Introduction
Nasopharyngeal carcinoma (NPC) is one of the most
common types of cancer in South East Asia, and remains a cause of
high morbidity in Southern China (1). In addition to its ability for rapid
growth, NPC has a tendency to invade the adjacent regions and
metastasize to the regional lymph nodes and distant organs. Despite
the sequela of radiation, radiotherapy remains the standard
treatment for NPC (2,3). However, only early stage NPC cases are
able to obtain effective results, including good prognosis and
function. The combination of radiotherapy and chemotherapy is
crucial in the therapy of locoregional advanced cases of NPC
(4). Although over 95% of biopsies
belong to the WHO type II or III classification, which are
sensitive to radiotherapy and chemotherapy, there are many
treatment-failure cases (5). Thus,
there is a requirement for new therapeutic targets and a better
understanding of the mechanisms involved in the metastasis of
NPC.
The Met receptor tyrosine kinase and its ligand,
hepatocyte growth factor (HGF), are overexpressed and/or activated
in a variety of human malignancies. The hepatocyte growth
factor/scatter factor (HGF/SF) and its receptor Met are important
in the development, homeostasis, tumorigenesis, angiogenesis,
invasion and metastasis of human malignancies (6). However, the mechanisms of the
HGF/c-Met signaling pathway that contribute to the invasiveness of
malignancies remain unknown. The Met receptor is frequently
overexpressed in NPC patients, and high expression is associated
with short patient survival (7).
The availability of the Met receptor has been shown to modify NPC
cell response to HGF. This finding enhanced our understanding of
the mechanisms of signaling transduction in the HGF-induced
progression of NPC. However, the role of the HGF/c-Met signaling
pathway in NPC has not been clearly elucidated. In this study, we
detected the effect of HGF/c-Met on proliferation and migration in
several NPC cell lines. Our results suggest the potential
administration in therapeutic combination with antibodies to c-Met
in NPC patients.
Materials and methods
Cell lines and reagents
Five human NPC cell lines (two well-differentiated
NPC cell lines, CNE-1 and HK-1, and three poorly differentiated NPC
cell lines, CNE-2, HONE-1, and SUNE-1) were maintained in RPMI-1640
(Invitrogen Life Technologies, Carlsbad, CA, USA), and were
supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA,
USA), 1 U/ml penicillin G, and 1 mg/ml streptomycin at 37°C and 5%
CO2. The S114 cells (NIH 3T3 cells transformed with
human HGF/SF and Met) (8) and the
SK-LMS-1 human leiomyosarcoma cell line (9) were maintained in DMEM (Gibco)
containing 10% FBS at 37°C and 5% CO2. S-114 cells
stably co-express human Met and HGF, resulting in autoactivation of
the Met receptor. The SK-LMS-1 cell line was derived from a human
leiomyosarcoma (smooth-muscle tumor); these cells express a high
level of the Met receptor, but only a small quantity of its ligand
HGF/SF, and respond mitogenically to exogenous HGF/SF. The SK-LMS-1
cell line was used as the control in the cell proliferation assay,
as it is able to proliferate under exogenous HGF. The HGF/SF was
prepared from S114 cells. Anti-HGF/SF neutralizing antibodies were
generated as previously described (10).
RT-PCR
Total RNA was extracted from cell line pellets using
TRIzol reagent, and reverse transcription was performed using the
SuperScript II RT kit (Invitrogen, Grand Island, NY, USA; no.
10928034) with total RNA (1 μg) according to the manufacturer's
instructions. The HGF mRNA expression levels were detected by
conventional RT-PCR with Taq DNA Polymerase, Recombinant
(Invitrogen, no. 10342-020). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as the internal control.
The specific primers for HGF and GAPDH were designed
by Primeriers software. The PCR reaction was performed according to
the manufacturer's instructions. The primers used were: HGF,
forward: 5′-CTACACTGGATTGATCAACTAT-3′ and reverse:
5′-AGTAGTTGTCTTAGGATTGTTG-3′; and GAPDH, forward:
5′-TTGCCATCAATGACCCCTTCA-3′ and reverse:
5′-CGCCCCACTTGATTTTGGA-3′.
The PCR conditions were as follows: amplification
reaction protocol was performed for 35 cycles consisting of 30 sec
at 94°C (denaturation), annealing 30 sec at 45°C and extension 30
sec at 72°C. The PCR products were separated on a 2% agarose gel,
stained with ethidium bromide and visualized by the Bio-Rad Imaging
System (Hercules, CA, USA).
Western blot analysis
S114, NIH 3T3, CNE-1, CNE-2, HK-1, HONE-1 and SUNE-1
cells were harvested and centrifuged. The whole cell lysates were
prepared by adding 2X SDS sample buffer (125 mmol/l Tris-HCl, pH
6.8, 2% SDS, 20% glycerol, 0.02 mg/ml bromophenol blue and 5%
mercaptoethanol). Equal amounts of protein (30 μg/sample as
determined by UV spectrometry) were electrophoresed on 12% SDS-PAGE
gels and transferred to nitrocellulose membranes. The membranes
were then blocked for 60 min at room temperature with 5% non-fat
dry milk/TBS-Tween-20 and reacted with the appropriate antibodies
for Met (1:500 dilution in blocking buffer) overnight at 4°C with
constant agitation. Following incubation with the primary antibody,
membranes were washed in TBS-Tween-20 and incubated with
horseradish peroxidase-conjugated secondary antibodies (1:5000
dilution in blocking buffer) for 1 h at room temperature. Proteins
were then visualized by incubation with enhanced chemiluminescence
detection reagents (Cell Signaling, No. 7072), followed by exposure
to radiograph film (Kodak, No. 6535876).
Fluorescence-activated cell sorting
(FACS) analysis
The whole cells, including S114, NIH 3T3, CNE-1,
CNE-2, HK-1, HONE-1 and SUNE-1, were blocked using 5% milk blocking
buffer for 30 min at 4°C, incubated with 2 mg/ml anti-hHGF
monoclonal antibody for 60 min at 4°C, and stained using 1:20
diluted FITC-labeled mouse anti-mouse secondary antibody for 15 min
at 4°C. The fluorescence intensity was analyzed with Cellquest
software (Becton Dickinson Bioscience, Franklin Lakes, NJ, USA).
The cells that were incubated with only secondary antibody were
analyzed as the controls.
Cell proliferation assay
For the proliferation assays, Cell Titer 96 AQ
Non-Radioactive Cell Proliferation Assay kits (Promega, Madison,
WI, USA; no. G5421) were used. SK-LMS-1, NIH 3T3, CNE-1, CNE-2,
HK-1, HONE-1 and SUNE-1 cells were plated in 96-well plates with
500 cells per well in triplicate in serum-free medium for 12 h.
Three doses of HGF (0.01, 0.1 and 1 μg/ml) were added 12 h later
while adhered to the bottom of the wells. The cells were incubated
with HGF for 24 h prior to the addition of the MTS reagent. The
plate was then read using a Dynex spectrophotometer. Each
experiment was repeated three times and the average and standard
error of the mean (SEM) were calculated.
Scratch wound closure assay
After being maintained in quiescence in serum-free
culture medium for 24 h, confluent cell monolayers of HONE-1 in
6-well plates were wounded by mechanical scraping with a 200 μl
pipette tip. Following washing to remove cell debris, cultures were
incubated in RPMI-1640 with or without HGF at a concentration of
0.1 μg/ml. Wound width was assessed at the time of scraping to
ensure that all wounds were the same at the start of the
experiment. The wound closure was recorded photographically over
time, using phase-contrast microscopy 24 and 48 h later.
Statistical analysis
The assays were performed in triplicate. Data were
expressed as the mean ± SD. Statistical analyses were performed
using analysis of variance by SPSS 14.0 software. P<0.05 was
considered to indicate a statistically significant difference.
Results
HGF gene expression in different NPC
cells
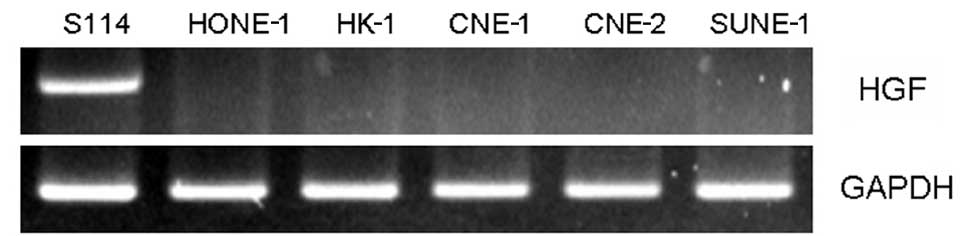
RT-PCR was used to determine HGF gene expression in
several NPC cell lines (CNE-1, CNE-2, HK-1, HONE-1 and SUNE-1).
There was no HGF gene expression in any of the NPC cell lines, with
the exception of the S114 cells, which served as the positive
controls (Fig. 1). The results
suggest that further study is required to determine the expression
of the HGF ligand to investigate the role of HGF in NPC cell
lines.
Met25 protein expression in different NPC
cells
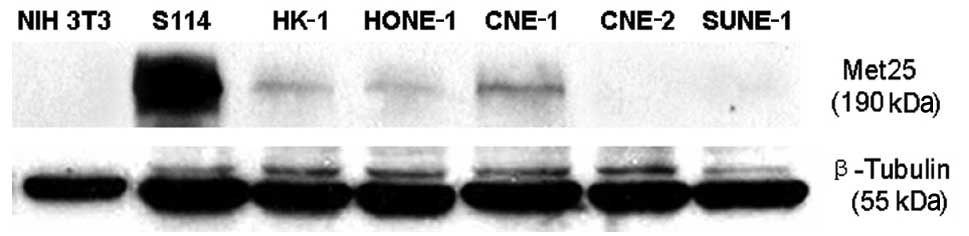
Since the HGF gene was not expressed in the NPC
cells, western blot analysis was used to detect the expression of
the HGF ligand, Met25 protein. A comparison of the S114 cells
(positive control) and NIH 3T3 cells (negative control) resulted in
the detection of Met25 protein expression in the HONE-1, HK-1 and
CNE-1 cells, but not in the CNE-2 and SUNE-1 cells (Fig. 2).
Anti-Met25 binding activity to the cell
surface
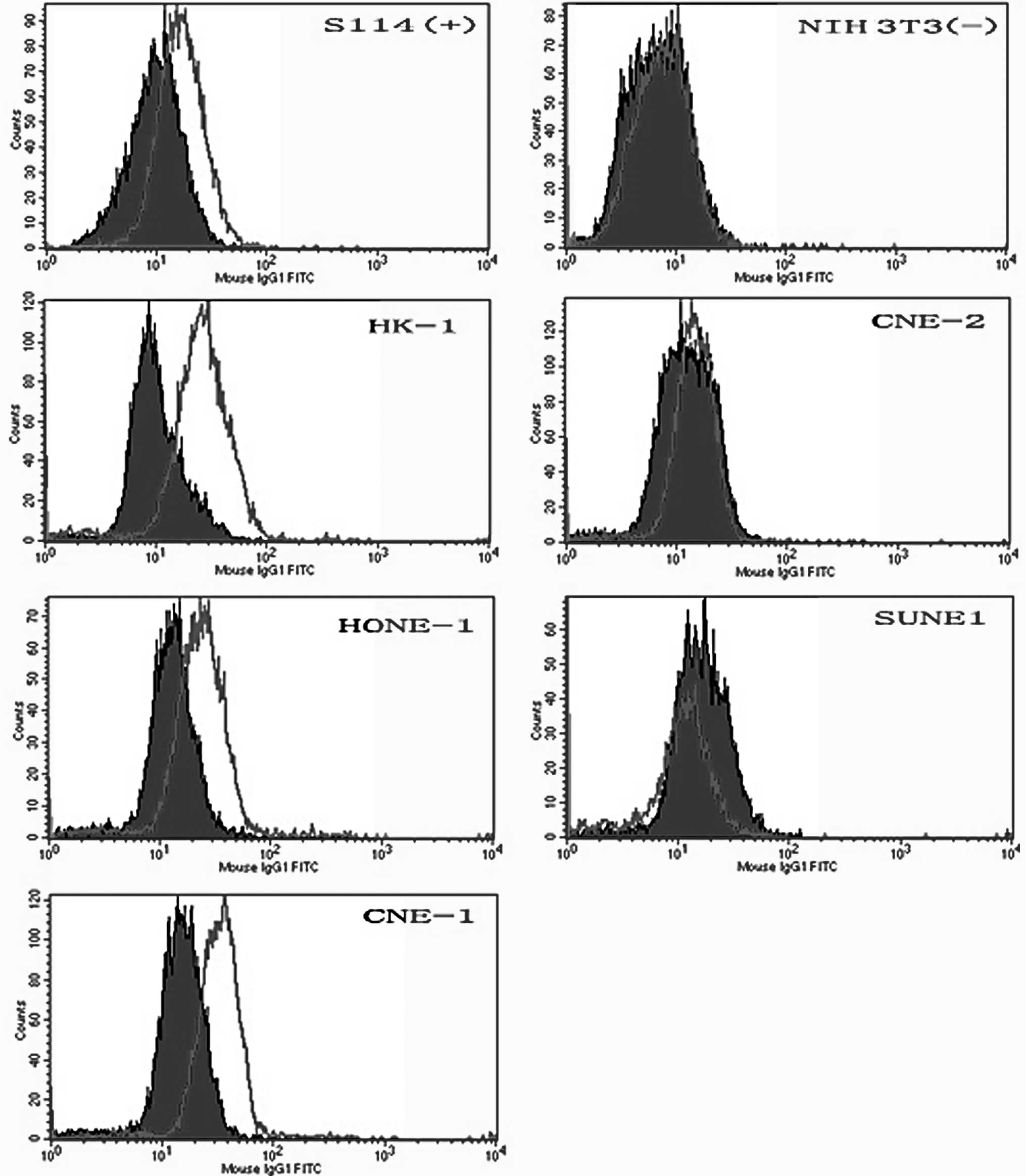
The specific binding activity of anti-Met25 mAb to
the Met protein on viable Met-expressing cells was determined by
FACS analysis. FITC-conjugated anti-Met25 mAb specifically bound
Met-expressing S114, HONE-1, HK-1 and CNE-1 cells (Fig. 3). No binding was observed in the NIH
3T3 cells, which were the negative controls. Similar results were
obtained from the two parts of the experiment by western blot and
FACS analyses.
HGF promotes the proliferation of NPC
cells
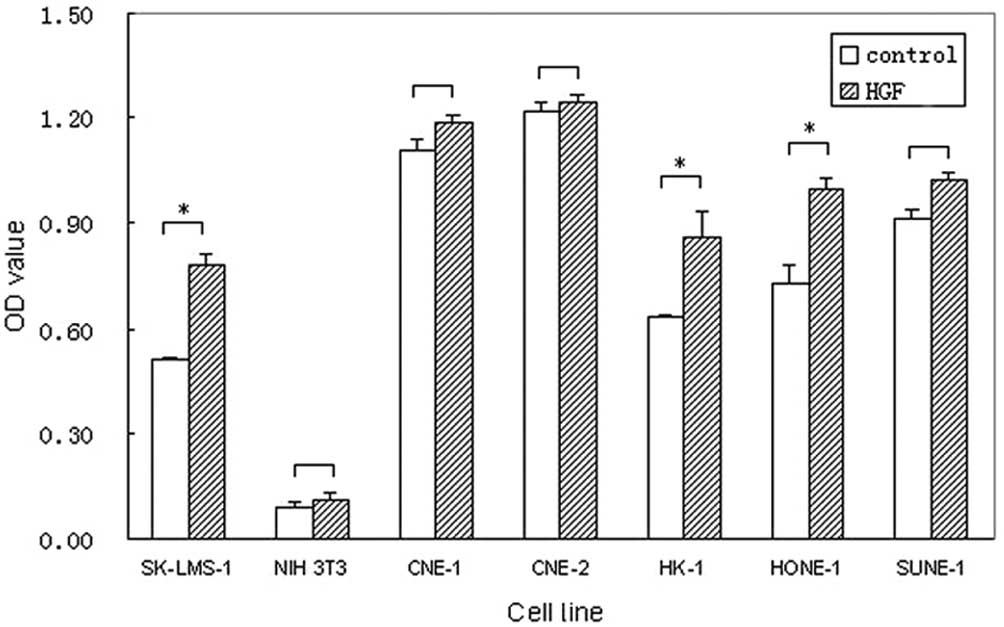
To investigate the role of HGF in NPC cells, HGF was
used as an exogenous factor to determine whether it was capable of
stimulating cell proliferation. Three different concentrations
(0.01, 0.1 and 1 μg/ml) of HGF were used. Concentrations of 0.1 and
1 μg/ml HGF promoted the proliferation of NPC cells, but not the
CNE-1, CNE-2 and SUNE-1 cells. The concentration of 1 μg/ml HGF had
similar effects on the cells compared with 0.1 μg/ml. However, 0.01
μg/ml HGF did not markedly stimulate the proliferation of all NPC
cells. As shown in Fig. 4,
exogenous HGF (0.1 μg/ml) promoted the proliferation in the NPC
cell lines with the exception of CNE-1, CNE-2 and SUNE-1, with
significant effects in HONE-1 and HK-1 (36.5 and 35.5%) (data of 1
and 0.01 μg/ml HGF were not shown).
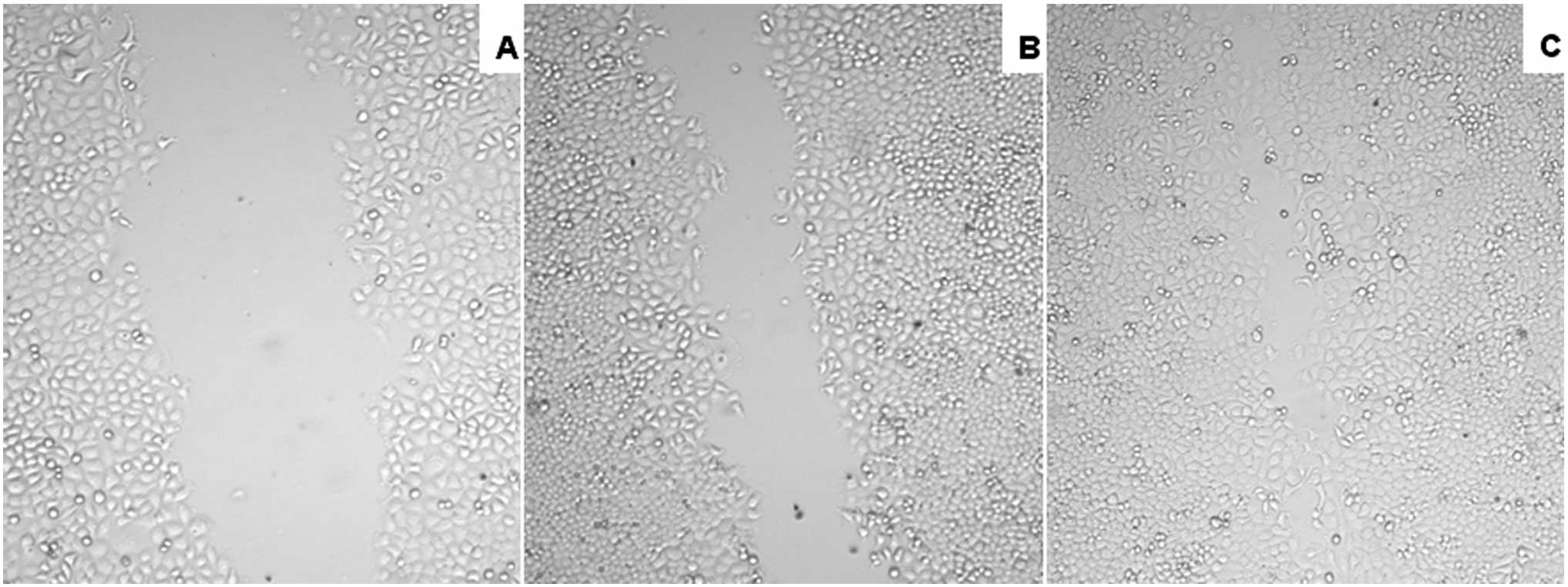
HGF promotes the healing of
scrapewound
The administration of HGF, a specific Met ligand,
significantly promoted wound closure in the experimental NPC cell
lines and had a profound effect on migration, which plays a pivotal
role in wound healing processes. Activation of Met increased
migration in in vitro scrape-wounding assays in HONE-1 cells
(Fig. 5).
Discussion
NPC is the most commonly diagnosed malignancy in
Southern China (1). The combination
of radiotherapy and chemotherapy is not always effective for early
resistant NPC and locoregional advanced cases, therefore other
treatment choices are required to prevent treatment failure,
including cases of distant metastasis. Targeted treatment has
become a new way to treat NPC due to its availability and safety.
The approach of adding epidermal growth factor receptor
(EGFR)-targeted therapy to radiotherapy and chemotherapy is being
actively studied for locoregionally advanced NPC, on the basis of
its overexpression and poor survival outcome (11).
The aim of this study was to determine the role of
HGF in several NPC cell lines, and to obtain evidence to improve
prognosis results and decrease sequela as radiation alone cannot
kill hypoxic cancer cells. The HGF/c-Met signal pathway is related
to the above, as hypoxia was able to activate this pathway and
result in migration and metastasis (12). HGF can lead to the phosphorylation
of two tyrosine residues at the c-terminus of the Met protein upon
binding to Met, which subsequently promotes cancer growth and
metastasis (13,14). Met phosphorylation also induces the
tyrosine phosphorylation of β-catenin, which causes β-catenin
dissociation from E-cadherin (15).
HGF may contribute to cell invasion by modulating
E-cadherin-mediated cell-cell adhesion through the downregulation
and internalization of E-cadherin.
The results of this study have demonstrated that the
selected NPC cells did not express the HGF gene, with S114 cells
serving as the positive control cell line. To investigate the role
of HGF in NPC cell lines, we detected the expression of the HGF
ligand, Met25 protein in the above cells. The results have shown
that only the HK-1, HONE-1 and CNE-1 cells expressed the Met
protein, whereas the CNE-2 and SUNE-1 cells did not express this
protein. These results were validated by similar results obtained
from the FACS analysis where only the HK-1, HONE-1 and CNE-1 cell
lines bound the anti-Met25 mAb, whereas the CNE-2 and SUNE-1 cells
did not exhibit binding activity. These results suggest that,
unlike the CNE-2 and SUNE-1 cells, the HK-1, HONE-1 and CNE-1 cells
may express segments of the Met protein, . This observation
indicates that HGF affects the cells that express the Met protein.
These results provide evidence that HGF may be selected as an
exogenous factor to investigate its role in promoting the
proliferation of NPC cells.
A study by Aune et al revealed that patients
with ovarian carcinomas had higher serum HGF levels than patients
with borderline and benign ovarian tumors (16). HGF in serum is an indicator of
ovarian carcinoma in females with a pelvic mass, and with poor
prognosis in advanced ovarian cancer. Similar results have been
demonstrated in studies evaluating HGF in gastric carcinoma,
colorectal cancer and hepatocellular carcinoma (17–19).
However, there has been no study evaluating HGF in NPC. In this
study, we aimed to determine whether the Met receptor was expressed
in several types of NPC cells despite no expression of the HGF
gene. Results suggested that the Met receptor was activated by its
paracrine ligand, HGF, from the interstitial tissues rather than by
an autocrine loop or its activating mutation.
To determine the functions of the HGF/c-Met pathway
in NPC cells, the effects of exogenous HGF were observed. Results
from proliferation assays revealed that HGF promoted the
proliferation of HK-1 and HONE-1 NPC cells. Additionally, exogenous
HGF was found to significantly increase the proliferation of the
HONE-1 and HK-1 cells (36.5 and 35.5%, respectively, P<0.05). To
validate the effect of HGF, healing of scrape-wounds on HONE-1
cells was determined. As over 95% of biopsies are classified as WHO
type II or III, which are poorly differentiated, the HONE-1 cell
line was selected for the migration experiments. HGF was found to
significantly promote wound closure in HONE-1 cells and had a
profound effect on the migration. The results indicate that Met may
be a good target for treatment, as it is capable of decreasing the
proliferation and migration of NPC cells.
In conclusion, our study has shown that exogenous
HGF promoted the proliferation of NPC cells, and that c-Met may
serve as a ligand for HGF and be an effective target for blocking
the function of HGF. This finding may therefore lead to the
development of new drugs for NPC treatment. Further investigations
are required to understand the mechanisms of the HGF/c-Met pathway
in NPC.
Acknowledgements
The authors thank Rich West for running the
fluorescence-activated cell sorting Calibur cytometer. This study
was supported by the National Natural Science Foundation of China
(81101671 and 30570785).
Abbreviations:
|
HGF
|
hepatocyte growth factor
|
|
NPC
|
nasopharyngeal carcinoma
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
PBS
|
phosphate-buffered saline
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Spano JP, Busson P, Atlan D, Bourhis J,
Pignon JP, Esteban C and Armand JP: Nasopharyngeal carcinoma: an
update. Eur J Cancer. 39:2121–2135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai SZ, Li WF, Chen L, et al: How does
intensity-modulated radiotherapy versus conventional
two-dimensional radiotherapy influence the treatment results in
nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys.
80:661–668. 2011. View Article : Google Scholar
|
|
3
|
Lee AW, Ng WT, Hung WM, et al: Major late
toxicities after conformal radiotherapy for nasopharyngeal
carcinoma-patient- and treatment-related risk factors. Int J Radiat
Oncol Biol Phys. 73:1121–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan AT, Leung SF, Ngan RK, et al: Overall
survival after concurrent cisplatin-radiotherapy compared with
radiotherapy alone in locoregionally advanced nasopharyngeal
carcinoma. J Natl Cancer Inst. 97:536–539. 2005. View Article : Google Scholar
|
|
5
|
Wei KR, Xu Y, Liu J, Zhang WJ and Liang
ZH: Histopathological classification of nasopharyngeal carcinoma.
Asian Pac J Cancer Prev. 12:1141–1147. 2011.PubMed/NCBI
|
|
6
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphoring receptors: cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian CN, Guo X, Cao B, et al: Met protein
expression level correlates with survival in patients with
late-stage nasopharyngeal carcinoma. Cancer Res. 62:589–596.
2002.PubMed/NCBI
|
|
8
|
Rong S, Oskarsson M, Faletto D, et al:
Tumorigenesis induced by coexpression of human hepatocyte growth
factor and the human Met protooncogene leads to high levels of
expression of the ligand and receptor. Cell Growth Differ.
4:563–569. 1993.PubMed/NCBI
|
|
9
|
Jeffers M, Rong S and Vande Woude GF:
Enhanced tumorigenicity and invasion-metastasis by hepatocyte
growth factor/scatter factor-met signaling in human cells
concomitant with induction of the urokinase proteolysis network.
Mol Cell Biol. 16:1115–1125. 1996.
|
|
10
|
Cao B, Su Y, Oskarsson M, et al:
Neutralizing monoclonal antibodies to hepatocyte growth
factor/scatter factor (HGF/SF) display antitumor activity in animal
models. Proc Natl Acad Sci USA. 98:7443–7448. 2001. View Article : Google Scholar
|
|
11
|
Chua DT, Wei WI, Wong MP, Sham JS,
Nicholls J and Au GK: Phase II study of gefitinib for the treatment
of recurrent and metastatic nasopharyngeal carcinoma. Head Neck.
30:863–867. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eder JP, Vande Woude GF, Boerner SA and
LoRusso PM: Novel therapeutic inhibitors of the c-Met signaling
pathway in cancer. Clin Cancer Res. 15:2207–2214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arriola E, Cañadas I, Arumí-Uría M, et al:
MET phosphorylation predicts poor outcome in small cell lung
carcinoma and its inhibition blocks HGF-induced effects in MET
mutant cell lines. Br J Cancer. 105:814–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Syed ZA, Yin W, Hughes K, Gill JN, Shi R
and Clifford JL: HGF/c-met/Stat3 signaling during skin tumor cell
invasion: indications for a positive feedback loop. BMC Cancer.
11:1802011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie LQ, Bian LJ, Li Z, Li Y, Li ZX and Li
B: Altered expression of E-cadherin by hepatocyte growth factor and
effect on the prognosis of nasopharyngeal carcinoma. Ann Surg
Oncol. 17:1927–1936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aune G, Lian AM, Tingulstad S, Torp SH,
Forsmo S, Reseland JE and Stunes AK: Increased circulating
hepatocyte growth factor (HGF): a marker of epithelial ovarian
cancer and an indicator of poor prognosis. Gynecol Oncol.
121:402–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka K, Miki C, Wakuda R, Kobayashi M,
Tonouchi H and Kusunoki M: Circulating level of hepatocyte growth
factor as a useful tumor marker in patients with early-stage
gastric carcinoma. Scand J Gastroenterol. 39:754–760. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toiyama Y, Miki C, Inoue Y, Okugawa Y,
Tanaka K and Kusunoki M: Serum hepatocyte growth factor as a
prognostic marker for stage II or III colorectal cancer patients.
Int J Cancer. 125:1657–1662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizuguchi T, Nagayama M, Meguro M, et al:
Prognostic impact of surgical complications and preoperative serum
hepatocyte growth factor in hepatocellular carcinoma patients after
initial hepatectomy. J Gastrointest Surg. 13:325–333. 2009.
View Article : Google Scholar
|