Introduction
Colorectal cancer (CRC) is one of the most common
malignancies and remains a major cause of cancer mortality in the
West (1). It is also a
multi-pathway disease with disparate subgroups exhibiting distinct
genetic and clinicopathological features, and probably different
outcomes (2). This may be the main
reason for the variability in treatment response observed among
patients of the same disease stage. Therefore, a combination of the
conventional TNM staging classification (at present, the major
prognostic indicator) with certain molecular markers involved in
CRC tumorigenesis, with verified prognostic and predictive impact,
is one of the main objectives of research worldwide (3).
Epidermal growth factor receptor (EGFR) is a
transmembrane glycoprotein member of the tyrosine-kinase receptor
family, encoded by the c-erB1 proto-oncogene and is considered as a
major regulator of several distinct cellular pathways. Activation
of EGFR promotes carcinogenesis, by increasing proliferation, cell
migration, angiogenesis and apoptosis inhibition (4–6). On
this basis, targeted therapies using anti-EGFR antibodies and
tyrosine-kinase inhibitors are now an approved treatment in
metastatic CRC (7,8). However, immunohistochemically assessed
EGFR expression has not been validated as a predictor of response
to this specific treatment. Moreover, the impact of EGFR expression
on the outcome of CRC patients is generally unclear (3). Methodological variability, indicated
by the wide range (18–97%) in the detected frequencies of EGFR in
CRC (9–14), may be responsible for this
effect.
Heterogeneity of CRC (2) should also be taken into account since
EGFR expression may be discordant among primary tumors, lymph nodes
and metastases (10,11). It may also be related to tumor stage
and grade, although the reported results on this issue are
inconsistent (9–14). However, there has been limited
attention regarding the association of EGFR with tumor site,
despite the considerable molecular and clinicopathological
differences between proximal (right-sided) and distal (left-sided)
CRC (15–18), suggesting the existence of two
distinct disease entities with different outcomes and treatment
responses (19,20).
In this study, differences regarding the
immunohistochemically assessed EGFR expression rate were examined
in a series of CRC cases previously investigated for segmental
differences in other molecular markers (18). We analyzed the correlation of EGFR
with stage and grade (i.e., the conventional prognostic indicators)
in the entire cohort and in the proximal and distal tumor subsets.
We also examined the correlation between EGFR and the previously
assessed p53 (18), considering the
central tumorigenic role of the latter marker along with its known
predilection for the distal tumor site (2,15,16,18).
Materials and methods
Study population
Hospital records of 147 unselected cases that
underwent surgery for CRC between 2000 and 2003 in the Second
Surgical Department of Tzaneio Hospital of Piraeus were
retrospectively examined. Following the omission of recurrences,
hereditary cases, synchronous cancers of double location and those
with unclear pathology reports or insufficient tissue for analysis,
119 patients (69 males, 50 females; mean age, 69.3 years; range,
32–90 years) were included in the study, providing a homogenous
sample of primary, sporadic and untreated cases. None of the cases
had undergone neo-adjuvant therapy, as it was not performed during
the selected study period at this hospital. The study was approved
by the Surgical department of the Athens Medical School.
Immunohistochemistry
Sections (5 μm) were obtained from paraffin-embedded
tissue blocks of primary tumor specimens. The immunoperoxidase
method was performed in three steps, using an Envision Dako kit
(Glostrup, Denmark). EGFR was assessed with anti-EGFR mouse
monoclonal antibody (dilution 1:200, Dako). Diaminobenzidine (DAB,
0.6%) was used as a chromogen and tissues were counter-stained with
hematoxylin. Normal epidermis with a known EGFR status served as a
positive control, whereas pre-immune rabbit serum was used as a
negative control.
Staining interpretation
Immunoreactivity was independently evaluated by two
observers (blinded to clinicopathological information) and
discrepancies between them were resolved by consensus. Any lesion
with distinctly visible staining [membranous and/or cytoplasmic
(9,11,12)]
was considered positive.
Multiple cutoffs and the scoring of staining
intensity (a less objective criterion), or complex scoring (i.e.,
combining percentages with intensity) were avoided as they all
potentially increase interobserver variability (21). Moreover, multiple stratification,
used for other markers (including p53) in our previous study
(18), was inappropriate due to the
relatively low proportion of EGFR positivity (see Results). The
selected threshold was similar to that used in previous studies
(1%), revealing strong prognostic and clinicopathological
correlations of EGFR (10,12,13).
Clinicopathological classification
Cases were classified according to the results of
their pathology report as stage I, II, III or IV using the TNM
classification, and Grade 1 (G1, well-differentiated), 2 (G2,
moderately differentiated) or 3 (G3, poorly differentiated) using
the WHO classification. The cases were also classified by site, as
proximal (cecum, ascending, transverse) and distal (descending,
sigmoid, rectum), in relation to the splenic flexure (15–18).
Moreover, considering the small size of certain
subsets and the fact that we aimed to examine the combined effect
of stage and grade on EGFR distribution, we stratified tumors into
three additional categories, modifying the corresponding
classification previously implemented by Resnic et al
(14): i) cases with at least one
indolent feature (stage I or G1), ii) cases with at least one
unfavorable feature (stage IV or G3) and iii) cases with
intermediate tumor characteristics (stages II-III with moderate
grade). Given the absence of tumors with completely conflicting
features (stage I/G3 or IV/G1) in our sample, there was no need for
exclusion of such cases.
Statistical analysis
The distribution of EGFR expression among various
clinicopathological variables was analyzed using the χ2
test (with Yates correction when necessary) and Fisher’s exact test
(appropriate for categorical comparisons between small subsets).
EGFR distribution by stage and grade (or their combination) was
separately examined in the proximal and distal subsets using the
same tests. Moreover, on the basis of the previously recorded data
for p53 (18), the distribution of
the various molecular combinations between EGFR and p53 was
similarly analyzed, particularly focusing on the pattern of tumors
combining EGFR with a high p53 expression level (>60%). Tests
were two-sided, with p values ≤0.05 considered to indicate a
statistically significant difference.
Results
Clinicopathological parameters and
immunohistochemistry
able I shows that moderate grade (86.5%), stage
II-III (79%) and distal tumor location (70%) were the prevailing
features in this sample. Positive EGFR expression was detected in
40 cases (34%), with typical immunostaining shown in Fig. 1. EGFR positivity was almost
uniformly distributed among the various clinicopathological
subsets. Even for tumor grade, the apparently considerable
variation of EGFR was not significant. No association was observed
between EGFR positivity and the previously assessed (18) high p53 staining found in 25% of
cases.
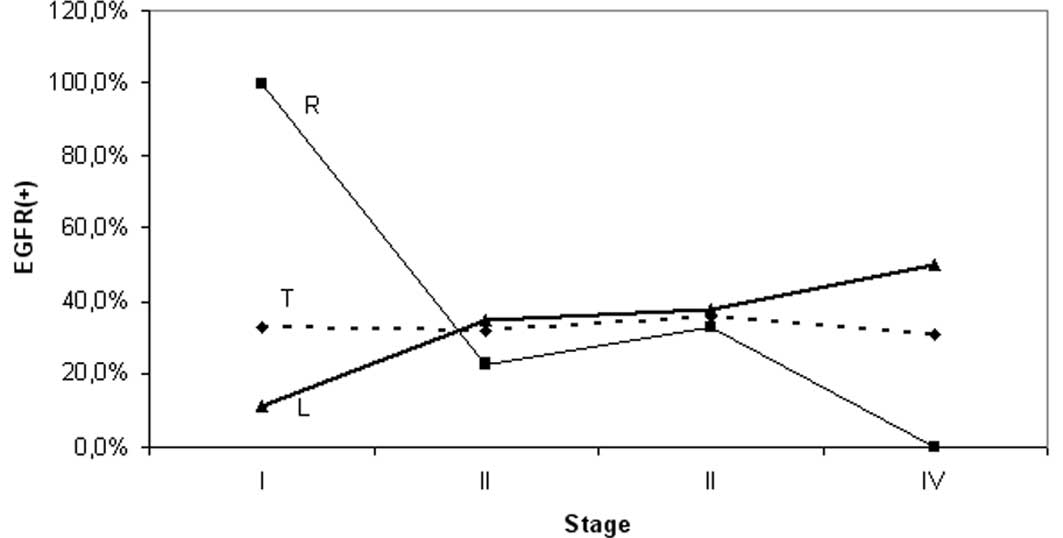
Pattern of staining variation by stage
and grade was different for each segment
EGFR expression was slightly higher in the distal
compared to the proximal site tumors (35 vs. 30.5%), but the
difference was insignificant (Table
I). However, the observed pattern of staining variation by
stage and grade was markedly different for each particular segment
(Table II); the frequency of EGFR
in the proximal subset varied between 100% (stage I) and 0% (stage
IV). Conversely, EGFR frequencies ranging from 11 (stage I) to 50%
(stage IV) were observed distally. Subset analysis revealed: i) a
significant difference of EGFR expression frequencies between stage
I and the other stages (II, III and IV) of the proximal segment
considered together (p=0.023) or separately (p=0.04, 0.07, 0.02,
respectively); and ii) a significant difference of the EGFR
staining frequency between proximal and distal tumors with stage I
disease (p=0.018), whereas the corresponding segmental difference
for stage IV did not reach the level of significance (p=0.1)
(Table II, Fig. 2).
 | Table IClinicopathological and
immunohistochemical features. |
Table I
Clinicopathological and
immunohistochemical features.
| Parameters | Cases | EGFR(+) | P-value |
|---|
|
|
| |
|---|
| n | (%)a | n | (%)b | |
|---|
| TNM stage | | | | | NS |
| I | 12 | (10) | 4 | (33) | |
| II | 50 | (42) | 16 | (32) | |
| III | 44 | (37) | 16 | (36) | |
| IV | 13 | (11) | 4 | (31) | |
| Grade | | | | | NS |
| Well (G1) | 7 | (6) | 4 | (57) | |
| Moderate (G2) | 103 | (86.5) | 35 | (34) | |
| Poor (G3) | 9 | (7.5) | 1 | (11) | |
| Combined
stage-grade | | | | | NS |
| Indolentc | 16 | (13.5) | 6 | (37.5) | |
|
Intermediated | 82 | (69) | 29 | (35) | |
|
Unfavorablee | 21 | (17.5) | 5 | (24) | |
| Tumor site | | | | | NS |
| Proximal
(right) | 36 | (30) | 11 | (30) | |
| Distal (left) | 83 | (70) | 29 | (35) | |
| Gender | | | | | NS |
| Male | 69 | (58) | 25 | (36) | |
| Female | 50 | (42) | 15 | (30) | |
| Age | | | | | NS |
| <70 | 56 | (47) | 19 | (34) | |
| >70 | 63 | (53) | 21 | (33) | |
| p53 staining | | | | | NS |
| Highf | 30 | (25) | 13 | (43) | |
| Lowg | 89 | (75) | 27 | (30) | |
| Total | 119 | (100) | 40 | (34) | |
 | Table IIEGFR segmental distribution by stage
and grade. |
Table II
EGFR segmental distribution by stage
and grade.
| Proximal | Distal | P-valuea |
|---|
|
|
| |
|---|
| n | EGFR(+) (%) | n | EGFR(+) (%) | |
|---|
| Stage |
| I | 3 | 3 (100) | 9 | 1 (11) | 0.018 |
| II | 13 | 3 (23) | 37 | 13 (35) | NS |
| III | 15 | 5 (33) | 29 | 11 (38) | NS |
| IV | 5 | - (0) | 8 | 4 (50) | 0.1 |
| Grade |
| Well (G1) | 4 | 3 (75) | 3 | 1 (33) | NS |
| Moderate (G2) | 25 | 7 (28) | 78 | 28 (36) | NS |
| Poor (G3) | 7 | 1 (14) | 2 | - (0) | NS |
| Combined stage -
grade |
| Indolentb | 5 | 4 (80) | 11 | 2 (18) | 0.035 |
|
Intermediatec | 20 | 6 (30) | 62 | 23 (37) | NS |
|
Unfavorabled | 11 | 1 (9) | 10 | 4 (40) | NS (0.12) |
| Total | 36 | 11 (30.5) | 83 | 29 (35) | NS |
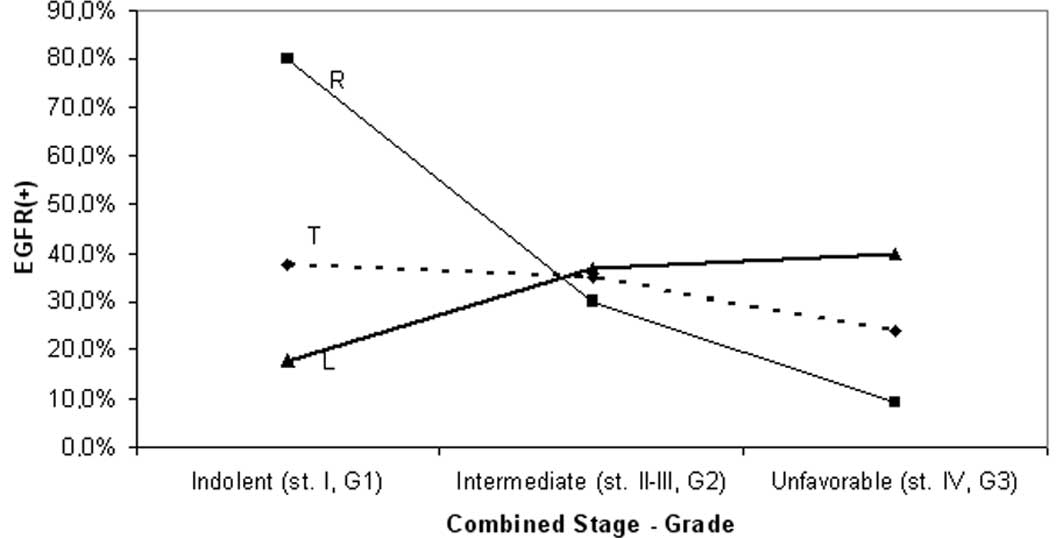
Moreover, a progressive reduction in EGFR frequency
was observed with worsening of grade. This pattern was recorded for
the overall series (from 57% in Grade 1 to 11% in Grade 3) and for
the proximal subset (from 75% in Grade 1 to 14% in Grade 3) but was
somewhat modified in the distal subset (Table II, Fig.
3). However, the observed differences of EGFR staining between
particular grades (of the same segment), or between proximal and
distal tumors of the same grade were not significant, although they
approached the level of significance in certain comparisons within
the proximal subset (G1 vs. G2–G3, p=0.075 and G1 vs. G3,
p=0.09).
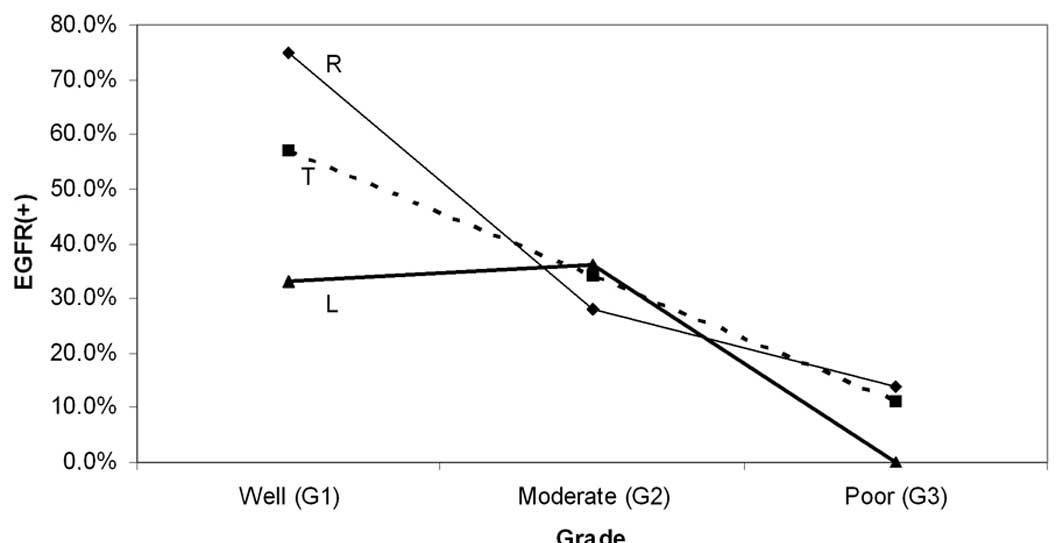 | Figure 3Variation of EGFR with grade. A
progressive reduction of EGFR(+) cases with worsening of tumor
grade is shown for the total sample (T) and the right-sided subset
(R), with the two groups showing similar variation patterns. The
corresponding pattern for left-sided tumors (L) was somewhat
differential. None of the observed differences of EGFR positivity,
between right and left subsets or between particular grades (G1,
G2, G3) of the same category (R, L, T) were significant. However,
the trend for EGFR(+) shown by the well-differentiated tumors
approached significance for the right subset (G1 vs. G2–G3, p=0.075
and G1 vs. G3, p=0.09) and the total sample (G1 vs. G3, p=0.08).
EGFR, epidermal growth factor receptor. |
EGFR expression was examined in three additional
tumor subsets including tumors with indolent (stage I or G1, 16
cases), unfavorable (stage IV or G3, 21 cases) and intermediate
(stage II–III with moderate grade, 82 cases) clinicopathological
features. The results of this analysis were similar to the
previously ascertained findings regarding EGFR stage distribution;
the indolent subset exhibited a significantly higher proportion of
EGFR positivity compared with the other subsets (80 vs. 22%,
p=0.022), although only for proximal cases. Additionally, the
frequency of EGFR(+) was significantly higher proximally than
distally for the indolent cases (80 vs. 37.5%, p=0.035; Table II and Fig. 4).
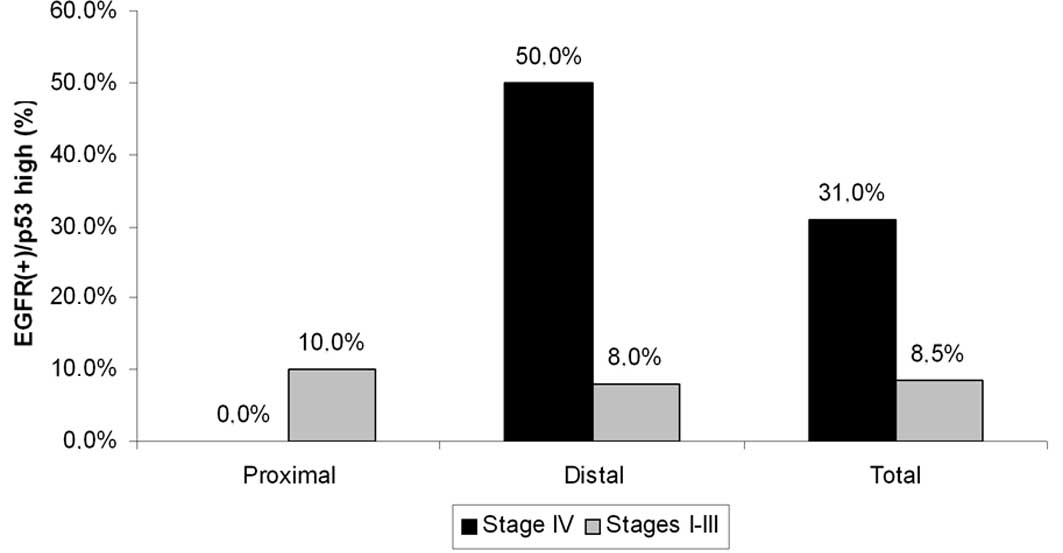
Molecular combinations were significantly
elevated in stage IV
Although the tumor site was found to be unrelated to
any molecular combination of EFGR with p53 (Table III), cases with EGFR(+)/p53 high
immunoreactivity (accounting for 11% of the total sample) were more
frequently detected in stage IV than in other stages (31 vs. 8.5%,
p=0.051). This trend was stronger and significant for distal tumors
(50 vs. 8%, p=0.004) and completely absent for corresponding
proximal tumors (Fig. 5).
 | Table IIIMolecular combinations between EGFR
and p53. |
Table III
Molecular combinations between EGFR
and p53.
| Total | Proximal | Distal | P-valuea |
|---|
|
|
|
| |
|---|
| n | % | n | % | n | % | |
|---|
| Marker |
| EGFR(+) | 40 | (34) | 11 | (30) | 29 | (35) | NS |
| p53 highb | 30 | (25) | 7 | (19.5) | 23 | (28) | NS |
| Marker
combination |
| EGFR(+)/p53
highb | 13 | (11) | 3 | (8) | 10 | (12) | NS |
| EGFR(−)/p53
lowc | 62 | (52) | 21 | (58) | 41 | (50) | NS |
| EGFR(−)/p53
highb | 17 | (14) | 4 | (11) | 13 | (15.5) | NS |
| EGFR(+)/p53
lowc | 27 | (22.5) | 8 | (22) | 19 | (23) | NS |
| Total | 119 | (100) | 36 | (100) | 83 | (100) | |
Discussion
The involvement of EGFR activation in a number of
cellular pathways promoting tumorigenesis may explain the benefit
from the recently implemented anti-EGFR therapies (i.e., cetuximab
or panitumab) (3,7,8).
Nonetheless, the prognostic and predictive value of EGFR status in
CRC remains uncertain (3). However,
the effect of EGFR on prognosis and treatment response may vary
among genetically different tumors; proximal and distal CRC have
been considered to evolve through different genetic pathways
[microsatellite instability/CpG island methylator phenotype
(MSI/CIMP) and chromosomal instability (CIN), respectively]
(15,16) with disparate clinicopathological
features (17–19) and possibly different outcomes
(19,20).
In the current study, we examined the impact of
tumor site on EGFR distribution in particular clinicopathological
variables. The observed variation in EGFR detection rate with
disease progression (from stage I to IV) was found to differ
between proximal and distal tumors, showing a reduction and
elevation of this rate, respectively. Proximal lesions also showed
a similar decrease in the proportion of EGFR positivity with
worsening of grade and, as expected, with the change of the
combination of stage and grade from indolent to unfavorable. By
contrast, for distally located tumors, the same change appeared to
have the opposite effect (elevation).
Notably, these trends were not present in the entire
cohort, with the exception of EGFR variation by grade, consistent
with previous results (11). This
lack of EGFR correlation with stage and grade has been also
reported by other authors (9,14,22),
whereas inconsistent findings are presented among studies
suggesting such connections (10,12,13).
Therefore, a separate investigation of proximal and distal CRC
appears to be necessary for a more accurate determination of the
effect of EGFR status on the progression, aggressiveness and,
probably, the outcome of the disease. In this respect, the fact
that EGFR status has failed to predict response in metastatic cases
that underwent anti-EGFR therapy may be partially explained by the
observed rarity of EGFR positivity in proximal metastatic disease.
Corresponding rarity of EGFR(+) in cases with poor grade may also
be relevant (11); aggressive
lesions are more commonly found in advanced stages, as well as at
the proximal site (23).
Moreover, tumors with the EGFR(+)/p53 high molecular
combination, exhibited a predilection for stage IV, which was
particularly pronounced in the distal subset. This observation may
be explained by the reported connection of p53 inactivation with
both distal site (15,18,24)
and higher stage (24),
particularly stage IV (25).
Nevertheless, the observed trend for metastatic disease (if
validated) could be clinically useful, facilitating the selection
of cases for chemotherapy and/or anti-EGFR therapy, based on
combined EGFR/p53 status and tumor location. Notably, as recently
reported, p53 mutation may predict response to cetuximab treatment
(26), suggesting that p53
inactivation is likely one of the mechanisms leading to EGFR
activation, as indicated by the 90% concordance between p53
mutations and the EGFR copy number increase (26).
Given that, at present, sufficient evidence of
prognostic and predictive significance for any single marker is
lacking, including EGFR and p53 (3,27), the
potential usefulness of marker combinations appears to be a more
promising approach (28,29). In this context, and as regards EGFR,
it has recently been reported that the effectiveness of anti-EGFR
therapy in metastatic CRC is decreased in cases with Ki-Ras
(30) or BRAF and PTEN mutations
(31). The impact of the tumor site
on these findings should also be examined, considering reported
associations of Ki-Ras mutations with metastatic disease and worse
outcome, particularly detected in distal tumors (32,33).
A limitation of our study is that the main findings
were detected in small subsets (stage I, IV - Grade 1, 3). However,
despite the modest size of our sample, our results were similar to
those of several relevant studies (9,10,12–14,22).
Although we confirmed these results in the expanded additional
subsets [created by combining stage and grade: interrelated
features differentially representing tumor growth potential
(34)], further investigation in a
larger sample is necessary. Another limitation is the long-standing
filing of paraffin blocks (7–10 years), which is shown to reduce
EGFR immunoreactivity (35). Such
an effect may explain the decreased EGFR(+) detection rate in our
sample (34%) compared to those seen in other studies with similar
thresholds (12,22,36),
ranging from 50 to 97%. However, Galizia et al (13), using a similar cut-off value, found
almost equal frequencies of EGFR(+) (35%), whereas other authors
(10,37) reported even lower rates (18 and
21.5%, respectively).
However, the simplicity of our methodology in EGFR
staining interpretation minimizes interobserver variability,
facilitates reproducibility and is appropriate for samples of this
size, with an observed EGFR(+) detection rate (34%). However, in
larger samples, or in those with higher detection rates and a wide
range in the observed percentages of positivity, the use of
multiple thresholds or complicated scoring systems may provide
better information (21).
Moreover, our data indicated the importance of
separate segmental analysis in revealing clinicopathological
correlations of EGFR; the uniform distribution of this marker among
stages in the entire cohort was resulted (mostly) from the combined
effect of the opposite trends in EGFR variation with disease
progression recorded for proximal and distal subsets. Similarly,
the counteraction between the indolent and the unfavorable tumor
subsets, exhibiting different segmental predilections of EGFR
positivity (for proximal and distal site, respectively),
contributed to the observed lack of segmental difference in overall
sample, perhaps explaining the reason for such differences having
rarely been reported (38). Even
more detailed analysis may be necessary; in particular, colon
segments (cecum, ascending and sigmoid) have recently been found
showing distinct clinicopathological features (39), possibly reflecting underlying
molecular disparities.
In conclusion, in this exploratory study we found
that the pattern of EGFR variation with disease progression and/or
aggressiveness differed according to tumor location. Although these
results support that proximal and distal CRC are different disease
entities, their potential impact on prognosis and treatment should
be investigated. Additional investigations may include: i)
meta-analyses of selected EGFR immunohistochemical studies with,
preferably, similar methodology; ii) large retrospective
site-specific analyses of EGFR predictiveness in patients receiving
anti-EGFR treatment; and iii) corresponding prospective studies. In
future, therapy decisions may be based on the combined
clinicopathological and molecular tumor status, possibly including
EGFR and tumor site.
Acknowledgements
We thank Drs Th. Vlassis, F. Georgiadis and I.
Elemenoglou for their help and Mrs. N. Vathi for valuable
assistance in the preparation of the manuscript.
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
CRC
|
colorectal cancer
|
|
G1
|
Grade 1
|
|
G2
|
Grade 2
|
|
G3
|
Grade 3
|
|
MSI
|
microsatellite instability
|
|
CIMP
|
CpG island methylator phenotype
|
|
CIN
|
chromosomal instability
|
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics. CA Cancer J Clin. 58:71–96. 2008.
|
|
2
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tejpar S: The use of molecular markers in
the diagnosis and treatment of colorectal cancer. Best Pract Res
Clin Gastroenterol. 21:1071–1087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zwick E, Hackel PO, Prenzel N and Ullrich
A: The EGF receptor as central transducer of heterologous signaling
systems. Trends Pharmacol Sci. 20:408–412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salomon D, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor - related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klapper LN, Kirschbaum MH, Sela M and
Yarden Y: Biochemical and clinical implications of the ErbB/HER
signaling network of growth factor receptors. Adv Cancer Res.
77:25–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tol J and Punt CJ: Monoclonal antibodies
in the treatment of metastatic colon cancer: a review. Clin Ther.
32:437–453. 2010. View Article : Google Scholar
|
|
8
|
Modjtahedi H and Essapen S: Epidermal
growth factor receptor inhibitors in cancer treatment: advances,
challenges, and opportunities. Anticancer Drugs. 20:851–855. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kountourakis P, Pavlakis K, Psyrri A, et
al: Clinicopathologic significance of EGFR and Her-2/neu in
colorectal adenocarcinomas. Cancer J. 12:229–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng Y, Kurland B, Wang J, et al: High
epidermal growth factor receptor expression in metastatic
colorectal cancer lymph nodes may be more prognostic of poor
survival than in primary tumor. Am J Clin Oncol. 32:245–252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McKay JA, Murray LJ, Curran S, et al:
Evaluation of the epidermal growth factor receptor (EGFR) in
colorectal tumours and lymph node metastases. Eur J Cancer.
38:2258–2264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spano JP, Lagorce C, Atlan D, et al:
Impact of EGFR expression on colorectal cancer patient prognosis
and survival. Ann Oncol. 16:102–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galizia G, Lieto E, Ferraraccio F, et al:
Prognostic significance of epidermal growth factor receptor
expression in colon cancer patients undergoing curative surgery.
Ann Surg Oncol. 13:823–835. 2006. View Article : Google Scholar
|
|
14
|
Resnic MB, Routhier J, Konkin T, Sabo E
and Pricolo VE: Epidermal growth factor receptor, c-MET,
beta-catenin, and p53 expression as prognostic indicators in stage
II colon cancer: a tissue microarray study. Clin Cancer Res.
10:3069–3075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iacopetta B: Are there two sites to
colorectal cancer? Int J Cancer. 101:403–408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugai T, Habano W, Jiao YF, Tsukahara M,
Takeda Y, Otsuka K and Nakamura S: Analysis of molecular
alterations in left- and right- sided colorectal carcinomas reveals
distinct pathways of carcinogenesis. J Mol Diagn. 8:193–201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nawa T, Kato J, Kawamoto H, et al:
Differences between right and left-sided colon cancer in patient
characteristics, cancer morphology and histology. J Gastroenderol
Hepatol. 23:418–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papagiorgis PC, Zizi AE, Tseleni S, et al:
Site impact on colorectal cancer biological behavior in terms of
clinicopathological and molecular features. J BUON. 16:84–92.
2011.PubMed/NCBI
|
|
19
|
Menguid R, Slidell MB, Wolfgang L, Chang
DC and Ahuja N: Is there a difference in survival between right-
versus left-sided colon cancers? Ann Surg Oncol. 15:2388–2394.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elsaleh H, Joseph D, Grieu F, Zeps N, Spry
N and Iacopetta B: Association of tumour site and sex with survival
benefit from adjuvant chemotherapy in CRC. Lancet. 355:1745–1750.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zlobec I, Steele R, Michel R, Compton C,
Lugli A and Jass J: Scoring of p53, VEGF, Bcl-2 and APAF-1
immunohistochemistry and interobserver reliability in colorectal
cancer. Mod Pathol. 19:1236–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JC, Wang ST, Chow NH and Yang HB:
Investigation of the prognostic value of coexpressed erbB family
members for the survival of colorectal cancer patients after
curative surgery. Eur J Cancer. 38:1065–1071. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takeuchi K, Kuwano H, Tsuzuki Y, Ando T,
Sekihara M, Hara T and Asao T: Clinicopathological characteristics
of poorly differentiated adenocarcinoma of the colon and rectum.
Hepatogastroenterology. 51:1698–1702. 2004.PubMed/NCBI
|
|
24
|
Russo A, Bazan V, Iacopetta D, Kerr D,
Soussi T and Gebbia N: The TP53 colorectal cancer international
collaborative study on the prognostic and predictive significance
of p53 mutation: influence of tumor site, type of mutation, and
adjuvant treatment. J Clin Oncol. 23:7518–7528. 2005. View Article : Google Scholar
|
|
25
|
Kastrinakis WV, Ramchurren N, Rieger KM,
Hest DT, Loda M, Steel G and Summerhayes IC: Increased incidence of
p53 mutations is associated with hepatic metastasis in colorectal
neoplastic progression. Oncogene. 11:647–652. 1995.PubMed/NCBI
|
|
26
|
Oden-Gangloff A, Di Fiore F, Bibeau F, et
al: TP53 mutations predict disease control in metastatic colorectal
cancer treated with cetuximab-based chemotherapy. Br J Cancer.
100:1330–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chun P and Wainberg A: Adjuvant
chemotherapy for stage II colon cancer: the role of molecular
markers in choosing therapy. Gastrointest Cancer Res. 3:191–196.
2009.PubMed/NCBI
|
|
28
|
O’Connell MJ, Lavery I, Yothers G, et al:
Relationship between tumor gene expression and recurrence in four
independent studies of patients with stage II/III colon cancer
treated with surgery alone or surgery plus adjuvant fluorouracil
plus leucovorin. J Clin Oncol. 28:3937–3944. 2010.PubMed/NCBI
|
|
29
|
Bacolod MD and Barany F: Molecular
profiling of colon tumors: the search for clinically relevant
biomarkers of progression, prognosis, therapeutics, and
predisposition. Ann Surg Oncol. 2011. View Article : Google Scholar
|
|
30
|
Allegra CJ, Jessup JF, Somerfield MR, et
al: American Society of Clinical Oncology provisional clinical
opinion: testing for KRAS gene mutations in patients with
metastatic colorectal carcinoma to predict response to
anti-epidermal growth factor receptor monoclonal antibody therapy.
J Clin Oncol. 27:2091–2096. 2009. View Article : Google Scholar
|
|
31
|
Laurent-Puig P, Cayre A, Manceau G, et al:
Analysis of PTEN, BRAF, and EGFR status in determining benefit from
cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin
Oncol. 27:5924–5930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elnatan J, Coh HS and Smith DR: C-KI-RAS
activation and the biological behaviour of proximal and distal
colonic adenocarcinomas. Eur J Cancer. 32A:491–497. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang MH, Lee IK, Si Y, Lee KS, Woo IS and
Byun JH: Clinical impact of K-ras mutation in colorectal cancer
patients treated with adjuvant FOLFOX. Cancer Chemother Pharmacol.
68:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dukes C and Bussey H: The spread of rectal
cancer and its effect on prognosis. Br J Cancer. 12:309–320. 1958.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Atkins D, Reiffen KA, Tegtmeier CL,
Winther H, Bonato MS and Störkel S: Immunohistochemical detection
of EGFR in paraffin-embedded tumor tissue: variation in staining
intensity due to choice of fixative and storage time of tissue
sections. J Histochem Cytochem. 58:893–901. 2004. View Article : Google Scholar
|
|
36
|
Tampellini M, Longo M, Cappia S, et al:
Co-expression of EGFR receptor, TGFa and S6 kinase is significantly
associated with colorectal carcinomas with distant metastases at
diagnosis. Virchows Arch. 450:321–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rigopoulos DN, Tsiambas E, Lazaris AC, et
al: Deregulation of EGFR/VEGF/HIF-1a signaling pathway in colon
adenocarcinoma based on tissue microarrays analysis. J BUON.
15:107–115. 2010.PubMed/NCBI
|
|
38
|
Messa C, Russo F, Caruso MG and Di Leo A:
EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta
Oncol. 37:285–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benedix F, Schmidt U, Mroczkowski P,
Gastinger I, Lippert H and Kube R: Colon carcinoma - classification
into right and left sided cancer or according to colonic subsite? -
Analysis of 29,568 patients. Eur J Surg Oncol. 37:134–139. 2011.
View Article : Google Scholar : PubMed/NCBI
|