Introduction
Pancreatic ductal adenocarcinoma is one of the most
lethal cancers with an overall 5-year survival rate of <5%
(1,2). Only a small proportion of pancreatic
cancers are eligible for radical resection and tumor recurrence is
common following curative resection due to its extremely malignant
potential and unusual resistance to chemotherapy and radiotherapy
(3). Therefore, novel molecular
biomarkers need to be developed and identified to improve early
diagnosis as well as predictive prognosis.
The tumor suppressor gene, RNA binding motif 5
(RBM5) (also called Luca15 or H37), is one of the ∼45 genes located
in the 370 kb tumor suppressor locus on chromosome 3p21.3. RBM5,
through pre-mRNA splicing of multiple target genes, has been shown
to function as a regulator of apoptosis. Its potential role in cell
cycle arrest and apoptosis has been demonstrated in several
malignancies, particularly non-small cell lung cancer (NSCLC) cells
(4,5). KRAS, also known as guanosine
triphosphatase (GTPase) KRAS, belongs to the RAS gene family which
encodes for a small protein with a molecular weight of 21 kDa with
GTPase activity. Mutation of a KRAS gene is an essential step in
the development of a number of cancers, including pancreatic
cancer. Overexpression of KRAS recruits and activates proteins
necessary for the propagation of growth factors and other molecular
signals, including c-RAF and phosphoinositide 3 (PI3)-kinase, as
well as inactivation of p53 and DPC4/Smad4, which are involved in
numerous signal transduction pathways (6–9).
Previously, a reverse correlation between RBM5 and KRAS was
reported in lung cancer tissues (10). In the present study, we investigated
the expression of RBM5 and KRAS at mRNA and protein levels and
their associations with clinicopathological features in pancreatic
ductal adenocarcinoma.
Materials and methods
Patients and sample preparation
In this study, we collected 45 cases of surgically
resected pancreatic ductal adenocarcinoma samples and adjacent
non-tumor tissues from July 2005 to June 2010. Following surgical
removal, all samples were immediately snap-frozen in liquid
nitrogen and stored at −80°C until total RNA was extracted.
Clinical data including age, gender, tumor pathological
characteristics and tumor stage were also collected based on
patient medical records. Tumors were staged according to the tumor
node metastasis classification (11). All tumor diagnosis was confirmed by
a pathologist using standard diagnostic criteria on the
pathological sections.
The study was approved by the Medical Ethics
Committee of Central South University, Xiangya hospital, Changsha,
China.Written informed patient consent was obtained from the
patient’s family.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The expression levels of RBM5 and epidermal growth
factor receptor (EGFR) mRNA were determined using a quantitative
RT-PCR technique (12). Briefly,
total RNA was isolated from samples using the TRIzol reagent
(Invitrogen, NY, USA) according to the manufacturer’s instructions.
Quantitative real-time PCR was performed on an ABI 7300 real-time
PCR system (Applied Biosystems, CA, USA) using SYBR-Green mix
(Applied Biosystems). Relative gene expression was calculated using
the ΔΔCt method, following the manufacturer’s instructions. All
reactions were carried out in triplicate. The primer sequences
were: glyceraldehyde 3-phosphate dehydrogenase (GAPDH),
5′-GGTGATGCTGGTGCTGA GTATGT-3′ and 5′-AAGAATGGGAGTTGCTGTTGAA
GTC-3′; RBM5, 5′-CAGCGCATATGGTTTGTCGG-3′ and
3′-TGCCCTTAAAGAGACAGGCG-5′; KRAS, 5′-ACT GGGGAGGGCTTTCTTTG-3′ and
5′-GGCATCATC AACACCCTGTCT-3′.
Detection of RBM5 and KRAS protein levels
by western blot analysis
Total cellular proteins from pancreatic tumor and
peritumoral tissues were extracted according to the protocol and
protein concentrations were determined using the Bradford method
(Bio-Rad, Hercules, CA, USA). An equal amount (50 μg) of
proteins were separated by sodium dodecyl sulphate-polyacrylamide
gel electrophoresis (SDS-PAGE) and subsequently transferred onto a
polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA,
USA). After washing with tris-buffered saline (TTBS), membranes
were incubated with the primary antibodies including anti-human
RBM5 and KRAS antibodies (Abcam, MA, USA) as well as an
anti-β-actin antibody (Santa Cruz Biotechnology Inc., Santa Cruz,
CA, USA) as a control, overnight at 40°C. Following this,
incubation with the secondary antibody, immunoglobulin G-horse
radish peroxidase (IgG-HRP; Abcam) was performed at room
temperature for 45 min. Finally, the results were visualized with
enhanced chemiluminescence (ECL) detection reagent and quantitated
by densitometry using the ImageQuant image analysis system
(Molecular Dynamics, Sunnyvale, CA, USA). A 50th
percentile cutoff point was used to define over- or underexpression
in pancreatic ductal adenocarcinomas.
Statistical analysis
A Wilcoxon matched pair test was used to compare the
mRNA and protein expression of RBM5 and KRAS between pancreatic
tumor and peritumoral tissues. Correlations between RBM5 and KRAS
expression were tested by the Spearman’s rank test. The
associations of RBM5 and KRAS expression with the
clinicopathological features were examined with the Chi-square test
or Fisher’s exact probability test. Tumor recurrence rates were
calculated using the Kaplan-Meier method and compared by means of
the log-rank test. Factors with tumor recurrence rate in the
univariate models were further evaluated in a multivariate Cox
regression model. All statistical analyses were performed using
SPSS software, version 19.0. (SPSS, Chicago, IL, USA). P≤0.05 was
considered to indicate a statistically significant difference.
Results
Expression of RBM5 decreases in
pancreatic ductal adenocarcinoma
To assess the expression of RBM5 mRNA and protein in
pancreatic ductal adenocarcinoma, we performed quantitative RT-PCR
and western blot analysis on 45 pairs of cancerous vs. peritumoral
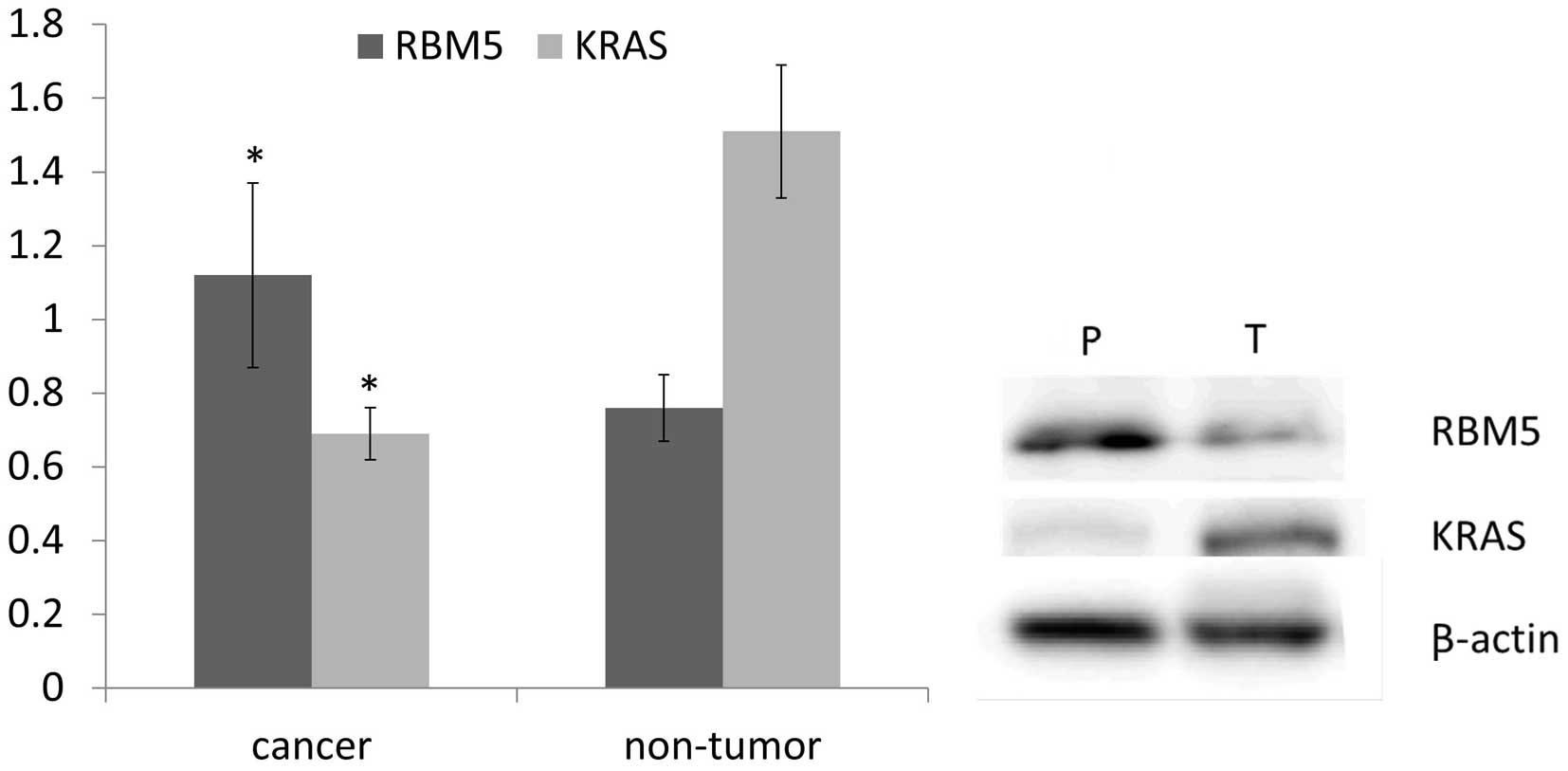
tissues. Compared to the peritumoral tissues, RBM5 was
downregulated in pancreatic cancers (P<0.05; Fig. 1). Consistent with the mRNA
expression level, the protein level of RBM5 detected by western
blot analysis, was significantly lower in cancerous tissues than
the peritumoral counterparts. Our results revealed that the
expression of RBM5 mRNA and protein is significantly decreased in
pancreatic ductal adenocarcinoma in comparison to peritumoral
tissues (P<0.05; Fig. 1).
KRAS expression increases in pancreatic
ductal adenocarcinoma
Quantitative RT-PCR and western blot analysis were
also performed to detect the expression of KRAS mRNA and protein in
45 pairs of pancreatic cancer vs. peritumoral tissues. The relative
KRAS mRNA expression was significantly higher in tumor tissues than
that in non-tumor tissues. Similarly, KRAS protein expression
detected by western blot analysis demonstrated a marked increase in
tumor tissues compared to peritumoral tissues. Our results revealed
that KRAS is significantly overexpressed in pancreatic ductal
adenocarcinoma in comparison to the non-tumor counterpart (Fig. 1).
RBM5 and KRAS protein expression
correlation in pancreatic ductal adenocarcinoma
By Spearman’s rank test, we analyzed the correlation
between protein expression of RBM5 and KRAS in 45 pancreatic ductal
adenocarcinomas. The results indicate that the expression of the
RBM5 protein is reversely correlated with the expression of the
KRAS protein in pancreatic ductal adenocarcinomas (R=−0.892,
P<0.01).
Association of RBM5 and KRAS expression
with clinical features of pancreatic ductal adenocarcinoma
The associations of RBM5 and KRAS expression with
clinicopathological features of pancreatic ductal cancers are
summarized in Table I. As shown in
the table, underexpression of RBM5 in pancreatic cancers was found
to be significantly associated with lymph node metastasis, distant
metastasis, Union for International Cancer Control (UICC) stage and
nerve and venous invasion (P<0.05). No significant differences
were identified between the RBM5 expression and the
clinicopathological parameters with respect to age, gender, tumor
size or cell differentiation (P>0.05). In contrast to RBM5,
overexpression of KRAS was significantly correlated with tumor
size, lymph node metastasis, UICC stage and nerve invasion
(P<0.05). No significant difference was found between age,
gender, distant metastasis, cell differentiation and venous
invasion (P>0.05).
 | Table ICorrelations between RBM5 and KRAS
expression and the clinicopathological features of pancreatic
cancer. |
Table I
Correlations between RBM5 and KRAS
expression and the clinicopathological features of pancreatic
cancer.
| | RBM5 expression
| KRAS expression
|
|---|
| Clinicopathological
features | n | Under | Over | P-value | Under | Over | P-value |
|---|
| Age (years) | | | | | | | |
| <65 | 25 | 14 | 11 | 0.947 | 13 | 12 | 0.8940 |
| ≥65 | 20 | 11 | 9 | | 10 | 10 | |
| Gender | | | | | | | |
| Male | 28 | 14 | 14 | 0.336 | 13 | 15 | 0.4200 |
| Female | 17 | 11 | 6 | | 10 | 7 | |
| Tumor size | | | | | | | |
| ≤2 cm | 16 | 11 | 5 | 0.348 | 13 | 3 | 0.0110 |
| 2–4 cm | 17 | 9 | 8 | | 6 | 11 | |
| >4 cm | 12 | 5 | 7 | | 4 | 8 | |
| Lymph node
metastasis | | | | | | | |
| Absent | 13 | 4 | 9 | 0.033 | 3 | 10 | 0.0165 |
| Present | 32 | 21 | 11 | | 20 | 12 | |
| Distant
metastasis | | | | | | | |
| Absent | 40 | 20 | 20 | 0.034 | 21 | 19 | 0.5980 |
| Present | 5 | 5 | 0 | | 2 | 3 | |
| Cell
differentiation | | | | | | | |
| Good | 18 | 11 | 7 | 0.179 | 12 | 6 | 0.0540 |
| Moderate | 13 | 9 | 4 | | 5 | 8 | |
| Poor | 14 | 5 | 9 | | 6 | 8 | |
| UICC stage | | | | | | | |
| I or II | 29 | 12 | 17 | 0.010 | 18 | 11 | 0.0480 |
| III or IV | 16 | 13 | 3 | | 5 | 11 | |
| Nerve invasion | | | | | | | |
| Absent | 26 | 10 | 16 | 0.007 | 18 | 8 | 0.0044 |
| Present | 19 | 15 | 4 | | 5 | 14 | |
| Venous
invasion | | | | | | | |
| Absent | 33 | 15 | 18 | 0.024 | 15 | 18 | 0.2080 |
| Present | 12 | 10 | 2 | | 8 | 4 | |
Tumor recurrence and expression levels of
RBM5 and KRAS
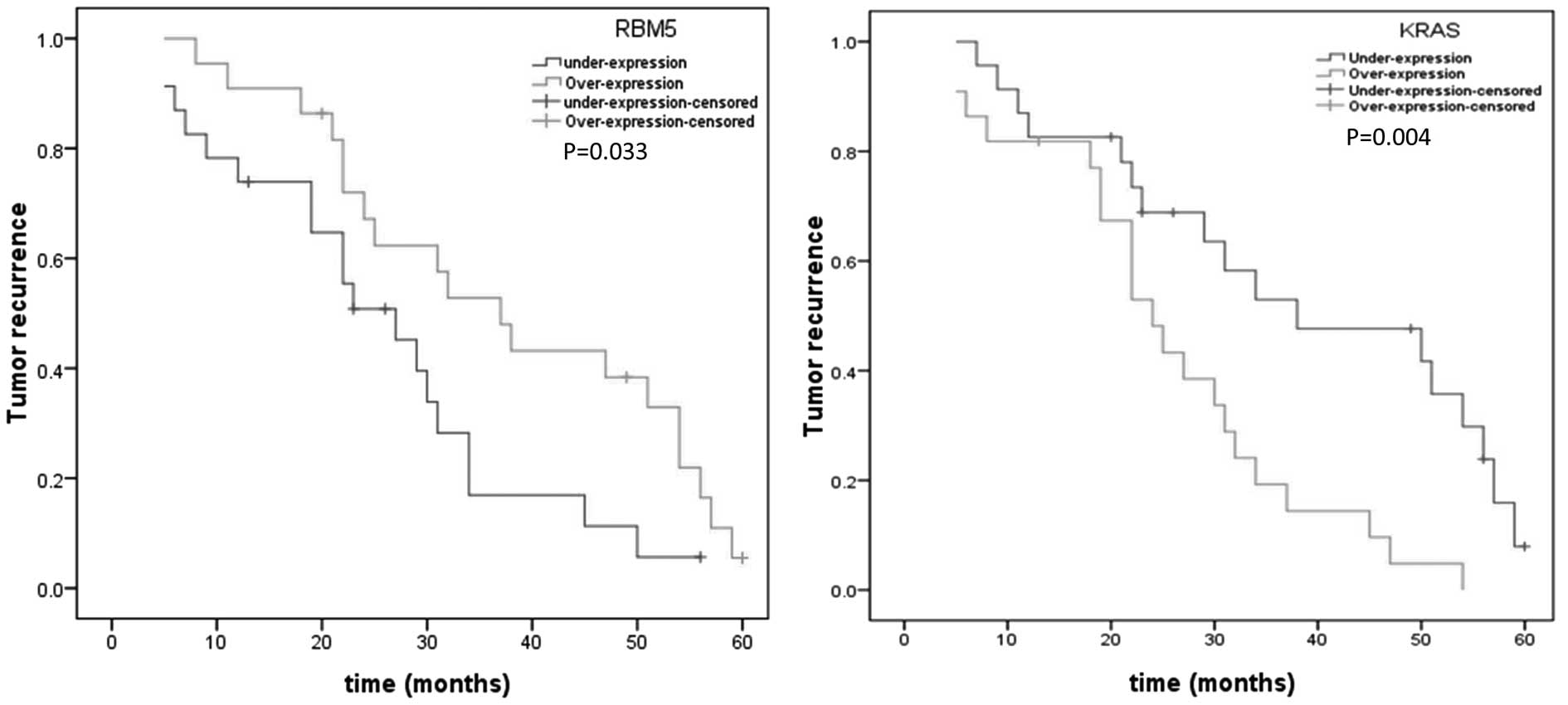
Tumor recurrence, analyzed by the Kaplan-Meier
method, suggested that underexpression of RBM5 had a greater
incidence of recurrence than overexpression (P=0.033; Fig. 2). Additionally, a significantly
increased tumor recurrence rate was related to overexpression of
KRAS in pancreatic cancers in comparison to that with
underexpression (P=0.004; Fig. 2).
Further multivariate analyses using Cox’s proportional hazards
model indicated that lymph node metastasis, tumor size (>4 cm),
advanced UICC stage and nerve invasion have predictive value with
regard to tumor recurrence of pancreatic cancer following radical
resection (Table II). However, RBM5
and KRAS expression was not an independent factor associated with a
higher rate of tumor recurrence.
 | Table IIMultivariate analysis of tumor
recurrence for pancreatic cancer following radical resection. |
Table II
Multivariate analysis of tumor
recurrence for pancreatic cancer following radical resection.
| Variable | HR | 95% CI | P-value |
|---|
| Tumor size (≤4 cm
vs. >4 cm) | 4.510 | 1.1–11.34 | 0.034 |
| Lymph node
metastasis (absent vs. present) | 4.030 | 1.17–13.88 | 0.027 |
| UICC stage (I and
II vs. III and IV) | 4.050 | 1.03–9.54 | 0.044 |
| Nerve invasion
(absent vs. present) | 5.482 | 1.14–26.3 | 0.033 |
Discussion
In previous years, numerous molecular markers have
been identified that are not only implicated in the occurrence and
developmental progress of pancreatic ductal adenocarcinoma but also
act as postoperative indictors for prognosis. Studies have shown a
reduced expression of RBM5 in several cancers, including breast
cancer, vestibular schwannoma and human non-small cell lung cancer
(10,13,14).
To our knowledge, this is the first study to investigate RBM5
expression at an RNA and protein level in pancreatic ductal
adenocarcinoma.
In the present study, significantly reduced RBM5
expression was observed in pancreatic cancer tissues compared to
peritumoral tissues. Additionally, underexpression of RBM5 had a
close association with unfavorable clinicopathological features,
including lymph node metastasis, distant metastasis, advanced UICC
stage and presence of nerve and venous invasion, suggesting its
potential role in tumor invasion and progression. Several studies
have showed that RBM5 is differentially expressed in human cancers
and is involved in tumorigenesis (15–18).
Oh et al(19) found that the
tumor suppressor RBM5/H37 alters the expression of genes involved
in metastasis, suggesting that a loss of RBM5 expression may
increase the metastatic potential of tumors. Our results, which
demonstrated a significant association between reduced RBM5
expression and metastasis-related clinicopathological features
support the above study. However, diverse effects of RBM5 have been
revealed in human cancers. A previous study revealed that RBM6-RBM5
transcription-induced chimerism leads to tumor-related increased
transcriptional activity of the RBM6 gene and has a close
association with breast tumor size, suggesting that it may be a
potential tumor differentiation marker (20). Taken together, it is clear that the
role of RBM5 in pancreatic cancer is still not fully
understood.
Oncogenic activation of the KRAS gene occurs in
>90% of pancreatic ductal carcinoma and malignant progression
from pancreatic intraepithelial carcinoma to a more aggressive form
of pancreatic cancer is accompanied by the early acquisition of
KRAS oncogene activation (21,22).
The high frequency of KRAS mutations in pancreatic cancers has been
proposed as a diagnostic tumor marker, as well as a prognostic
indicator. Consistent with other studies, our results revealed that
pancreatic cancer tissues are presented with elevated KRAS
expression compared to non-tumor tissues (23–25).
Moreover, our results also demonstrated a close correlation between
KRAS overexpression and unfavorable clinicopathological factors,
including larger tumor size, lymph node metastasis, advanced UICC
stage and the presence of nerve invasion. However, taking into
account the contrasting results regarding the prognostic utility of
KRAS mutations, KRAS currently cannot be recommended for clinical
application to determine prognosis in patients with pancreatic
adenocarcinoma cancer (26–28). Further studies are required to
understand the clinical implication and mechanism of KRAS in
pancreatic cancer.
The correlation between RBM5, EGFR and KRAS has been
demonstrated in several publications (4,13,29).
RBM5 expression is altered as a result of changes in KRAS and the
EGFR dimerization partner, human epidermal growth factor receptor 2
(HER2). A previous study demonstrated a reverse correlation between
RBM5 expression and EGFR and KRAS expression in NSCLCs (10). However, another study indicated that
reduced EGFR expression has no correlation with any change in RBM5
expression at either the RNA or protein level (30). Our results revealed that the
expression of RBM5 and KRAS is negatively correlated in pancreatic
cancer. Additionally, underexpression of RBM5 and overexpression of
KRAS in pancreatic cancer has a close association with metastasis
and invasion-related clinicopathological features, suggesting their
collaboration in prompting tumor ability of invasion and
metastasis.
Cancer recurrence is a major concern in patients
with pancreatic ductal adenocarcinoma following radical resection.
The differentially expressed RBM5 and KRAS in pancreatic cancer and
their significant associations with postoperative recurrence of
pancreatic cancer suggests their potential use as predictors of
clinical implication. However, neither low RBM5 expression nor high
KRAS expression were proved to be an independent factor associated
with higher recurrence rate when corrected with age, gender, tumor
size, lymphatic involvement, distant metastasis, cell
differentiation, UICC stage and nerve and venous invasion. Our
results are in accordance with the majority of studies, which
suggest that KRAS mutations have no significant association with
prognostic survival in pancreatic cancer patients (31–33).
However, a number of conventional clinico-pathological parameters
in our study, including lymph node metastasis, tumor size (>4
cm), advanced UICC stage and nerve invasion, are implicated as
independent prognostic indicators for pancreatic cancer.
Collectively, expression of RBM5 and KRAS in
pancreatic ductal adenocarcinomas is significantly decreased and
increased, respectively, compared to non-tumor tissues.
Furthermore, we revealed that poor clinicopathological features and
high tumor recurrence rate are significantly associated with a low
expression of RBM5 and high expression of KRAS. Further research is
required to determine the role of RBM5 in metastasis and invasion
of pancreatic cancer.
References
|
1
|
Warshaw AL and Fernandez-del Castillo C:
Pancreatic carcinoma. N Engl J Med. 326:455–465. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
3
|
Yeo TP, Hruban RH, Leach SD, et al:
Pancreatic cancer. Curr Probl Cancer. 26:176–275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sutherland LC, Wang K and Robinson AG:
RBM5 as a putative tumor suppressor gene for lung cancer. J Thorac
Oncol. 5:294–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh JJ, Razfar A, Delgado I, et al: 3p21.3
tumor suppressor gene H37/Luca15/RBM5 inhibits growth of human lung
cancer cells through cell cycle arrest and apoptosis. Cancer Res.
66:3419–3427. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heidorn SJ, Milagre C, Whittaker S, et al:
Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor
progression through CRAF. Cell. 140:209–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suda K, Tomizawa K and Mitsudomi T:
Biological and clinical significance of KRAS mutations in lung
cancer: an oncogenic driver that contrasts with EGFR mutation.
Cancer Metastasis Rev. 29:49–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneider G and Schmid RM: Genetic
alterations in pancreatic carcinoma. Mol Cancer. 2:152003.
View Article : Google Scholar
|
|
9
|
Kobayashi T, Ishida J, Musashi M, et al:
p53 transactivation is involved in the antiproliferative activity
of the putative tumor suppressor RBM5. Int J Cancer. 128:304–318.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang H, Zhang J, Shao C, et al:
Differential expression of RBM5, EGFR and KRAS mRNA and protein in
non-small cell lung cancer tissues. J Exp Clin Cancer Res.
31:362012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH and Fleming ID: TNM
Classification of Malignant Tumors. Vol 80 (5th edition). Union
Internationale Contre le Cancer and the American Joint Committee on
Cancer. 1803–1804. 1997.
|
|
12
|
Zhou X, Hao Q, Liao J, Zhang Q and Lu H:
Ribosomal protein S14 unties the MDM2-p53 loop upon ribosomal
stress. Oncogene. 2012.PubMed/NCBI
|
|
13
|
Rintala-Maki ND, Goard CA, Langdon CE, et
al: Expression of RBM5-related factors in primary breast tissue. J
Cell Biochem. 100:1440–1458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Welling DB, Lasak JM, Akhmametyeva E,
Ghaheri B and Chang LS: cDNA microarray analysis of vestibular
schwannomas. Otol Neurotol. 23:736–748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YS, Hwan JD, Bae S, Bae DH and Shick
WA: Identification of differentially expressed genes using an
annealing control primer system in stage III serous ovarian
carcinoma. BMC Cancer. 10:5762010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maarabouni MM and Williams GT: The
antiapoptotic RBM5/LUCA-15/H37 gene and its role in apoptosis and
human cancer: research update. Scientific World Journal.
6:1705–1712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rintala-Maki ND, Abrasonis V, Burd M and
Sutherland LC: Genetic instability of RBM5/LUCA-15/H37 in MCF-7
breast carcinoma sublines may affect susceptibility to apoptosis.
Cell Biochem Funct. 22:307–313. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao C, Zhao L, Wang K, Xu W, Zhang J and
Yang B: The tumor suppressor gene RBM5 inhibits lung adenocarcinoma
cell growth and induces apoptosis. World J Surg Oncol. 10:1602012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oh JJ, Taschereau EO, Koegel AK, et al:
RBM5/H37 tumor suppressor, located at the lung cancer hot spot
3p21.3, alters expression of genes involved in metastasis. Lung
Cancer. 70:253–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang K, Ubriaco G and Sutherland LC:
RBM6-RBM5 transcription-induced chimeras are differentially
expressed in tumours. BMC Genomics. 8:3482007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang R and Tang D: Autophagy in pancreatic
cancer pathogenesis and treatment. Am J Cancer Res. 2:383–396.
2012.PubMed/NCBI
|
|
22
|
Ying H, Kimmelman AC, Lyssiotis CA, et al:
Oncogenic Kras maintains pancreatic tumors through regulation of
anabolic glucose metabolism. Cell. 149:656–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rall CJ, Yan YX, Graeme-Cook F, et al:
Ki-ras and p53 mutations in pancreatic ductal adenocarcinoma.
Pancreas. 12:10–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hruban RH, van Mansfeld AD, Offerhaus GJ,
et al: K-ras oncogene activation in adenocarcinoma of the human
pancreas. A study of 82 carcinomas using a combination of
mutant-enriched polymerase chain reaction analysis and
allele-specific oligonucleotide hybridization. Am J Pathol.
143:545–554. 1993.
|
|
25
|
Grunewald K, Lyons J, Frohlich A, et al:
High frequency of Ki-ras codon 12 mutations in pancreatic
adenocarcinomas. Int J Cancer. 43:1037–1041. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garcea G, Neal CP, Pattenden CJ, Steward
WP and Berry DP: Molecular prognostic markers in pancreatic cancer:
a systematic review. Eur J Cancer. 41:2213–2236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Castells A, Puig P, Mora J, et al: K-ras
mutations in DNA extracted from the plasma of patients with
pancreatic carcinoma: diagnostic utility and prognostic
significance. J Clin Oncol. 17:578–584. 1999.PubMed/NCBI
|
|
28
|
Finkelstein SD, Przygodzki R, Pricolo VE,
et al: K-ras-2 topographic genotyping of pancreatic adenocarcinoma.
Arch Surg. 129:367–372; discussion 372–363, 1994.
|
|
29
|
Gazdar AF, Gao B and Minna JD: Lung cancer
cell lines: Useless artifacts or invaluable tools for medical
science? Lung Cancer. 68:309–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Masilamani TJ, Rintala-Maki ND, Wang K and
Sutherland LC: Downregulating activated epidermal growth factor
receptor has no effect on RBM5 expression. Chin Med J (Engl).
125:2378–2381. 2012.PubMed/NCBI
|
|
31
|
Song MM, Nio Y, Dong M, et al: Comparison
of K-ras point mutations at codon 12 and p21 expression in
pancreatic cancer between Japanese and Chinese patients. J Surg
Oncol. 75:176–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dergham ST, Dugan MC, Kucway R, et al:
Prevalence and clinical significance of combined K-ras mutation and
p53 aberration in pancreatic adenocarcinoma. Int J Pancreatol.
21:127–143. 1997.PubMed/NCBI
|
|
33
|
Motojima K, Urano T, Nagata Y, Shiku H,
Tsunoda T and Kanematsu T: Mutations in the Kirsten-ras oncogene
are common but lack correlation with prognosis and tumor stage in
human pancreatic carcinoma. Am J Gastroenterol. 86:1784–1788.
1991.PubMed/NCBI
|