Introduction
Hepatocellular carcinoma (HCC) is the fifth most
prevalent cancer and the third most common cause of cancer
mortality worldwide (1,2). Surgical resection and liver
transplantation are available for the treatment of early-stage HCC,
but the prognosis of HCC remains poor due to the high level of
tumor invasion, frequent intrahepatic spread, presence of
extrahepatic metastasis and resistance to chemotherapy (3,4).
Although the introduction of sorafenib provided successful
treatment for advanced HCC (5,6), no
established standard chemotherapeutic agents or molecular targeted
agents are currently available for use as either neoadjuvant or
adjuvant therapy. Additionally, sorafenib does not sufficiently
eradicate cancer cells. Therefore, novel and more effective agents
are required to improve the outcomes in patients with HCC.
CD44, a major adhesion molecule for the
extracellular matrix, is involved in a wide variety of
physiological processes, including leukocyte homing and activation,
wound healing and cell migration (7,8).
Through alternative mRNA splicing, cells produce numerous CD44
protein isoforms. The standard isoform (CD44s) is expressed
predominantly in hematopoietic cells and normal epithelial cell
subsets, whereas variant isoforms (CD44v) are expressed by certain
epithelial cells, during embryonic development, lymphocyte
maturation and activation and in several types of carcinoma. The
finding that the expression of a CD44v induced a metastatic
phenotype in locally growing tumor cells attracted considerable
interest (9). Expression of the
CD44v6 isoform was detected in patients with high-grade
non-Hodgkin’s lymphoma and found to correlate with a poor prognosis
(10). In colorectal cancer
patients, the expression of CD44s and the CD44v correlated with a
poor prognosis and may be considered as a strong prognostic factor
in patients (11). In patients with
cervical cancer, a high level of expression of CD44v6 is associated
with a poor prognosis (12).
Similarly, in breast cancer patients, the expression of variants
containing the exons v3, v6 and v7/8 correlates with more
aggressive stages of the disease (13). However, there are certain tumor
types, including neuroblastomas and prostate cancer, in which the
absence of CD44 expression correlates with transformation and a
poor prognosis (14,15). As such, while numerous studies have
analyzed the expression of CD44 isoforms in human tumors of
different origins, their results were often controversial.
Therefore, we assessed the association between
CD44v6 and the invasive capacity of human HCC cell lines. The
clinical significance of CD44v6 was also investigated by
immunohistochemical analysis in patients with HCC.
Materials and methods
Cell lines and culture conditions
The PLC/PRF/5, HuH1, HLF and HLE human HCC cell
lines were purchased from the Japanese Collection of Research
Bioresources (Osaka, Japan). SK HEP-1 was purchased from the
American Type Culture Collection (Manassas, VA, USA). The cells
were routinely maintained in Dulbecco’s modified Eagle’s medium
(Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Invitrogen). The cells were incubated at 37°C in a 5%
CO2 air-humidified atmosphere.
Protein extraction and western blot
analysis
The protein extraction from cultivated cells and the
western blot analyses were performed as previously described
(16,17). In brief, the cells were lysed in a
cell lysis buffer containing 25 mM Tris (pH 7.4), 100 mM NaCl and
1% Tween-20. Equal amounts of the proteins were loaded onto 10%
gels and separated by SDS-PAGE. The resolved proteins were
electrophoretically transferred to polyvinylidene fluoride (PVDF)
membranes (Bio-Rad, Inc., Hercules, CA, USA). The membranes were
blocked with 5% low-fat dry milk in TBS-T [25 mM Tris (pH 7.4), 125
mM NaCl, 0.4% Tween-20] for 1 h at room temperature, followed by
overnight incubation with the primary antibody at 4°C. The blots
were extensively washed with TBS-T and incubated at a 1:2000
dilution of HRP-conjugated secondary antibody (Santa Cruz
Biotechnology and Cell Signaling, Santa Cruz, CA, USA) diluted in
TBS-T for 1 h at room temperature. The membranes were washed and
visualized using a chemiluminescent detection reagent kit (ECL; GE
Healthcare Corp., Piscataway, NJ, USA). A primary antibody against
CD44v6 (1:1000 dilution; Bender Medsystems, San Diego, CA, USA) was
used in this study.
RNA extraction and quantitative
RT-PCR
Total RNA extraction, complementary DNA (cDNA)
synthesis and quantitative real-time PCR were performed as
described previously (16,17). The total RNA was extracted from the
cells using the RNeasy Mini kit (Qiagen, Hilden, Germany) and cDNA
was synthesized with the SuperScript III transcriptor first strand
cDNA synthesis system for RT-PCR (Invitrogen), according to the
manufacturer’s instructions. Quantitative reverse transcription PCR
(qRT-PCR) was performed using a LightCycler 480 II instrument
(Roche, Mannheim, Germany). To determine the difference in the gene
expression levels between the specimens, 2−ΔΔCt was used
to measure the fold changes among the samples (18). To perform qRT-PCR, primers were
designed using the Universal Probe Library (Roche) according to the
manufacturer’s instructions. The primer sequences used in the
real-time PCR were: CD44, 5′-GCAGTCAACAGTCGAAGAAGG-3′,
5′-TGTCCTCCACAGCTCCATT-3′ and universal probe no. 29; and GAPDH,
5′-AGCCACATCGCTCAGACAC-3′, 5′-GCCCAATACGACCAAATCC-3′ and universal
probe no. 60.
Cell invasion assay
The in vitro cell invasion assay was
performed as previously described (17). In brief, the invasion of tumor cells
that migrated through transwell inserts with a uniform layer of BD
Matrigel basement membrane matrix (BD Biosciences) was assessed
according to the manufacturer’s instructions. The cells were seeded
at 5×104 in 500 μl of serum-free medium in the upper
chamber of the insert and medium containing 10% FBS was added in
the lower chamber. After 22 h, the non-invading cells were removed
with a cotton swab, the invading cells were stained with 1%
toluidine blue and the cells were counted under a microscope.
Patients and treatment
From the 235 consecutive patients who had undergone
curative hepatic resection between 2004 and 2007 in the Department
of Gastroenterological Surgery, Graduate School of Medical Sciences
(Kumamoto University, Kumamoto, Japan), 150 primary HCC samples
were analyzed in this study. None of the patients had received any
preoperative anticancer treatment. The pathological diagnoses and
the clinicopathological factors of patients were established based
on the general rules of primary liver cancer of the Liver Cancer
Study Group of Japan (19,20) and the American Joint Committee on
Cancer (AJCC)/International Union Against Cancer (UICC) staging
system (21). The median duration
of follow-up after surgery was 44 months. This study was approved
by the Human Ethics Review Committee of the Graduate School of
Medicine, Kumamoto University (Kumamoto, Japan).
Immunohistochemistry (IHC) and
scoring
The sample processing and IHC procedures were
performed as previously described (17). Endogenous peroxidase activity was
blocked using 3% hydrogen peroxide. The sections were incubated in
diluted antibodies. A subsequent reaction was performed with a
biotin-free HRP enzyme-labeled polymer from the Envision Plus
detection system (Dako Co., Carpinteria, CA, USA). A positive
reaction was visualized with a 3,3′-diaminobenzidine solution,
followed by counterstaining with Meier’s hematoxylin. The primary
antibody for CD44v6 (1:100 dilution; Bender Medsystems) was used
for the study. IHC staining was independently scored by two
pathologists. For membranous CD44v6, the results were classified
ranging from 0 to 3+: 0, no staining; 1+, 1–25%; 2+, 26–50%; and
3+, >50% staining of the specimen. The 1, 2 and 3+ specimens
were considered to be positive IHC results.
Statistical analysis
The data are presented as the mean ± SD. Independent
Student’s t-tests were used to compare the continuous variables
between the two groups. Categorical variables were compared using
the χ2 test. The overall and disease-free survival were
calculated using the Kaplan-Meier method and compared using the
log-rank test. The statistical analyses were performed as indicated
with a statistical analysis software program (Excel Statistics,
Social Survey Research Information Co., Tokyo, Japan). P<0.05
was considered to indicate a statistically significant result.
Results
The expression of CD44v6 is associated
with the invasive phenotype of HCC cells
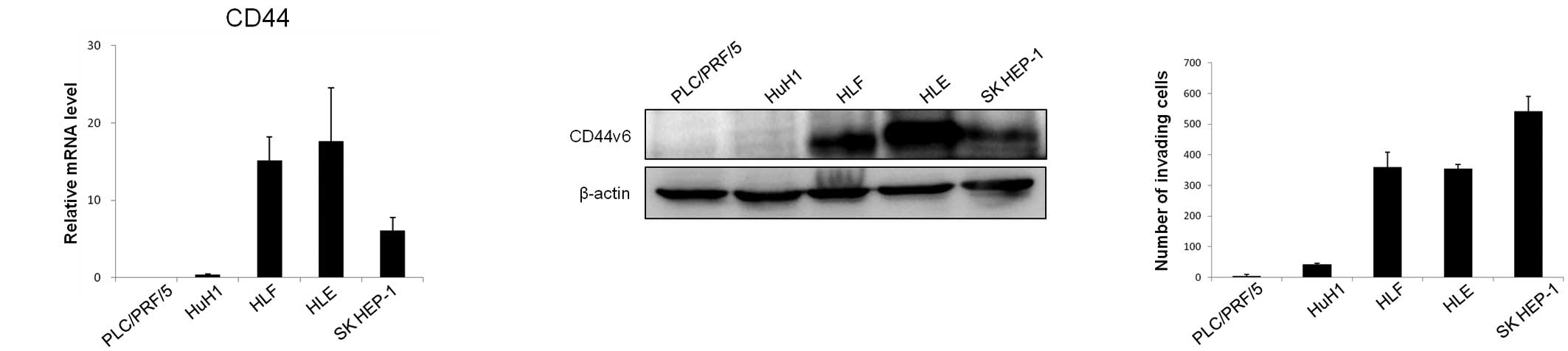
We examined the expression of CD44v6 and its ability
for invasiveness in five HCC cell lines (PLC/PRF/5, HuH1, HLF, HLE
and SK HEP-1). The HLF, HLE and SK HEP-1 cells showed a high
expression of CD44, while the PLC/PRF/5 and HuH1 cells showed a low
expression of CD44 at the mRNA level (Fig. 1A). The mRNA levels in the cells
corresponded to the protein levels, with cells that had high levels
of CD44 mRNA also showing a high expression of the CD44v6 protein
(Fig. 1B). The HLF, HLE and SK
HEP-1 cells showed high invasiveness, whereas the PLC/PRF/5 and
HuH1 cells showed low invasiveness (Fig. 1C). These results suggest that CD44v6
expression is correlated with the invasive phenotype in HCC
cells.
The expression of CD44v6 in
hepatocellular carcinoma
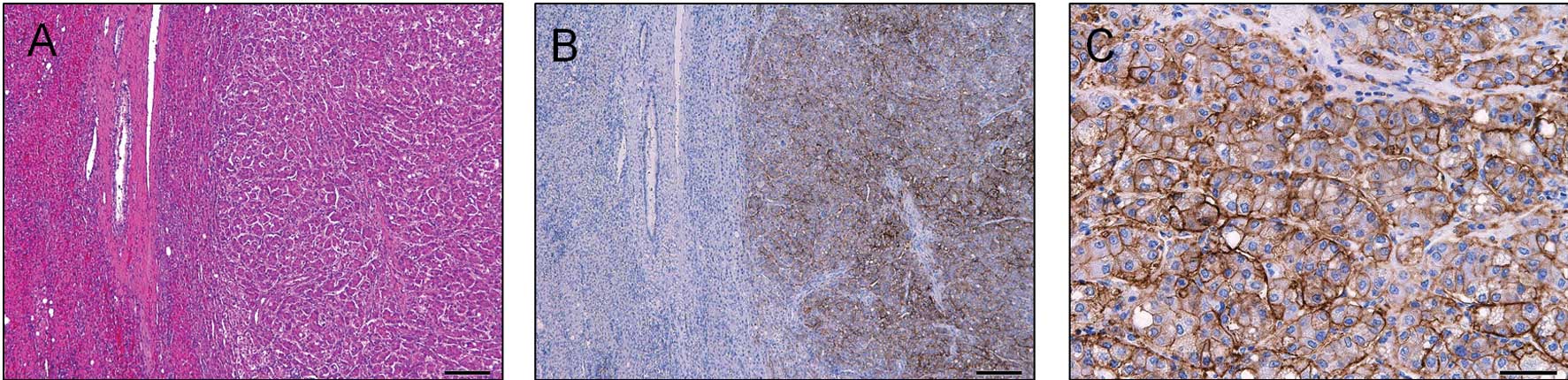
To investigate the clinical significance of the
expression of CD44v6, we analyzed the protein levels of CD44v6 in
the tumor and the adjacent liver tissue by IHC in samples from 150
HCC patients. CD44v6 was mainly expressed in the tumor cell
membrane (Fig. 2). A total of 46
cases (30.7%) were diagnosed as positive for CD44v6 expression
(Table I). CD44v6 was not expressed
in the adjacent liver tissue in any of the patients (Table I).
 | Table IExpression of CD44v6 in the tumors and
adjacent liver sections of 150 HCC patients. |
Table I
Expression of CD44v6 in the tumors and
adjacent liver sections of 150 HCC patients.
| CD44v6 score |
|---|
|
|
|---|
| Tissue | 0 | 1+ | 2+ | 3+ |
|---|
| Tumor | 104 | 32 | 9 | 5 |
| Adjacent liver | 150 | 0 | 0 | 0 |
The clinical significance of CD44v6 in
hepatocellular carcinoma
No significant correlation was found between the
level of CD44v6 expression and the clinicopathological factors
(Table II). However, results
showed a weak correlation between a low level of CD44v6 expression
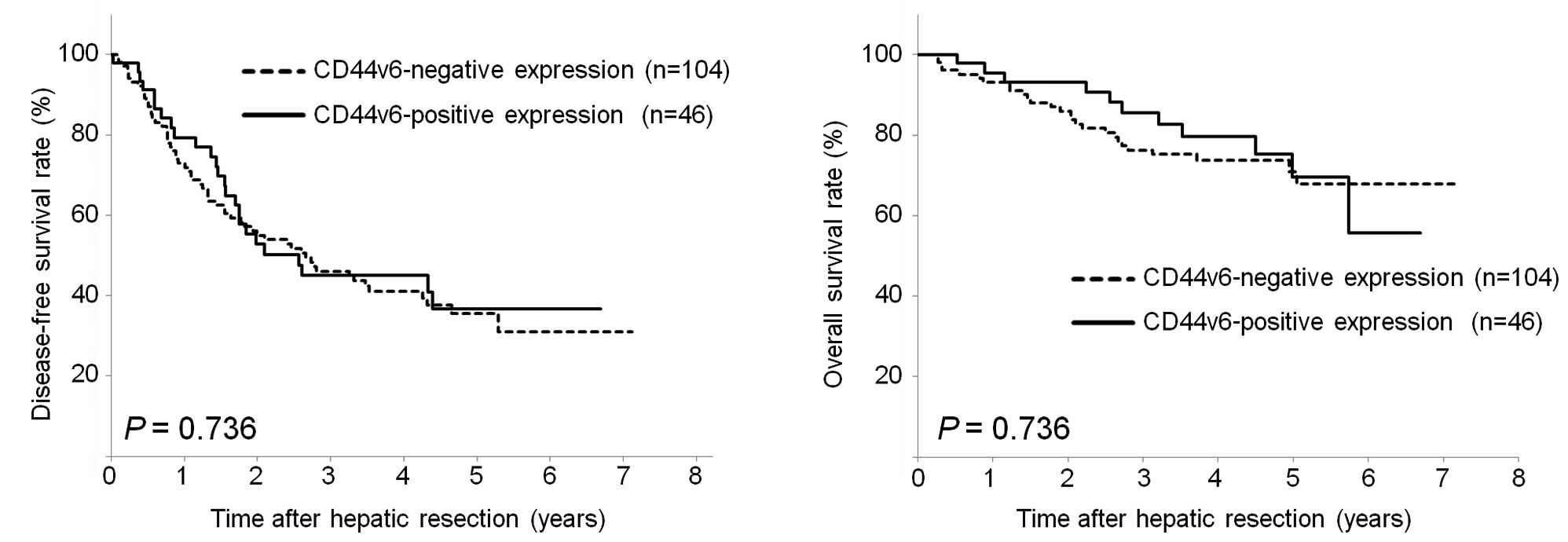
and vascular invasion in HCC patients (P=0.080). Kaplan-Meier
curves revealed that a high CD44v6 expression was not significantly
associated with disease-free survival (P=0.736, Fig. 3A) or overall survival (P=0.736,
Fig. 3B).
 | Table IICorrelation between the expression of
CD44v6 and the clinicopathological factors of 150 HCC patients. |
Table II
Correlation between the expression of
CD44v6 and the clinicopathological factors of 150 HCC patients.
| Clinicopathological
factors | CD44v6 positive
(n=46) | CD44v6 negative
(n=104) | P-value |
|---|
| Age ≤60/>60
(years) | 10/36 | 34/70 | 0.174 |
| Male/female | 38/8 | 85/19 | 0.897 |
| HBs-Ag
negative/positive | 32/14 | 75/29 | 0.750 |
| HCV-Ab
negative/positive | 23/23 | 58/46 | 0.513 |
| Child-Pugh
classification A/B | 41/5 | 94/10 | 0.813 |
| AFP ≤20/>20
(ng/ml) | 24/22 | 52/52 | 0.806 |
| PIVKA-II ≤107/>107
(mAU/ml) | 24/22 | 51/53 | 0.723 |
| Tumor size ≤3/>3
(cm) | 16/30 | 34/70 | 0.802 |
| Tumor number
1/≥2 | 33/13 | 73/31 | 0.848 |
| Tumor encapsulation
absent/present | 5/41 | 12/92 | 0.905 |
| Tumor differentiation
moderate, well/poor | 37/9 | 84/20 | 0.962 |
| LCSGJ TNM stage 1,
2/3, 4 | 27/19 | 62/42 | 0.916 |
| AJCC/UICC TNM stage
1, 2/3, 4 | 40/6 | 82/22 | 0.240 |
| Vascular invasion
absent/present | 45/1 | 93/11 | 0.080 |
Discussion
In the present study, the expression CD44v6 was
found to be correlated with the invasive phenotype of HCC cell
lines. However, the level of CD44v6 expression was not found to be
correlated with the survival or clinicopathological factors of
patients with HCC. Instead, a low expression level of CD44v6 tended
to be associated with vascular invasion.
In a previous study, Endo and Terada reported that a
high CD44v6 expression in patients with HCC was significantly
correlated with the presence of vascular invasion and a poor
prognosis (22), which was not in
agreement with our results. Both the present study and that by Endo
and Terada analyzed the expression of CD44v6 by IHC in samples from
patients with HCC following hepatectomy. The proportion of
CD44v6-positive cases in our study was similar to that in the study
of Endo and Terada (30.6 vs. 34%). However, these authors did not
report the characteristics of the patients. Therefore, the
discrepancy in the results may be due to differences in the
characteristics of the patients included in the studies.
CD44v6 acts as an essential co-receptor for the
activation of Met. In several cell lines, CD44v6 catalyzes the
formation of a complex with Met and its ligand (23). In addition, CD44v6 binding to the
extracellular matrix also activates the PI3K-Akt pathway and
regulates Met transcription. These observations suggest that the
role of CD44v6 in the invasive and malignant phenotype are linked
to its collaboration with receptor tyrosine kinases and c-Met.
However, the CD44s isoform is essential for the response to TGF-β
in the epithelial-mesenchymal transition (EMT) and an increase in
CD44s expression was found to be accompanied by a loss of variant
isoforms in breast cancer (24).
The EMT is a developmental process in which epithelial cells lose
their polarity and acquire the migratory properties of mesenchymal
cells. The EMT has been shown to be a pivotal mechanism in cancer
invasion and metastasis (25,26).
In conclusion, our study suggests that the
expression level of CD44v6 is correlated with the invasiveness of
hepatocellular carcinoma in vitro, but does not appear to be
clinically significant. Instead, a low expression of CD44v6 showed
a tendency to be associated with the vascular invasion of HCC.
Future experiments should investigate the role of the various CD44
isoforms, including the CD44s isoform, in HCC cell lines and in
patients with HCC.
References
|
1
|
Jou J and Diehl AM: Epithelial-mesenchymal
transitions and hepatocarcinogenesis. J Clin Invest. 120:1031–1034.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics. CA Cancer J Clin. 59:225–249. 2009.
|
|
3
|
Poon RT, Ng IO, Fan ST, et al:
Clinicopathologic features of long-term survivors and disease-free
survivors after resection of hepatocellular carcinoma: a study of a
prospective cohort. J Clin Oncol. 19:3037–3044. 2001.PubMed/NCBI
|
|
4
|
Eguchi S, Kanematsu T, Arii S, et al:
Recurrence-free survival more than 10 years after liver resection
for hepatocellular carcinoma. Br J Surg. 98:552–557.
2011.PubMed/NCBI
|
|
5
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villanueva A and Llovet JM: Targeted
therapies for hepatocellular carcinoma. Gastroenterology.
140:1410–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zöller M: CD44: can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011.PubMed/NCBI
|
|
9
|
Günthert U, Hofmann M, Rudy W, et al: A
new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991.
|
|
10
|
Stauder R, Eisterer W, Thaler J and
Günthert U: CD44 variant isoforms in non-Hodgkin’s lymphoma: a new
independent prognostic factor. Blood. 85:2885–2899. 1995.
|
|
11
|
Wielenga VJ, van der Neut R, Offerhaus GJ
and Pals ST: CD44 glycoproteins in colorectal cancer: expression,
function, and prognostic value. Adv Cancer Res. 77:169–187. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kainz C, Kohlberger P, Tempfer C, et al:
Prognostic value of CD44 splice variants in human stage III
cervical cancer. Eur J Cancer. 31A:1706–1709. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Götte M and Yip GW: Heparanase,
hyaluronan, and CD44 in cancers: a breast carcinoma perspective.
Cancer Res. 66:10233–10237. 2006.PubMed/NCBI
|
|
14
|
Shtivelman E and Bishop JM: Expression of
CD44 is repressed in neuroblastoma cells. Mol Cell Biol.
11:5446–5453. 1991.PubMed/NCBI
|
|
15
|
Gao AC, Lou W, Dong JT and Isaacs JT: CD44
is a metastasis suppressor gene for prostatic cancer located on
human chromosome 11p13. Cancer Res. 57:846–849. 1997.PubMed/NCBI
|
|
16
|
Hiyoshi Y, Kamohara H, Karashima R, et al:
MicroRNA-21 regulates the proliferation and invasion in esophageal
squamous cell carcinoma. Clin Cancer Res. 15:1915–1922. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okabe H, Beppu T, Hayashi H, et al:
Hepatic stellate cells accelerate the malignant behavior of
cholangiocarcinoma cells. Ann Surg Oncol. 18:1175–1184. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liver Cancer Study Group of Japan. The
General Rules for the Clinical and Pathological Study of Primary
Liver Cancer. Kanehara; Tokyo: 2009
|
|
20
|
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y
and Makuuchi M: Staging of hepatocellular carcinoma: assessment of
the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772
patients in Japan. Ann Surg. 245:909–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vauthey JN, Lauwers GY, Esnaola NF, et al:
Simplified staging for hepatocellular carcinoma. J Clin Oncol.
20:1527–1536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Endo K and Terada T: Protein expression of
CD44 (standard and variant isoforms) in hepatocellular carcinoma:
relationships with tumor grade, clinicopathologic parameters, p53
expression, and patient survival. J Hepatol. 32:78–84. 2000.
View Article : Google Scholar
|
|
23
|
Orian-Rousseau V, Chen L, Sleeman JP,
Herrlich P and Ponta H: CD44 is required for two consecutive steps
in HGF/c-Met signaling. Genes Dev. 16:3074–3086. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown RL, Reinke LM, Damerow MS, et al:
CD44 splice isoform switching in human and mouse epithelium is
essential for epithelial-mesenchymal transition and breast cancer
progression. J Clin Invest. 121:1064–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|