Introduction
Ovarian cancer is associated with a high mortality
due to the absence of effective screening strategies to identify
patients at high risk or who have already developed early
neoplastic lesions still amenable to treatment (1). The current management of advanced
ovarian cancer includes cytoreductive surgery followed by
combination chemotherapy; however, the long-term survival of
ovarian cancer patients remains unsatisfactory (2). Despite advances in surgery and
chemotherapy, novel treatment strategies are required to further
benefit patients (3). Taxanes are
widely used to treat patients with cancer of the lung, breast,
stomach, endometrium or ovary (4).
At present, chemotherapy in combination with Taxol is the standard
first-line therapy for patients with advanced ovarian cancer
(5); however, tolerance to Taxol in
ovarian cancer cells has been observed (6) and the mechanisms of resistance are not
yet fully understood.
Subbaramaiah et al observed that taxanes have
the ability to promote transcription of the cyclooxygenase (COX)-2
gene and to stabilize the COX-2 messenger RNA transcript (7). Sorokin (8) identified that enforced expression of
COX-2 causes enhancement in multidrug resistance (MDR) expression
and functional activity. Therefore, upregulation of COX-2 induced
by taxanes may attenuate the antitumor effect of taxanes. COX-2 is
one of the key enzymes that catalyze the rate-limiting step in
prostaglandin (PG) biosynthesis from arachidonic acid, and an
elevated expression of COX-2 is associated with tumor growth,
invasion (9), migration (10), increased stage, reduced survival
rate (11) and chemoresistance
(12) of ovarian cancers. A number
of studies have demonstrated that COX-2-selective inhibitors
inhibit the COX enzymes, downregulate the level of PGE2
and decrease the production of vascular endothelial growth factor
(VEGF) in tumors. Additionally, they have anti-angiogenic effects
on the neovasculature and attenuate tumor growth (9,13).
Therefore, early results revealed enhanced anticancer activity from
the addition of COX-2 inhibitors to taxane in non-small cell lung
cancer (NSCLC) and human endothelial cells by inhibiting PG
production and enhancing anti-angiogenic effects (14,15).
COX-1, another key enzyme that catalyzes the
rate-limiting step in PG biosynthesis from arachidonic acid, is
overexpressed in ovarian cancer (16) and is considered the dominant pathway
responsible for generating PGs in epithelial ovarian cancers
(17). COX-1-selective inhibitors
demonstrate potent antitumor activity in ovarian tumors by
influencing cell proliferation and apoptosis and decreasing the
production of VEGF in tumors (17,18).
In our previous study, we observed that a combination of COX-1 and
COX-2-selective inhibitors have better chemopreventive properties
on ovarian cancer than when administered alone (19). However, no studies have reported on
the addition of COX-1 inhibitors to taxane on ovarian cancer
treatment. Consequently, we investigated the effect of combining
Taxol and COX inhibitors on tumor growth, angiogenesis and
apoptosis in a human ovarian cancer xenograft.
Materials and methods
Human ovarian tumors in nude mice
SKOV-3 cells were used for tumor growth studies
in vivo. The SKOV-3 cells were purchased from the China
Center for Type Culture Collection and grown in the recommended
media under standard conditions. SKOV-3 cells were implanted
subcutaneously in the dorsal skin (2×106 cells) of
female athymic nude mice (nu/nu, 7–8 weeks old). When the tumors
became visible (7 days after inoculation), the mice were randomly
separated into eight groups (n=6): control, SC-560, celecoxib,
Taxol, SC-560/Taxol, celecoxib/Taxol, SC-560/celecoxib and
SC-560/celecoxib/Taxol. The study was approved by the ethics
committee of Nanjing Medical University of Hangzhou Hospital,
Hangzhou, China.
Dose and administration time of
drugs
COX inhibitors, SC-560 (Sigma, St. Louis, MO, USA)
and celecoxib (Pfizer, Groton, CT, USA) were administered by gavage
and Taxol (Bristol-Myers Squibb SRL, Italy) was administered by
intraperitoneal injection in a 0.5 ml suspension of 0.5%
methylcellulose (Sigma) and 0.025% Tween-20 (Sigma) at a dose of 3
mg/kg (SC-560) and 100 mg/kg (celecoxib) twice a day and 20 mg/kg
(Taxol) once a week. The doses of COX inhibitors were selected for
their specificity in inhibiting COX isotypes (20). In the control group, mice were
treated with physiological saline under similar conditions. Drugs
or the vehicle control were administered for a period of 21 days,
beginning one week after the tumors became palpable.
Measurement of tumor volume
The tumor dimensions were measured twice a week
using a linear caliper and tumor volume was calculated using the
following equation: volume (mm3) = a × b2/2,
where a is the largest diameter and b is the smallest diameter
(21). The animals were weighed
weekly throughout the study. On day 28, all mice were sacrificed
and tumor tissue samples were collected and fixed in 10%
phosphate-buffered formalin solution for immunohistology or stored
at −80°C until analyzed. The tumor tissue samples were snap-frozen
in liquid nitrogen prior to their storage at −80°C.
Reverse transcription-polymerase chain
reaction (RT-PCR) for VEGF mRNA
Total RNA was extracted using TRIzol reagents
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. Isolated RNA was electrophoresed
through 1.0% agarose-formaldehyde gels to verify the quality of the
RNA. The first strand cDNA was generated by reverse transcription.
After a sufficient amount of cDNA was obtained, we performed PCR
amplification using a real-time PCR cycler (ABI 7500, Applied
Biosystems Company, Foster City, CA, USA). VEGF 189, 165 and 121
were routinely detected in this series of ovarian cancer. The
sequences of PCR primers were: VEGF 121, 5′-ACTCGGAT
GCCGACACGGGA-3′ and 5′-CCTGGCCTTGCTTGCTC CCC-3′; VEGF 165,
5′-CCAGGATCCTCTGCCCGCCT-3′ and 5′-GCGGCTTCCGGCACCTACAG-3′; VEGF
189, 5′-GGCAAAAGTTGCGAGCCGCC-3′ and 5′-TGGATG GACCGGGAGCAGGG-3′;
β-actin, 5′-GGGTGACGAGGC CCAGAGCA-3′ and 5′-GGG
GCCACACGCAGCTCATT-3′. The amplification system included 50
μl of SYBR-Green mix (32.5 μl), ddH2O
(14.5 μl), cDNA (2 μl), forward primer (0.5
μl) and reverse primer (0.5 μl). The reaction
conditions were as follows: stage 1, 50°C for 2 min (1 cycle);
stage 2, 95°C for 5 min (1 cycle); stage 3, 95°C for 0.25 min
followed by 60°C for 0.75 min (40 cycles); stage 4, 95°C for 0.25
min, then 60°C for 1 min and lastly, 95°C for 0.25 min followed by
60°C for 0.25 min (1 cycle). The results of real-time PCR were
analyzed by the DCt method: ΔCT = CTselected gene −
CTβ-actin. Relative quantitation (RQ) = 2−ΔCT
× 100%. The results of real-time PCR were presented as the ratio
between the selected genes and β-actin transcripts.
Immunohistochemistry for MVD
Formalin-fixed paraffin-embedded tumor sections (6
μm) were subjected to immunostaining using CD34
antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA).
Sections were deparaffinized and hydrated by sequential immersion
in xylene and grade alcohol solutions. The sections were then
incubated with 3% hydrogen peroxide in methanol solution for 34 min
to block endogenous peroxidase activity. For antigen retrieval,
slides were pressured in the pressure cooker for 2×10 min. For
CD34 staining, the sections were immersed in normal goat
serum for 34 min. Immunohistochemical staining was performed using
the streptavidin-biotin method. Microvessel density (MVD) was
evaluated according to the method first described by Weidner et
al(22). The entire tumor
section was first carefully scanned at low magnification with light
microscopy (magnification, ×40) to find the area that presented the
most intense neovascularization. Since the immunohistochemistry of
CD34 demonstrated slight heterogeneity within the same
tumor, the five most highly vascularized areas (hot spots) were
selected in ×200 magnification fields. The mean of five counts was
calculated and used in statistical analyses.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate (dUTP)-biotin
nick end labeling (TUNEL) assay
Apoptosis was measured in tissue sections by TUNEL
assay. TUNEL assay allows easy demonstration of cell death as a
result of apoptosis. The tissue samples were fixed in 4%
paraformaldehyde for 24 h, dehydrated and embedded in paraffin in
the conventional manner. The paraffin-embedded tissues were cut
into 4 μm-thick sections. Following deparaffinization in a
graded alcohol series, the tissue sections were covered with 20
μg/ml proteinase K PBS(−) for 15 min at room temperature,
followed by blocking of endogenous peroxidase activity. The samples
were then incubated with terminal deoxynucleotidyl transferase
(TdT) enzyme and biotin-16-dUTP in TdT buffer containing 0.01%
bovine serum albumin for 1.5 h at 37°C in a humidity chamber.
Biotin-16-dUTP nucleotides that had been incorporated into DNA
fragments were detected using the ABC method with diaminobenzidine
(DAB) as the chromogen. In each tissue specimen, five high-power
fields (magnification, ×400) were randomly selected. The apoptotic
index was calculated in these fields as the percentage of positive
cells, using the following equation: Apoptotic index = (number of
positive cells/total number of cells) × 100% (23).
Statistical analyses
Statistical analysis was performed with SPSS
software version 17.0 (SPSS Inc., Chicago, IL, USA). Statistical
significance among the control and drug-treated groups on tumor
growth was determined by least significant difference (LSD) t-test.
We used a Tukey’s honest significance difference (Tukey HSD) test
for evaluation of the inhibitory activity on tumor cell MVD, VEGF
mRNA expression and the increase in apoptosis. Correlations between
VEGF score and MVD were estimated using the Karl Pearson
coefficient of correlation. All experimental data were expressed as
mean ± standard error (SE). P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of ovarian cancer growth
To test whether SC-560, celecoxib or Taxol inhibits
ovarian cancer growth, we used the human ovarian carcinoma cell
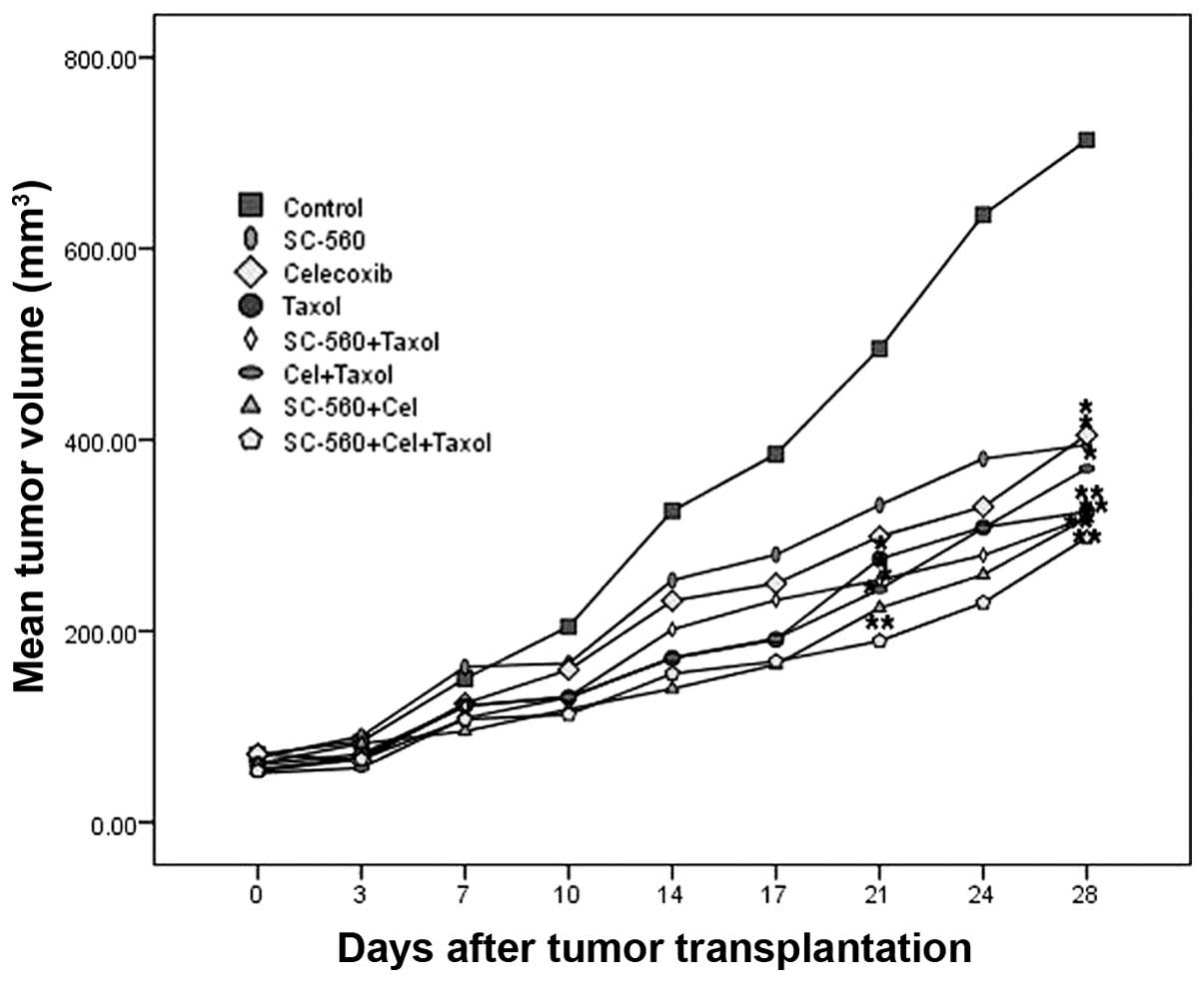
line SKOV-3. The data in Fig. 1
show the relative effect of SC-560, celecoxib and Taxol treatment.
The whole experiment was continued for 28 days. After 7 days to
allow tumor establishment, mice were treated with SC-560, celecoxib
and Taxol. The tumor growth increased in the control group whereas
the growth was substantially suppressed in the treatment groups.
After three weeks of treatment with SC-560, celecoxib or Taxol, a
mean tumor volume of 331.72, 298.85 and 275.59 mm3 was
observed, respectively, while the mean tumor volume of the control
group was 495.30 mm3. At the end date of administration,
all the treatment groups, with the exception of the SC-560 and
celecoxib groups, had already demonstrated significant inhibitory
effects on mean tumor volume (P<0.05). Moreover, at the end of
the experiment, all treatment groups demonstrated notable effects
on the inhibition of ovarian cancer growth; however, the inhibitory
rate in these groups had no difference from each other
(P>0.05).
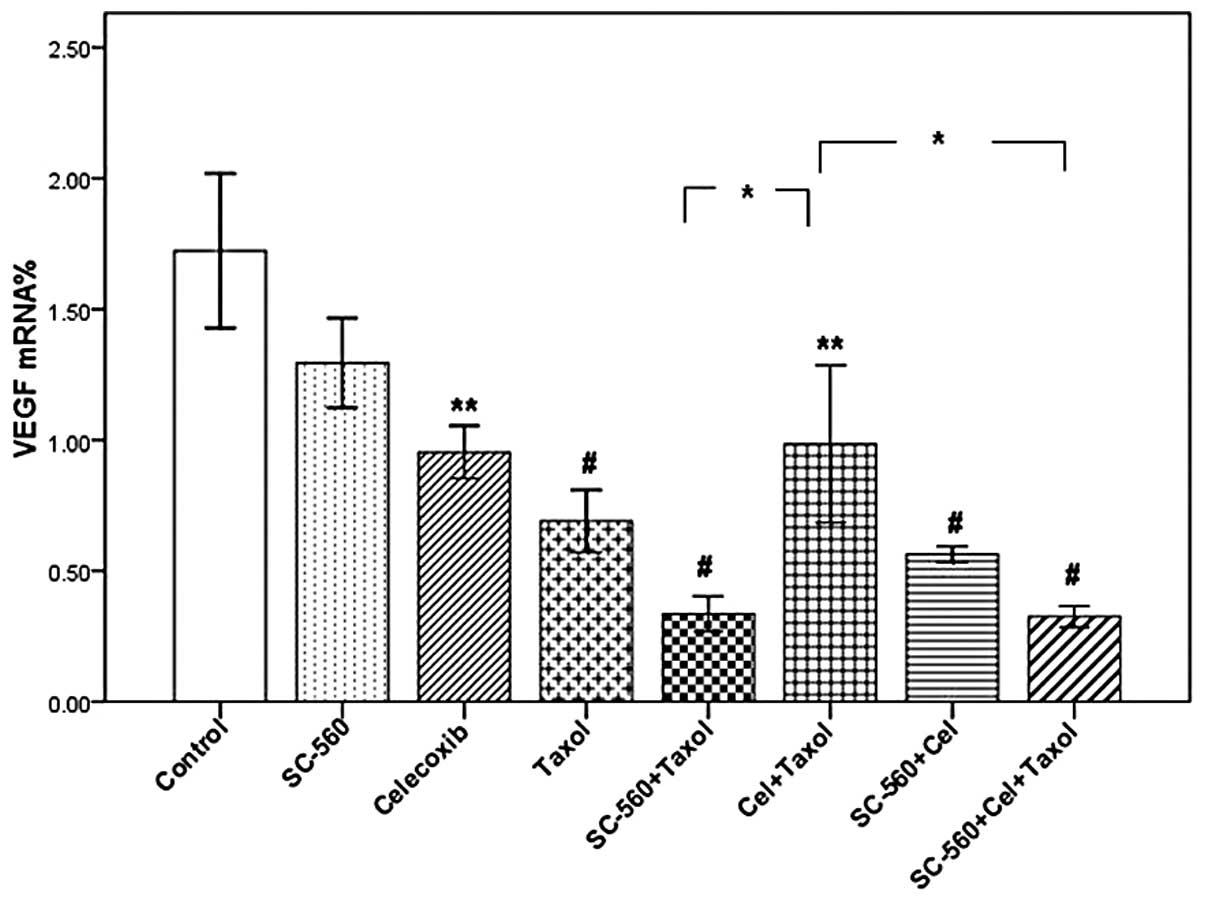
VEGF mRNA expression level
In this study, we measured VEGF mRNA levels in
xenograft tumors by real-time PCR analysis. Three molecular
isoforms of VEGF were generated by alternative splicing, rendering
proteins containing 189, 165 and 121 amino acid residues. Real-time
PCR analysis indicated the ΔCT of VEGF in the eight groups
(Table I). As shown in Fig. 2, although the levels of VEGF mRNA in
the SC-560/Taxol and SC-560/celecoxib/Taxol groups demonstrated a
decreasing tendency when compared with the Taxol group, the
difference was not statistically significant. However, the VEGF
mRNA levels in these two groups were significantly lower than that
in the celecoxib/Taxol group (P<0.05).
 | Table IΔCt of VEGF in the eight groups. |
Table I
ΔCt of VEGF in the eight groups.
| Group | VEGF 121 | VEGF 165 | VEGF 189 |
|---|
| Control | 6.94±0.23 | 4.58±0.26 | 6.34±0.23 |
| SC-560 | 7.13±0.30 | 5.34±0.30 | 6.54±0.21 |
| Celecoxib | 7.10±0.23 | 5.19±0.20 | 7.99±0.11 |
| Taxol | 7.87±0.32 | 6.28±0.49 | 7.79±0.29 |
| SC-560 + Taxol | 9.14±0.33 | 7.00±0.48 | 8.99±0.23 |
| Celecoxib +
Taxol | 8.31±0.64 | 4.60±0.43 | 8.04±0.40 |
| SC-560 +
celecoxib | 8.50±0.22 | 5.91±0.21 | 8.05±0.25 |
| SC-560 + celecoxib
+ Taxol | 8.52±0.35 | 7.67±0.24 | 8.81±0.45 |
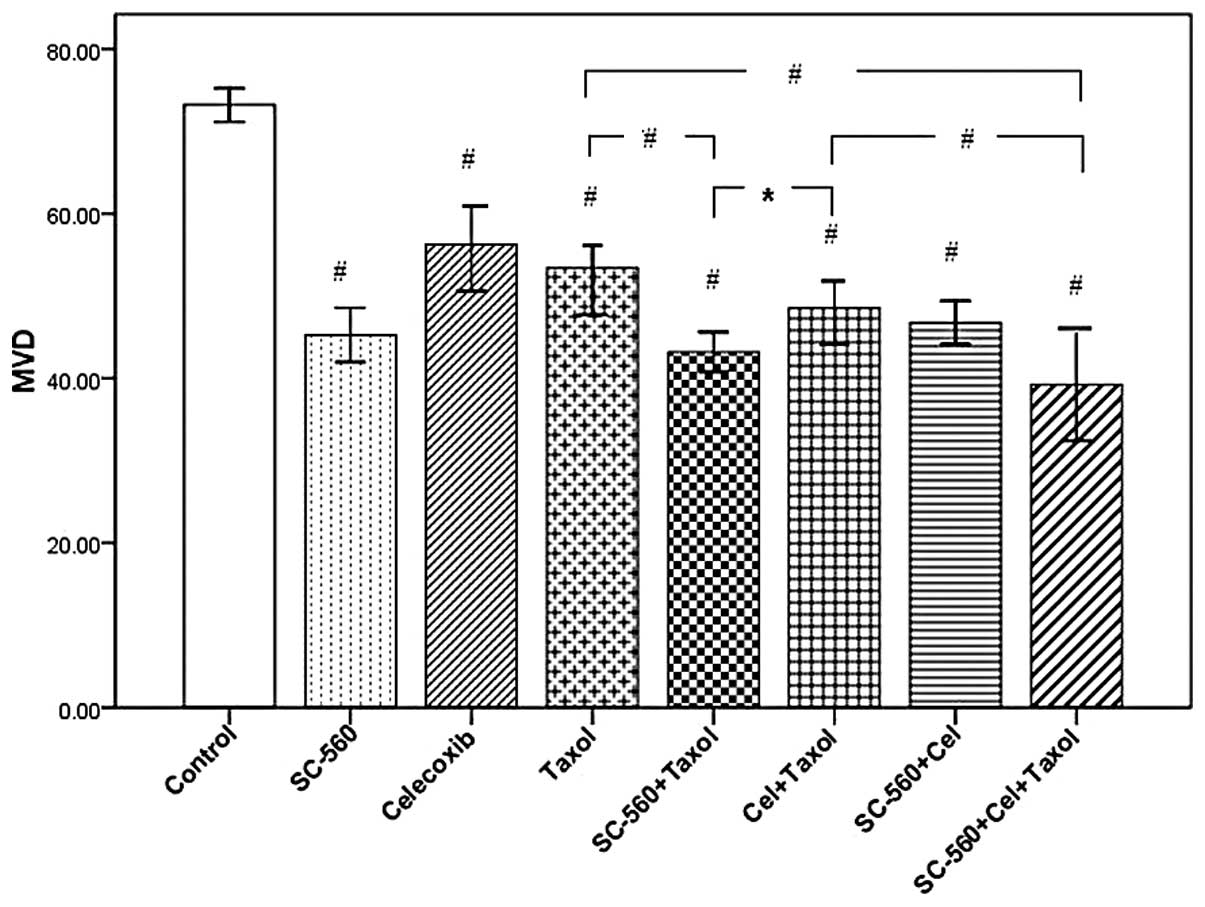
Effect on tumor blood vessels
To evaluate the anti-angiogenic therapeutic efficacy
of these three drugs, we histologically examined the residual
tumors. Frozen tumor sections were immunohistochemically stained
with an endothelial specific antibody against CD34.
Immunohistochemical analysis identified a decrease in the number of
CD34-positive microvessels of frozen tumor sections in
mice treated with SC-560, celecoxib and Taxol. MVD in tumor tissues
were reduced from 73.20±0.80 in the control group to 53.43±2.22,
43.20±0.94, 48.53±1.70 and 39.57±2.03 in the Taxol, SC-560/Taxol,
celecoxib/Taxol and SC-560/celecoxib/Taxol-treated groups,
respectively. The data in Fig. 3
show that sections from tumors in all drug-treated mice displayed a
marked reduction in MVD compared with the vehicle-treated mice
(P<0.001). In addition, the MVD values in the SC-560/Taxol and
SC-560/celecoxib/Taxol groups displayed a distinguished reduction
when compared with Taxol-treated mice (P<0.001), which were also
significantly lower than that in the celecoxib/Taxol group
(P<0.05 and P<0.001, respectively). However, there was no
difference between the SC-560/Taxol and SC-560/celecoxib/Taxol
groups.
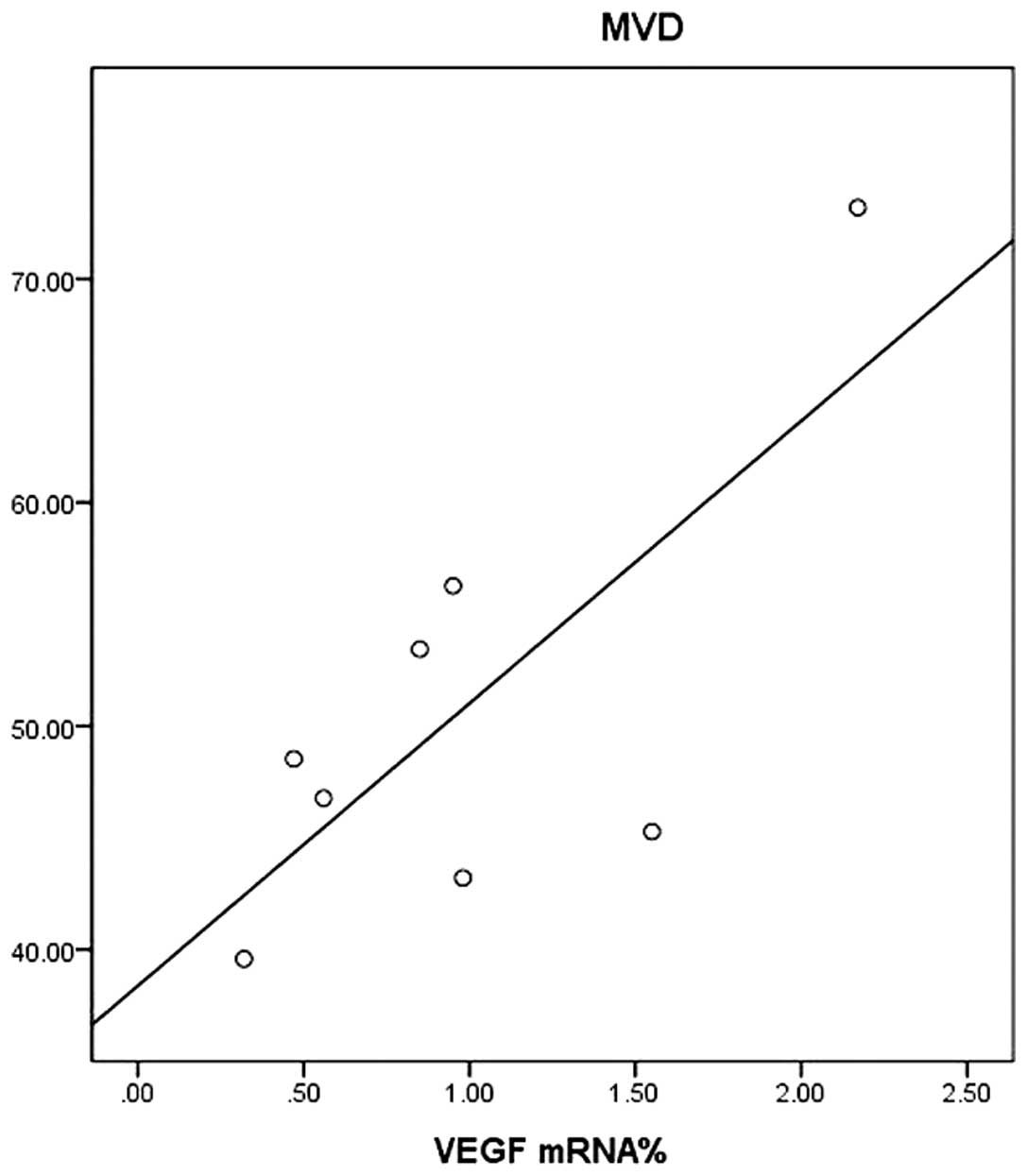
Correlation between VEGF and MVD
Linear equations were created to show the
correlation between MVD and VEGF (Fig.
4). The analysis revealed a positive correlation between the
expressions of VEGF mRNA and MVD (correlation coefficient, r=0.737,
P<0.05).
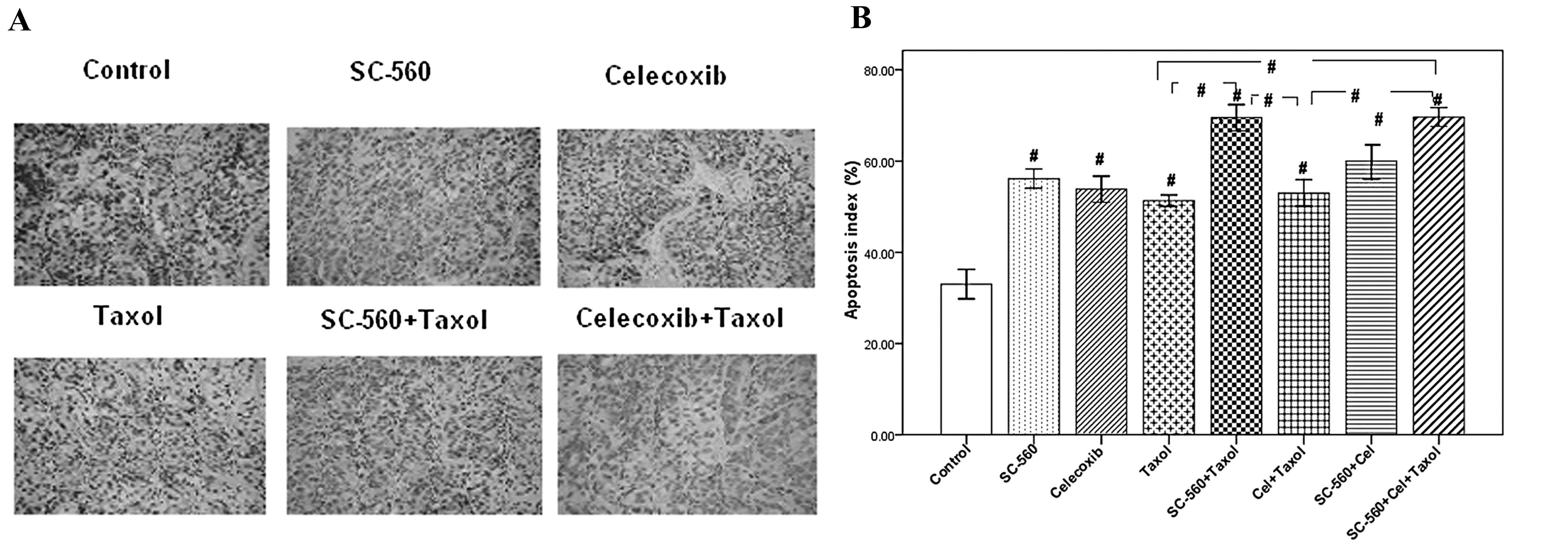
Effect on tumor cell apoptosis
We assessed cell apoptosis in the eight groups by
TUNEL assay. Fig. 5A shows six
representative images of tumor cell apoptosis. The number of
apoptotic cells was more frequent in tumor sections of the
treatment groups than in those of the control group. Data for the
apoptotic index of the eight groups are shown in Fig. 5B. The apoptotic index in all
drug-treated groups were significantly different to that of the
control group (33.00±3.22%; P<0.001). The apoptotic indices in
the SC-560/Taxol (69.50±2.87%) and SC-560/celecoxib/Taxol groups
(69.67±2.08%) demonstrated a significant increase at the end of
treatment compared with the Taxol group (51.33±1.26%; P<0.001).
These were also significantly higher than that in the
celecoxib/Taxol group (P<0.001). However, the apoptotic index
between the SC-560/Taxol and SC-560/celecoxib/Taxol groups
demonstrated no significant difference (P>0.05).
Discussion
The main finding in the present study was that
SC-560 enhances the anti-angiogenic and pro-apoptotic effect of
Taxol and these effects are better than those observed with
celecoxib.
In this study, the mean tumor volumes in the
treatment groups were significantly lower than in the
vehicle-treated mice at the end of treatment. The effects of SC-560
and celecoxib administered alone on inhibiting tumor growth were
similar to that of Taxol. Taxanes are anti-microtubule agents that
have strong anti-neoplastic effects. It is well known that Taxol is
deemed to be the standard first-line therapy for patients with
advanced ovarian cancer (5). A
number of studies revealed that taxanes upregulate the COX-2 level
in tumor cells and enhance MDR1 expression and functional activity
(7,24); therefore, the addition of COX-2
inhibitors to Taxol is widely used for antitumor treatment
(12,25,26).
Sorokin identified that COX-2 inhibitors decrease the function of
MDR1-enhanced accumulation of chemotherapy agents and decrease the
resistance of tumors to chemotherapeutic drugs, thus enhancing the
anti-tumor efficacy of Taxol (8).
Furthermore, the combination of a COX-2-selective inhibitor and
Taxol has been used in phase II trials of solid tumor treatment
(27–29). However, research on Taxol in
combination with COX-1-selective inhibitors used for the
chemotherapy of ovarian cancer has not been conducted. COX
inhibitors, which are selected based on definitive mechanisms
relevant to tumorigenesis, have beneficial applications in human
cancer chemoprevention trials. The combination of COX-1 and
COX-2-selective inhibitors performed better anti-tumor effects than
when administered alone (19,30).
In this study, we added SC-560 and celecoxib to Taxol. Although the
mean tumor volumes in the combination groups were significantly
different from the vehicle-treated mice, no difference was observed
between these groups and the Taxol group. This may be associated
with the difference in dosage, the frequency of administration and
the length of the experimental time. Therefore, this requires
further investigation.
Ovarian cancer growth is angiogenesis-dependent and
an increased production of angiogenic growth factors, including
VEGF, is prognostically significant even during the early stages of
the disease. VEGF is the most important of all the growth factors
involved in tumor angiogenesis. Strong VEGF expression is suggested
to play an important role in the tumor progression of ovarian
carcinoma (31). A line of evidence
reveals that levels of VEGF have been correlated with tumor
response and survival rate in malignancies (32,33).
In addition, the importance of angiogenesis in tumor progression
has been highlighted, demonstrating that the angiogenic potential
of tumors assessed by MVD directly correlates with poor prognosis
(34). In the present study, we
analyzed the levels of VEGF mRNA and values of MVD to assess the
anti-angiogenic effect of these three drugs, as well as to observe
whether combined administration produces better anti-angiogenic
effects compared to a single administration. Our previous study
identified that SC-560 inhibits the COX-associated upregulation of
VEGF and reduces MVD (35). In the
present study, the value of MVD in the SC-560/Taxol group was
significantly different to the Taxol group and the level of VEGF
mRNA in this group also demonstrated a decreasing tendency when
compared with the Taxol group. In addition, the MVD value and VEGF
mRNA level in the SC-560/Taxol group were significantly lower than
that in the celecoxib/Taxol group. A number of studies identified
that COX-1, not COX-2, mRNA and protein levels are elevated in
human ovarian cancers. COX-1 is the dominant pathway responsible
for generating PGs in epithelial ovarian cancers in mice.
Additionally, COX-1 may contribute to carcinoma development in the
ovary through stimulation of neovascularization and selective
inhibition of COX-1, not COX-2, inhibits arachidonic
acid-stimulated VEGF production (16–18,36).
These results suggest that SC-560, when combined with Taxol,
enhances the anti-angiogenic effect of Taxol and that this effect
is better than with celecoxib treatment.
Unrestricted cell proliferation and reduced
apoptosis are hallmarks of cancer cells (19). Apoptosis is a multistep process and
an increasing number of genes have been identified to be involved
in the control or execution of apoptosis (37). Taxanes induce an unbalance between
microtubule polymerization and depolymerization, which finally
leads to cell cycle arrest and apoptosis (38). COX inhibitors induce apoptosis by
inhibiting the production of COXs, reducing the PGE2
levels and changing gene expression (39–42).
In the present study, the apoptotic index in the SC-560/Taxol group
was significantly different from the Taxol and celecoxib/Taxol
groups, which suggests that SC-560 has a more pronounced effect on
enhancing the proapoptotic activity of Taxol than celecoxib. This
was consistent with the result that SC-560 had a greater effect on
enhancing the anti-angiogenic effect of Taxol than celecoxib. This
may be associated with the result observed by Gupta et al
that COX-1, not COX-2, is overexpressed in ovarian cancer (16).
The present findings demonstrate that the
combination of SC-560 and Taxol has a better effect on suppressing
angiogenesis and promoting cell apoptosis than Taxol alone and
these effects were better than the combination of celecoxib and
Taxol.
References
|
1
|
Ozols RF: Future directions in the
treatment of ovarian cancer. Semin Oncol. 29:32–42. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yokoyama Y, Sakamoto T, Sato S and Saito
Y: Evaluation of cytoreductive surgery with pelvic and paraaortic
lymphadenectomy and intermittent cisplatin-based combination
chemotherapy for improvement of long-term survival in ovarian
cancer. Eur J Gynaecol Oncol. 20:361–366. 1999.
|
|
3
|
Ozols RF: Recurrent ovarian cancer:
evidence-based treatment. J Clin Oncol. 20:1161–1163.
2002.PubMed/NCBI
|
|
4
|
Kohler DR and Goldspiel BR: Evaluation of
new drugs: Paclitaxel (taxol). Pharmacotherapy. 14:3–34. 1994.
|
|
5
|
Pignata S, Scambia G, Ferrandina G, et al:
Carboplatin plus paclitaxel versus carboplatin plus pegylated
liposomal doxorubicin as first-line treatment for patients with
ovarian cancer: the MITO-2 randomized phase III trial. J Clin
Oncol. 29:3628–3635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Qu Y, Niu XL, Sun WJ, Zhang XL and
Li LZ: Autocrine production of interleukin-8 confers cisplatin and
paclitaxel resistance in ovarian cancer cells. Cytokine.
56:365–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Subbaramaiah K, Hart JC, Norton L and
Dannenberg AJ: Microtubule-interfering agents stimulate the
transcription of cyclooxygenase-2. Evidence for involvement of
ERK1/2 AND p38 mitogen-activated protein kinase pathways. J Biol
Chem. 275:14838–14845. 2000. View Article : Google Scholar
|
|
8
|
Sorokin A: Cyclooxygenase-2: potential
role in regulation of drug efflux and multidrug resistance
phenotype. Curr Pharm Des. 10:647–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uddin S, Ahmed M, Hussain A, Assad L,
Al-Dayel F, Bavi P, Al-Kuraya KS and Munkarah A: Cyclooxygenase-2
inhibitior inhibits PI3-K/AKT kinase activity in epithelial ovarian
cancer. Int J Cancer. 126:382–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu P, Su Y, Guo S, Teng L, Xu Y, Qi J,
Gong H and Cai Y: Over-expression of COX-2 induces human ovarian
cancer cells (CAOV-3) viability, migration and proliferation in
association with PI3-k/Akt activation. Cancer Invest. 26:822–829.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Athanassiadou P, Grapsa D, Athanassiades
P, Gonidi M, Athanassiadou AM, Tsipis A and Patsouris E: The
prognostic significance of COX-2 and survivin expression in ovarian
cancer. Pathol Res Pract. 204:241–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferrandina G, Ranelletti FO, Martinelli E,
Paglia A, Zannoni GF and Scambia G: Cyclooxygenase-2 (Cox-2)
expression and resistance to platinum versus platinum/paclitaxel
containing chemotherapy in advanced ovarian cancer. BMC Cancer.
6:1822006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masferrer JL, Leahy KM, Koki AT, Zweifel
BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ and
Seibert K: Antiangiogenic and antitumor activities of
cyclooxygenase-2 inhibitors. Cancer Res. 60:1306–1311.
2000.PubMed/NCBI
|
|
14
|
Olsen SR: Taxanes and COX-2 inhibitors
from molecular pathways to clinical practice. Biomed Pharmacother.
59(Suppl 2): S306–S310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Merchan JR, Jayaram DR, Supko JG, He X,
Bubley GJ and Sukhatme VP: Increased endothelial uptake of
paclitaxel as a potential mechanism for its antiangiogenic effects:
potentiation by COX-2 inhibition. Int J Cancer. 113:490–498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta RA, Tejada LV, Tong BJ, Das SK,
Morrow JD, Dey SK and Dubois RN: Cyclooxygenase-1 is overexpressed
and promotes angiogenic growth factor production in ovarian cancer.
Cancer Res. 63:906–911. 2003.PubMed/NCBI
|
|
17
|
Daikoku T, Wang D, Tranguch S, Morrow JD,
Orsulic S, DuBois RN and Dey SK: Cyclooxygenase-1 is a potential
target for prevention and treatment of ovarian epithelial cancer.
Cancer Res. 65:3735–3744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daikoku T, Tranquch S, Trofimova IN,
Dinulescu DM, Jacks T, Nikitin AY, Connolly DC and Dey SK:
Cyclooxygenase-1 is overexpressed in multiple genetically
engineered mouse models of epithelial ovarian cancer. Cancer Res.
66:2527–2531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W, Wang J, Jiang HR, Xu XL, Zhang J,
Liu ML and Zhai LY: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on ovarian carcinoma in vivo.
Int J Mol Sci. 12:668–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williams CS, Watson AJ, Sheng H, Helou R,
Shao J and DuBois RN: Celecoxib prevents tumor growth in vivo
without toxicity to normal gut: lack of correlation between in
vitro and in vivo models. Cancer Res. 60:6045–6051. 2000.PubMed/NCBI
|
|
21
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell proliferation
associated human nuclear antigen defined by the monoclonal antibody
Ki-67. J Immunol. 133:1710–1715. 1984.PubMed/NCBI
|
|
22
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
a new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
del Vecchio MT, Leoncini L, Buerki K,
Kraft R, Megha T, Barbini P, Tosi P and Cottier H: Diffuse
controcytic and/or centroblastic malignant non-Hodgkins lymphomas:
comparison of mitotic and pyknotic (apoptotic) indices. Int J
Cancer. 47:38–43. 1991.PubMed/NCBI
|
|
24
|
Ratnasinghe D, Daschner PJ, Anver MR,
Kasprzak BH, Taylor PR, Yeh GC and Tangrea JA: Cyclooxygenase-2,
P-glycoprotein-170 and drug resistance; is chemoprevention against
multidrug resistance possible? Anticancer Res. 21:2141–2147.
2001.PubMed/NCBI
|
|
25
|
Altorki NK, Keresztes RS, Port JL, Libby
DM, Korst RJ, Flieder DB, Ferrara CA, Yankelevitz DF, Subbaramaiah
K, Pasmantier MW and Dannenberg AJ: Celecoxib, a selective
cyclo-Oxygenase-2 inhibitor, enhances the response to preoperative
paclitaxel and carboplatin in early-stage non-small-cell lung
cancer. J Clin Oncol. 21(14): 2645–2650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gasparini G, Meo S, Comella G, Stani SC,
Mariani L, Gamucci T, Avallone A, LoVullo S, Mansueto G, Bonginelli
P, Gattuso D and Gion M: The combination of the selective
cyclooxygenase-2 inhibitor celecoxib with weekly paclitaxel is a
safe and active second-line therapy for non-small cell lung cancer
a phase II study with biological correlates. Cancer J. 11:209–216.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Altorki NK, Christos P, Port JL, Lee PC,
Mirza F, Spinelli C, Keresztes RS, Beneck D, Paul S, Stiles BM,
Zhang Y and Schrump DS: Preoperative taxane-based chemotherapy and
celecoxib for carcinoma of the esophagus and gastroesophageal
junction: results of a phase 2 trial. J Thorac Oncol. 6:1121–1127.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhatt RS, Merchan J, Parker R, Wu HK,
Zhang L, Seery V, Heymach JV, Atkins MB, McDermott D and Sukhatme
VP: A phase 2 pilot trial of low-dose, continuous infusion, or
“metronomic” paclitaxel and oral celecoxib in patients with
metastatic melanoma. Cancer. 116:1751–1756. 2010.
|
|
29
|
Mutter R, Lu B, Carbone DP, Csiki I,
Moretti L, Johnson DH, Morrow JD, Sandler AB, Shyr Y, Ye F and Choy
H: A phase II study of celecoxib in combination with paclitaxel,
carboplatin, and radiotherapy for patients with inoperable stage
IIIA/B non-small cell lung cancer. Clin Cancer Res. 15:2158–2165.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kitamura T, Itoh M, Noda T, Matsuura M and
Wakabayashi K: Combined effects of cyclooxygenase-1 and
cyclooxygenase-2 selective inhibitors on intestinal tumorigenesis
in adenomatous polyposis coli gene knockout mice. Int J Cancer.
109:576–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamamoto S, Konishi I, Mandai M, Kuroda H,
Komatsu T, Nanbu K, Sakahara H and Mori T: Expression of vascular
endothelial growth factor (VEGF) in epithelia ovarian neoplasms:
correlation with clinicopathology and patient survival and analysis
of serum VEGF levels. Br J Cancer. 76:1221–1227. 1997. View Article : Google Scholar
|
|
32
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Folkman J: Angiogenesis: an organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Xu RJ, Lin ZY, Zhuo GC and Zhang HH:
Effects of a cyclooxygenase-1-selective inhibitor in a mouse model
of ovarian cancer, administered alone or in combination with
ibuprofen, a nonselective cyclooxygenase inhibitor. Med Oncol.
26:170–177. 2009. View Article : Google Scholar
|
|
36
|
Dore M, Cote LC, Mitchell A and Sirois J:
Expression of prostaglandin G/H synthase type 1, but not type 2, in
human ovarian adenocarcinomas. J Histochem Cytochem. 46:77–84.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gastman BR: Apoptosis and its clinical
impact. Head Neck. 23:409–425. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Foa R, Norton L and Seidman AD: Taxol
(paclitaxel): A novel antimicrotubule agent with remarkable
anti-neoplastic activity. Int J Clin Lab Res. 24:6–14. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leahy KM, Ornberg RL, Wang Y, Zweifel BS,
Koki AT and Masferrer JL: Cyclooxygenase-2 inhibition by celecoxib
reduces proliferation and induces apoptosis in angiogenic
endothelial cells in vivo. Cancer Res. 62:625–631. 2002.PubMed/NCBI
|
|
40
|
Daikoku T, Tranguch S, Chakrabarty A, Wang
D, Khabele D, Orsulic S, Morrow JD, Dubois RN and Dey SK:
Extracellular signal-regulated kinase is a target of
cyclooxygenase-1-peroxisome proliferator-activated receptor-delta
signaling in epithelial ovarian cancer. Cancer Res. 67:5285–5292.
2007. View Article : Google Scholar
|
|
41
|
Bottone FG Jr, Martinez JM, Alston-Mills B
and Eling TE: Gene modulation by Cox-1 and Cox-2 specific
inhibitors in human colorectal carcinoma cancer cells.
Carcinogenesis. 25:349–357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu J, Song X, Lin HP, Young DC, Yan S,
Marquez VE and Chen CS: Using cyclooxygenase-2 inhibitors as
molecular platforms to develop a new class of apoptosis-inducing
agents. J Natl Cancer Inst. 94:1745–1757. 2002. View Article : Google Scholar : PubMed/NCBI
|