting the Akt pathway. Downstream, NOC
induces FOXO3a activity and leads to increased expression of Bim,
which induces apoptosis in breast cancer cells. This study
demonstrated that NOC inhibits Akt activation, thereby preventing
FOXO3a phosphorylation (inactivation) in breast cancer cells. This
reveals a new mechanism of NOC-induced apoptosis of breast cancer
cells.
In summary, our study demonstrated that NOC induces
apoptosis in breast cancer cells by promoting FOXO3a activity. This
results in the expression of Bim via inactivation of Akt. Further
studies are required to assess the anticancer activity of NOC in
vivo. A thorough understanding of the mechanisms of NOC may
lead to discovery and development of novel therapeutic molecules
for the treatment and prevention of human breast cancer.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation (no. 81172375); the
Municipal Bureau of Science and Technology of Changsha, Hunan,
China (no. K1104060-31); the Project of Scientific Research of
Hunan Province, the Administration Bureau of Traditional Chinese
Medicine (no. 2010081); the Project of Scientific Research of Hunan
Province, the Department of Education (no. 10C0975) and the Major
Project Item of Scientific Research of Hunan Province, the
Department of Education (no. 09A054).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Qiu MZ, Xu RH, Ruan DY, et al: Incidence
of anemia, leukocytosis, and thrombocytosis in patients with solid
tumors in China. Tumour Biol. 31:633–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang T, Chen X, Qu L, Wu J, Cui R and
Zhao Y: Chrysin and its phosphate ester inhibit cell proliferation
and induce apoptosis in Hela cells. Bioorg Med Chem. 12:6097–6105.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SJ, Yoon JH and Song KS: Chrysin
inhibited stem cell factor (SCF)/c-Kit complex-induced cell
proliferation in human myeloid leukemia cells. Biochem Pharmacol.
74:215–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woo KJ, Jeong YJ, Park JW and Kwon TK:
Chrysin-induced apoptosis is mediated through caspase activation
and Akt inactivation in U937 leukemia cells. Biochem Biophys Res
Commun. 325:1215–1222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, VanAlstyne PC, Irons KA, Chen S,
Stewart JW and Birt DF: Individual and interactive effects of
apigenin analogs on G2/M cell-cycle arrest in human colon carcinoma
cell lines. Nutr Cancer. 48:106–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Q, Zhao XH and Wang ZJ: Flavones and
flavonols exert cytotoxic effects on a human oesophageal
adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing
apoptosis. Food Chem Toxicol. 46:2042–2053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kachadourian R, Leitner HM and Day BJ:
Selected flavonoids potentiate the toxicity of cisplatin in human
lung adenocarcinoma cells: a role for glutathione depletion. Int J
Oncol. 31:161–168. 2007.PubMed/NCBI
|
|
9
|
Saarinen N, Joshi SC, Ahotupa M, et al: No
evidence for the in vivo activity of aromatase-inhibiting
flavonoids. J Steroid Biochem Mol Biol. 78:231–239. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walle T, Otake Y, Brubaker JA, Walle UK
and Halushka PV: Disposition and metabolism of the flavonoid
chrysin in normal volunteers. Br J Clin Pharmacol. 51:143–146.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng X, Meng WD, Xu YY, Cao JG and Qing
FL: Synthesis and anticancer effect of chrysin derivatives. Bioorg
Med Chem Lett. 13:881–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao XC, Tian L, Cao JG and Liu F:
Induction of apoptosis by 5,7-dihydroxy-8-nitrochrysin in breast
cancer cells: the role of reactive oxygen species and Akt. Int J
Oncol. 37:1345–1352. 2010.PubMed/NCBI
|
|
13
|
Gomes AR, Brosens JJ and Lam EW: Resist or
die: FOXO transcription factors determine the cellular response to
chemotherapy. Cell Cycle. 7:3133–3136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Gorp AG, Pomeranz KM, Birkenkamp KU,
Hui RC, Lam EW and Coffer PJ: Chronic protein kinase B (PKB/c-akt)
activation leads to apoptosis induced by oxidative stress-mediated
Foxo3a transcriptional up-regulation. Cancer Res. 66:10760–10769.
2006.PubMed/NCBI
|
|
15
|
Essafi A, Fernandez de Mattos S, Hassen
YA, et al: Direct transcriptional regulation of Bim by FoxO3a
mediates STI571-induced apoptosis in Bcr-Abl-expressing cells.
Oncogene. 24:2317–2329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reuter S, Eifes S, Dicato M, Aggarwal BB
and Diederich M: Modulation of anti-apoptotic and survival pathways
by curcumin as a strategy to induce apoptosis in cancer cells.
Biochem Pharmacol. 76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Polier G, Ding J, Konkimalla BV, et al:
Wogonin and related natural flavones are inhibitors of CDK9 that
induce apoptosis in cancer cells by transcriptional suppression of
Mcl-1. Cell Death Dis. 2:e1822011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kok SH, Cheng SJ, Hong CY, et al:
Norcantharidin-induced apoptosis in oral cancer cells is associated
with an increase of proapoptotic to antiapoptotic protein ratio.
Cancer Lett. 217:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kops GJ, Dansen TB, Polderman PE, et al:
Forkhead transcription factor FOXO3a protects quiescent cells from
oxidative stress. Nature. 419:316–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dansen TB and Burgering BM: Unravelling
the tumor-suppressive functions of FOXO proteins. Trends Cell Biol.
18:421–429. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang H and Tindall DJ: Dynamic FoxO
transcription factors. J Cell Sci. 120:2479–2487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CC, Yang HL, Way TD, et al: Inhibition
of cell growth and induction of apoptosis by Antrodia
camphorata in HER-2/neu-overexpressing breast cancer cells
through the induction of ROS, depletion of HER-2/neu, and
disruption of the PI3K/Akt signaling pathway. Evid Based Complement
Alternat Med. 7028572012.
|