Introduction
Cervical cancer remains one of the leading causes of
cancer-related mortality in women despite medical advances and the
availability of vaccination programmes (1). Promoter hypermethylation of tumor
suppressor genes (TSGs) has long been contested as a probable cause
of cancer and its progression (2).
Several TSGs are inactivated by this mechanism (3). The tissue inhibitor of
metalloproteinases-2 (TIMP2) is known to antagonize matrix
metalloproteinase activity and to suppress tumor growth,
angiogenesis, invasion and metastasis. The TIMP2 gene is
known to be expressed in normal human tissues, whereas its
expression is downregulated in glioblastomas and metastatic lung
tumors (4). TIMP2
overexpression had an inhibitory effect on tumor growth and
angiogenesis in a breast cancer mouse model (5). In addition, overexpression of
TIMP2 has been shown to restrict the invasiveness of various
tumor cell types in vitro (6,7).
Suzuki et al (8) observed
methylated TIMP2 in the colorectal cancer cell line RKO.
However, methylation of TIMP2 was not commonly found in
primary colorectal tumors. The frequency of the hypermethylated
TIMP2 gene in cervical cancer was found to be 47% by Ivanova
et al (9), whereas
methylation-specific PCR (MSP) and sodium bisulfite analysis of
genomic DNA of the HeLa cell line revealed an unmethylated promoter
and expression of TIMP2. SiHa and Caski cervical cancer cell
lines had a methylated promoter with downregulated expression of
corresponding gene activity and methylation of the 5′ region of the
TIMP2 gene. Therefore, in the present study, we aimed to
study the role of the promoter hypermethylation and associated
TIMP2 expression in cervical cancer cell lines.
Materials and methods
Cell culture
The cervical cancer cell lines HeLa, SiHa and Caski
were procured from NCCS (Pune, India) and maintained in RPMI-1640
(Sigma, St. Louis, MO, USA) supplemented with 10% FBS (Life
Technologies, Israel) and 10,000 units of penicillin and
streptomycin (Sigma) at 37°C and 5% CO2 (10).
Treatment of cell lines
HeLa, SiHa and Caski cell lines were treated with
5-aza-2′-deoxycytidine (Sigma) (positive control) at 20-μM
concentration for 4 days with change of media along with
5-aza-2′-deoxycytidine every 48 h. Untreated cells were used as a
control to analyse the promoter methylation status of the
TIMP2 gene.
Methylation specific PCR (MSP)
DNA was isolated using standard phenol:chloroform
extraction and quantified using an ND1000 spectrophotometer (Thermo
Scientific). DNA (1 μg) was subjected to bisulfite modification
using EZ gold methylation kit (Zymo Research). Bisulfite-modified
DNA was used for MSP of the TIMP2 gene with a set of primers
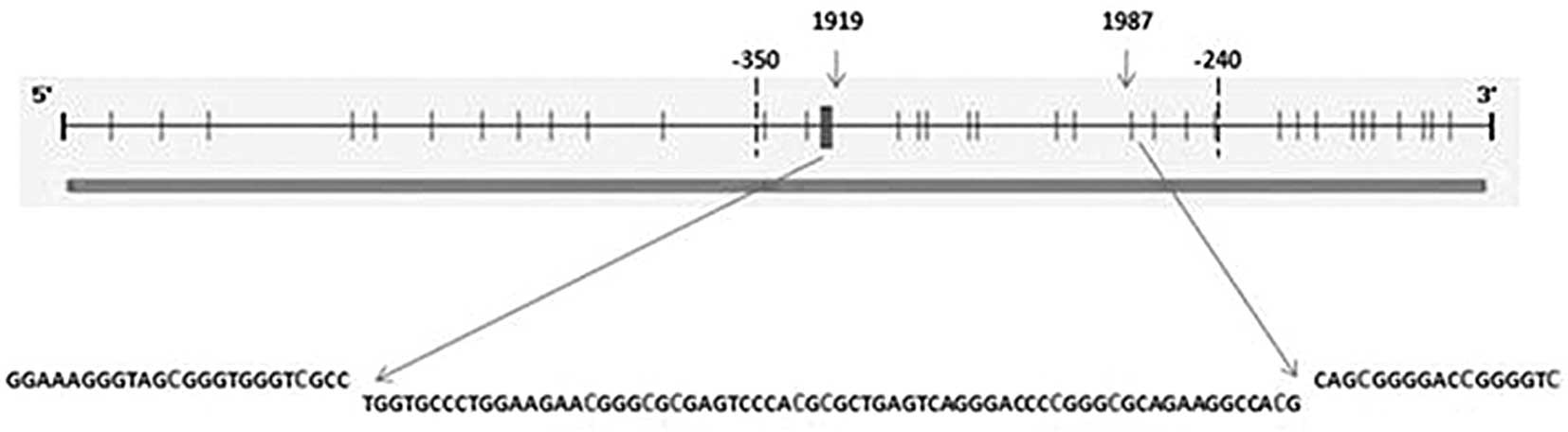
(9) spanning regions 1919–1987
(−325 to −257), relative to the transcription start site. MSP was
performed as detailed by Ivanova et al (9). The PCR products were analysed on 3%
agarose gel. MSP was carried out in duplicate.
Reverse transcription PCR (RT-PCR)
Total RNA was isolated from cultured cells using TRI
reagent (Sigma) and was treated with RNAse-free DNAseI (Fermentas)
to eliminate any DNA contamination. cDNA was synthesized using a
First Strand Revertaid cDNA synthesis kit (Fermentas). RT-PCR was
carried out for TIMP2, β-actin and GAPDH genes
using the following primers: TIMP2 (forward: 5′-TGGCCT
TTATATTTGATCCACAC-3′, reverse: 5′-AAAAATCCAAAC GGAAACAAAAT-3′);
β-actin (forward: 5′-CATGTACGT TGCTATCCAGGC-3′, reverse:
5′-CTCCTTAATGTCACG CACGAT-3′); GAPDH (forward:
5′-CAAGGTCATCCA TGACAACTTTG-3′, reverse: 5′-GTCCACCACCCTGTTGCT
GTAG-3′), under the following conditions: initial denaturation at
94°C for 3 min followed by 28 cycles (94°C for 30 sec, 60°C for 45
sec, 72°C for 45 sec) and final extension at 72°C for 5 min.
β-actin and GAPDH were regarded as the internal
control. Densitometric analysis of the bands was performed using 1D
analysis software with average density as a parameter, calculated
using intensity (INT U)/mm2 with a sensitivity of 10
(Bio-Rad, Hercules, CA, USA). Fold change in expression was
calculated for each band using the formula: Fold change = average
density of test gene/average density of internal control.
Results
MSP
MSP for the TIMP2 gene with primers specific
to methylated DNA was carried out with untreated and 5′
aza-2′-deoxycytidine treated cells, which resulted in the
amplification of a 68-bp amplicon in untreated cells of the HeLa,
SiHa and Caski cell lines, whereas no such band was observed in 5′
aza-2′-deoxycytidine treated cells. Primers specific for
unmethylated DNA responded only with samples treated with 5′
aza-2′-deoxycytidine, resulting in amplification of the 68-bp
amplicon (Fig. 1A).
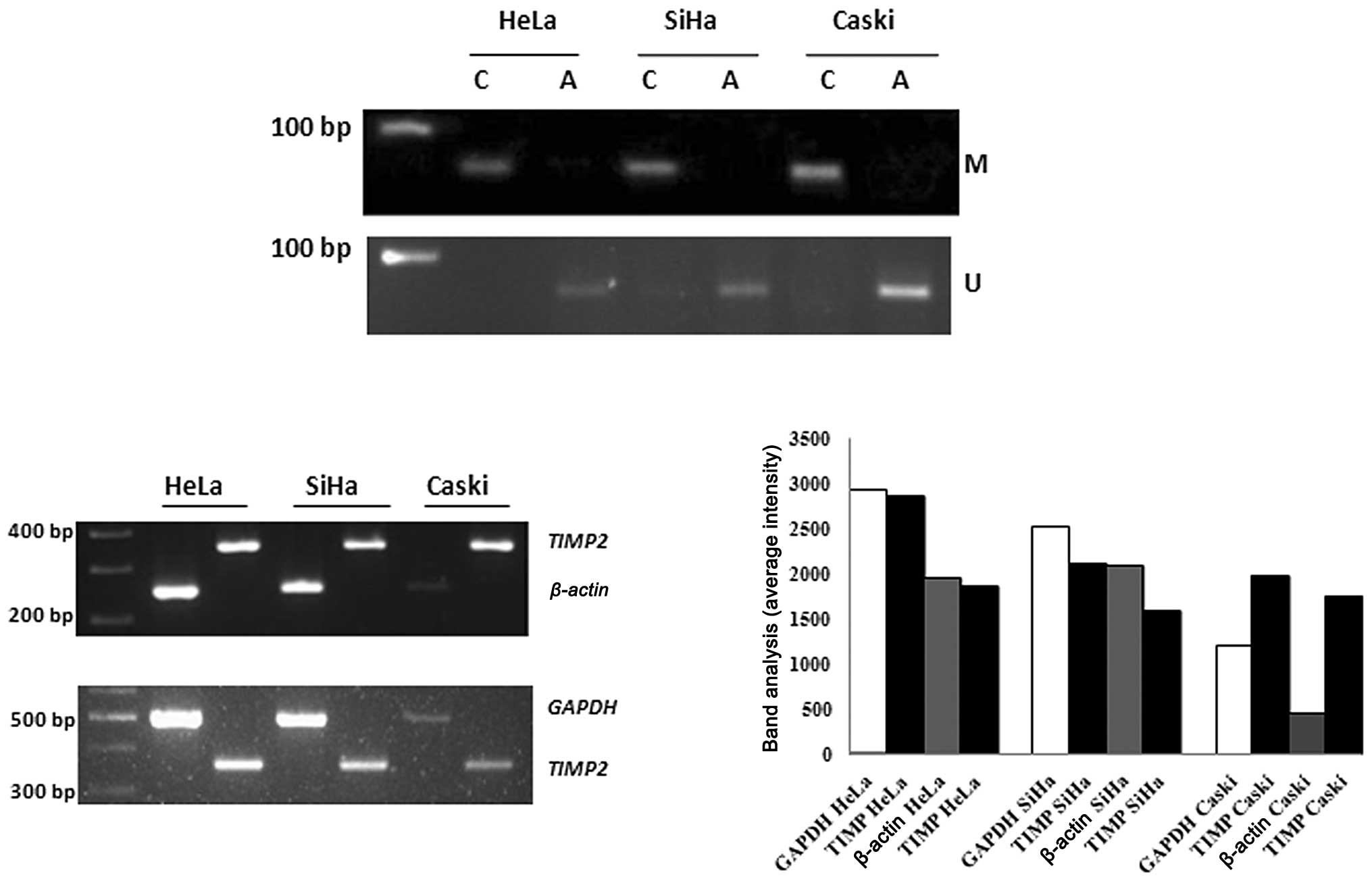 | Figure 1(A) M, methylation-specific band; U,
unmethylation-specific band; C, control samples; A,
5-aza-2′-deoxycytidine treated samples. From left: lane 1, 100 bp
DNA ladder; lanes 2–3, TIMP2 MSP in the HeLa cell line;
lanes 4–5, TIMP2 MSP in the SiHa cell line; lanes 6–7,
TIMP2 MSP in the Caski cell line. (B) RT-PCR was carried out
to analyse the expression of TIMP2 in the HeLa, SiHa and
Caski cell lines, respectively. From left: lane 1, 100 bp DNA
ladder; lanes 2–3, comparative expression analysis of TIMP2,
β-actin and GAPDH in the HeLa cell line; lanes 4–5,
comparative expression analysis of TIMP2, β-actin and
GAPDH in the SiHa cell line; lanes 6–7, comparative
expression analysis of TIMP2, β-actin and
GAPDH in the Caski cell line. (C) Comparative densitometric
analysis of TIMP2 expression with β-actin and
GAPDH in the HeLa, SiHa and Caski cell lines. |
RT-PCR
RT-PCR for the TIMP2 gene was carried out for
the HeLa, SiHa and Caski cell lines to assess the impact of
promoter hypermethylation on the expression of the TIMP2
gene in untreated cells. Expression of the TIMP2 gene was
found to be normal in the HeLa cell line. In the case of the SiHa
cell line, the TIMP2 gene exhibited downregulation, which
was not found to be significant at RT-PCR level, whereas
TIMP2 was found to be markedly upregulated in the Caski cell
line, which was confirmed following a comparison of two internal
controls (Fig. 1B). As per the
analysis, the expression of the TIMP2 gene was found to be
3.7- and 1.6-fold in the Caski cell line, 0.95- and 0.98-fold in
the HeLa cell line, and 0.76- and 0.83-fold (Fig. 1C) compared to β-actin and
GAPDH, respectively.
Discussion
The TIMP2 gene, an endogenous inhibitor of
matrix metalloproteinase (MMP), plays a significant role in
cell invasion and tumorigenesis and is downregulated or silenced in
various human cancer cell lines. The percentage ratio of
TIMP2 expression compared to that of MMP has always
been debated. High expression of both TIMP2 and MMP9
has been observed in invasive cancer cells of the cervix and the
surrounding stromal cells, whereas the expression of both
TIMP2 and MMP9 is hardly detectable in cervical
intraepithelial neoplasias (CIN) by hybridization and in
situ RT-PCR (11,12). The fact that TIMP2 is an
inhibitor of MMPs means that it belongs to a significant
family of tumor suppressor genes whose function requires
comprehensive evaluation. During our ongoing study on DNA
methylation, we selected the TIMP2 gene and focused on a
possible reversal of hypermethylation and subsequent
reactivation.
Since there was only one published study by Ivanova
et al available (9) relating
TIMP2 promoter hypermethylation to cervical cancer and,
specifically, cervical cancer cell lines, a TIMP2 promoter
hypermethylation study spanning the 1919-1987 region, i.e., −325 to
−257 relative to the transcription start site was undertaken.
However, the results were very dissimilar to those already
reported. The promoter of the TIMP2 gene was found to be
methylated in the HeLa cell line using MSP, which was confirmed
after treatment of the HeLa cell line with 5′ aza-2′-deoxycytidine
leading to the disappearance of the 68 bp methylation-specific
band. Primers specific for unmethylated DNA responded only to 5′
aza-2′-deoxycytidine treated samples, which again confirmed the
promoter hypermethylation, contrary to the existing study.
Similarly, methylation of the TIMP2 gene was confirmed in
the SiHa and Caski cell lines. Upon meta-analysis of the
TIMP2 sequence (GenBank accession number: U 44381), eight
CpG sites were found in the 1919–1987 (−325 to −257) region and 13
CpG sites were found in total when the range was extended to −350
to −240, as in the study carried out by Ivanova et al
(9), relative to the transcription
start site (Fig. 2). The CpG sites
within the −350 to −240 region in the HeLa cell line were found to
be unmethylated, correlating with the normal expression of the
TIMP2 gene in the HeLa cell line. In the case of the SiHa
cell line, seven CpG sites were hemimethylated, three were
methylated and three were found to be unmethylated, whereas in the
Caski cell line all CpG sites were found to be methylated with the
exception of one or two sites that correlated with the
downregulated gene expression of TIMP2 in the SiHa and Caski
cell lines. In the present study, the transcription of the
TIMP2 gene was found to be highly upregulated in the Caski
cell line despite the promoter being hypermethylated. High
expression in the Caski cell line was also confirmed with another
housekeeping gene, GAPDH, in addition to β-actin;
however, there was a consistent expression of the TIMP2 gene
in the HeLa cell line, whereas the downregulated expression was
observed in the SiHa cell line despite promoter hypermethylation,
which was moderately observable at the RT-PCR level.
High expression of TIMP2 in HPV-16 positive
SiHa and Caski cell lines has been reported (13) but discussion is lacking on the
effect and role of promoter methylation status of the TIMP2
gene in these cervical cancer cell lines. Our study clearly showed
that the CpG sites within the 1919–1987 (−325 to −257) region,
relative to the transcription start site of the TIMP2
promoter, do not regulate the expression of the TIMP2 gene
in the Caski and HeLa cell lines. However, the expression in the
SiHa cell line indicated downregulation but was not found to be
significant. Five CpG sites still remain in the −350 to −240
region, relative to the transcription start site, of which two CpG
sites are situated upstream of −325 and the remaining three CpG
sites are situated downstream of −247. The methylation profile of
specific CpG sites within a promoter, located towards the
transcription start site are involved in the regulation of gene
expression (14). Since the
TIMP2 gene is expressed in cervical cancer cell lines, the
involvement of other CpG sites situated downstream of −247,
including the remaining three CpG sites is significant. It is
speculated that other CpG sites located towards the transcription
start site play a more significant role in regulating the
expression of the TIMP2 gene in cervical cancer cell lines
and specifically the Caski cell line. In conclusion, we did not
find a clear-cut correlation between promoter hypermethylation and
expression of TIMP2 gene in cervical cancer cell lines,
indicating that the interplay of methylation and location of CpG
sites in the promoter near the transcription site may be important
for gene expression.
Acknowledgements
This study was supported by the Council of
Scientific and Industrial Research (CSIR), New Delhi, India.
References
|
1
|
Guerry SL, De Rosa CJ, Markowitz LE, et
al: Human papillomavirus vaccine initiation among adolescent girls
in high-risk communities. Vaccine. 29:2235–2241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
I-Wen T, Pei-Chi H, Kuan-Der L, et al:
Targeted methylation of two tumor suppressor genes is sufficient to
transform mesenchymal stem cells into cancer stem/initiating cells.
Cancer Res. 71:4653–4663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esteller M, Corn PG, Baylin SB, et al: A
gene hypermethylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
4
|
Mohanam S, Wang SW, Rayford A, et al:
Expression of tissue inhibitors of metalloproteinases: negative
regulators of human glioblastoma invasion in vivo. Clin Exp
Metastasis. 13:57–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hajitou A, Sounni NE, Devy L, et al:
Down-regulation of vascular endothelial growth factor by tissue
inhibitor of metalloproteinase-2: effect on in vivo mammary tumor
growth and angiogenesis. Cancer Res. 61:3450–3457. 2001.PubMed/NCBI
|
|
6
|
DeClerck YA, Perez N, Shimada H, et al:
Inhibition of invasion and metastasis in cells transfected with an
inhibitor of metalloproteinases. Cancer Res. 52:701–708.
1992.PubMed/NCBI
|
|
7
|
Valente P, Fassina G, Melchiori A, et al:
TIMP-2 over-expression reduces invasion and angiogenesis and
protects B16F10 melanoma cells from apoptosis. Int J Cancer.
19:246–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki H, Gabrielson E, Chen W, et al: A
genomic screen for genes upregulated by demethylation and histone
deacetylase inhibition in human colorectal cancer. Nat Genet.
31:141–149. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ivanova T, Vinokurova S, Petrenko A, et
al: Frequent hypermethylation of 5′ flanking region of TIMP-2 gene
in cervical cancer. Int J Cancer. 108:882–886. 2004.
|
|
10
|
Freshney RI: Culture of Animal Cells A
Manual of Basic Techniques. John Wiley & Sons; New York:
1994
|
|
11
|
Davidson B, Goldberg I, Kpopolovic J, et
al: MMP-2 and TIMP-2 expression correlates with poor prognosis in
cervical carcinoma: a clinicopathologic study using
immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol.
73:372–382. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nuovo GJ, MacConnell PB, Simsir A, et al:
Correlation of the in situ detection of polymerase chain
reaction-amplified metalloproteinase complementary DNAs and their
inhibitors with prognosis in cervical carcinoma. Cancer Res.
55:267–275. 1995.
|
|
13
|
Silva Cardeal LB, Brohem CA, Silveira
Corrêa TC, et al: Higher expression and activity of
metalloproteinases in human cervical carcinoma cell lines is
associated with HPV presence. Biochem Cell Biol. 84:713–719.
2006.PubMed/NCBI
|
|
14
|
Jianduan L, Zhengyan Z, Miri B, et al:
IGSF4 promoter methylation and expression silencing in human
cervical cancer. Gynecol Oncol. 96:150–158. 2005. View Article : Google Scholar : PubMed/NCBI
|