Introduction
The incidence of esophageal adenocarcinoma (EAC) and
Barrett’s esophagus (BE) has been on the rise (1) and overall survival for EAC,
particularly when it is diagnosed at an advanced stage, is poor
(2). Although esophagectomy remains
the mainstay of curative treatment, it is reported to be associated
with a high mortality rate of 8–20% depending on the hospital
volume (3). Lymph node metastasis
is common even in the early stages of EAC since the esophagus
characteristically receives lymphatic supply networks.
Additionally, a subset of patients who have muscularis mucosae
invasion have the potential for lymph node metastasis, which
eventually leads to advanced EAC (4). This evidence provides a rationale for
rigorous surveillance and the complete removal of early EAC in
BE.
Epithelial malignancy, or carcinoma, comprises
cohesive epithelial cells linked to one another by E-cadherin-based
cell-cell junctions and it is initially separated from the stroma
by the basement membrane. Local invasion through the epithelial
basement membrane is the first stage of metastasis, which leads to
intravasation into lymph vessels. To gain access to the stromal
layer, epithelial cells have to undergo a weakening of cell-cell
adhesion and a series of transfers among different cell
compartments. Mounting evidence points to a crucial role for
epithelial mesenchymal transition (EMT) in tumor progression. EMT
involves the disruption of the intercellular junctions causing
dissociation from surrounding cells and the acquisition of a
migratory mesenchymal-like characteristic, enabling epithelial
cells to migrate away from the original tissue and to invade into
stromal components (5). The loss of
E-cadherin expression has been identified in gastrointestinal
cancers and is reported to be associated with cancer progression
and poor prognosis (6–8). The E-cadherin promoter is frequently
repressed by specific transcriptional repressors including
Snail, Slug and Twist, and some of these
repressors are specifically recognized at the invasive front of
human cancers (9).
A direct link between EMT and cancer stem cells
(CSC) was recently reported. CSCs refer to a subset of tumor cells
that have the ability to self-renew and continually sustain
tumorigenesis. Cells undergoing EMT may be the precursors to
metastatic cancer cells, possibly even metastatic CSCs (10). CD133, originally identified as a
cell surface marker of primitive hematopoietic stem cells, is a
marker of tumor-initiating cells in a number of human cancers
(11–13).
We hypothesized that E-cadherin transcriptional
factors could also be expressed at the invading edge of early EAC.
If these factors were expressed, the evidence would add legitimacy
to the complete removal of early EAC. At present, no studies have
assessed the expression of transcriptional factors in specimens of
EAC. The aim of this study was to investigate the expression of
Snail, Slug, Twist and CD133 in tissue samples from early EAC
patients, and assess whether the invading edges of the tumor in the
submucosa were stained for each antibody to transcriptional factor
proteins.
Materials and methods
Patient tissue
Patients who were referred for esophagectomy
following endoscopic surveillance during the period 1997 to 2007
were identified. The patients were identified as having mucosal
cancer by endoscopic ultrasonography and were assessed by a
multidisciplinary team, including experienced thoracic surgeons.
The patients did not receive any chemotherapy or radiotherapy as
adjuvant therapy prior to or following surgery. The Institutional
Review Board approved all surgical specimens used for this study.
Ten surgically treated early EAC patients including seven
non-metastatic patients (p-T1N0M0, n=7; p-T1N1M0, n=2; p-T2N1M0,
n=1) were analyzed in this study (TNM definitions: T1, tumor
invades lamina propria or submucosa; T2, tumor invades muscularis
propria; N1, regional lymph node metastasis). Slides with specimens
were formalin-fixed and paraffin-embedded.
Pathology assessment
The pathology assessment was performed according to
our protocol as previously published (14). The diagnosis of EAC was confirmed by
at least two experienced gastrointestinal pathologists. In this
study, all cases were reviewed again by a single study pathologist
(TTW) with expertise in BE-associated neoplasia.
Immunohistochemistry
Immunohistochemistry for Snail, Slug, Twist and
CD133 was performed as follows: Slides were first deparaffinized
and rehydrated with xylene and ethanol, followed by a water rinse.
Antigen retrieval was then performed with sodium citrate buffer (10
mM sodium citrate, 0.05% Tween-20, pH 6.0). Selected blocks were
sliced into four sections for immunohistochemical analysis. Three
sections were incubated in a primary antibody to Snail, Slug or
CD133 (rabbit polyclonal antibody, Abcam, MA, USA) overnight at
4°C, then washed with Tris-buffered saline (TBS). Enzyme-conjugated
secondary antibody was applied to the slides in TBS with 1% bovine
serum albumin (BSA), and incubated for 1 h at room temperature. The
slides were then rinsed three times with TBS. The other section was
washed and incubated for 1–2 h with primary antibody to Twist
(Twist2 antibody, mouse monoclonal, Novus Biological, Littleton,
CO, USA) in 1% BSA-phosphate-buffered saline (PBS) at room
temperature, washed with TBS and applied with secondary antibody to
the slide in 1% BSA-PBS. The slide was also rinsed three times with
PBS.
Methods
The antibody distribution was visualized by a
polarized light microscopy (Axio Scope.A1, Carl Zeiss, USA) and
assessed whether the invading edges of the tumor in the submucosa
were stained for each antibody. We also evaluated whether there
were differences in the intensity of staining between cancer cells
in the mucosa and invasive cancer cells in the submucosa. The
slides were scored by a method described in a previous study
(15) for i) intensity of staining
(0, negative; 1, weak; 2, moderate and 3, intense), ii) percentage
of epithelial cells staining (0, 0–5%; 1, 6–25%; 2, 26–50%; 3,
51–75% and 4, 76–100%) and iii) percentage of invasive cancer cells
staining (same as epithelial cells). Cell localization (nucleus,
cytoplasm or cell surface) and uniformity (focal or general) were
also assessed.
Results
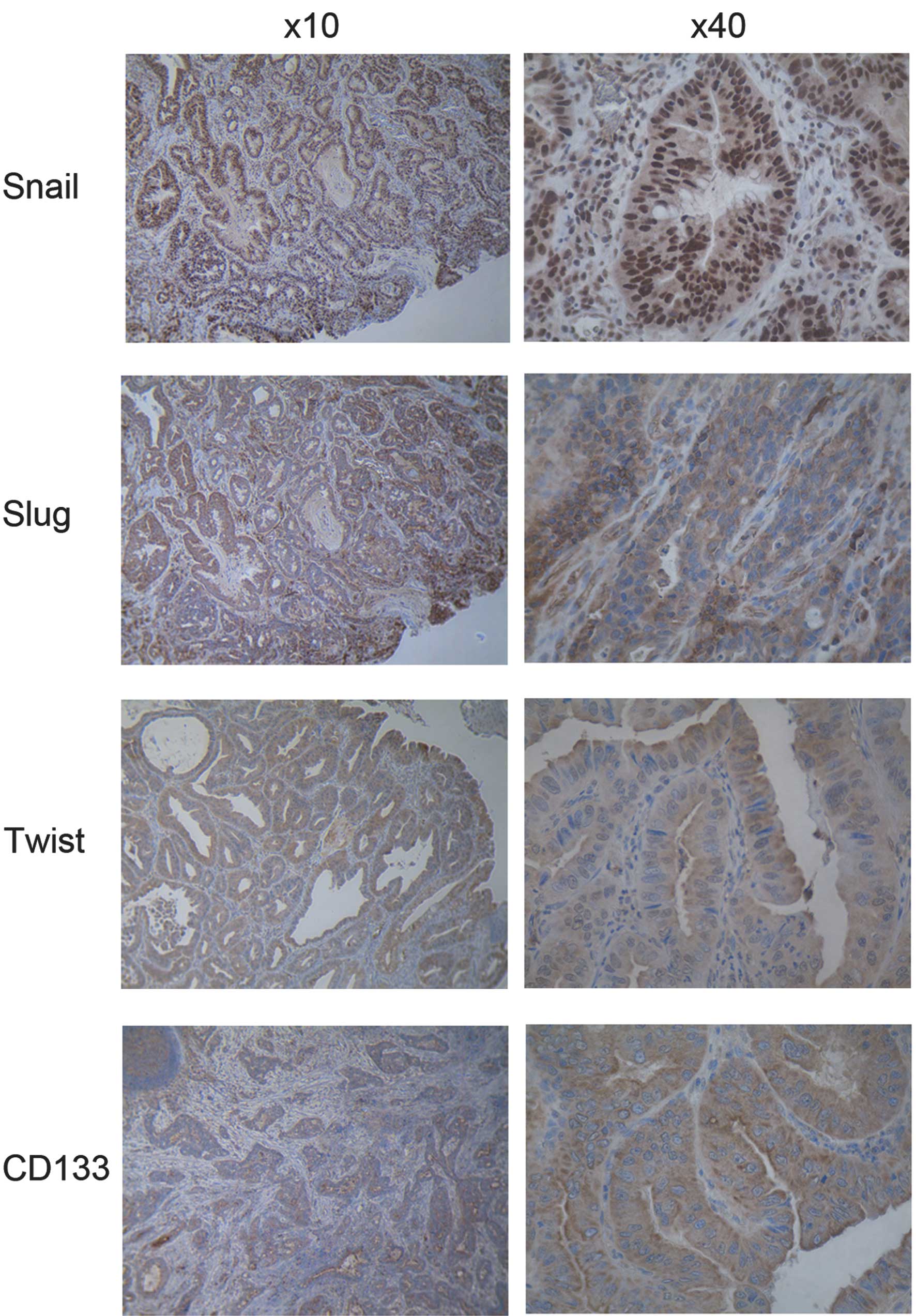
The four proteins were expressed in cancer cells
with uniform staining in both the mucosa and submucosa with Snail
being more localized in the nucleus, whereas Slug, Twist and CD133
were detected exclusively in the cytoplasm in all of the EAC
specimens (Fig. 1). The intensity
of staining of metastatic cancer cells in the submucosa was similar
to that in the mucosa. Semi-quantitative scored analyses of Snail,
Slug, Twist and CD133 for overall intensity were 2.5, 2.8, 1.9 and
2.4, respectively. For cancer cells in the mucosa, the scores were
4.0, 3.8, 3.3 and 3.2, respectively, whereas for invading cells in
the submucosa the scores were 4.0, 3.6, 3.1 and 3.6, respectively.
The intensity of staining and the semi-quantitative score were
similar between the non-metastatic and metastatic patients
(Table I).
 | Table ISemi-quantitative scored analyses of
percentage of stained cells. |
Table I
Semi-quantitative scored analyses of
percentage of stained cells.
| Snail | Slug | Twist | CD133 |
|---|
| Cancer cells above
muscularis mucosa | 4.0 | 3.8 | 3.3 | 3.2 |
| Invading cells below
muscularis mucosa | 4.0 | 3.6 | 3.1 | 3.6 |
| Non-metastatic
patients | 4.0 | 3.8 | 3.1 | 3.5 |
| Metastatic
patients | 4.0 | 3.5 | 3.2 | 3.2 |
Discussion
Tumor metastasis is essential in predicting the
prognosis of cancer patients and is responsible for more than 90%
of all cancer mortality (16). EAC
is well known for its high incidence of lymphatic dissemination
even in the early stages of the disease. Moreover, almost 30% of
T1b tumors have positive lymph node metastasis (17). The lamina propria in the esophagus
contains lymphatic and blood vessels unlike the rest of the
gastrointestinal tract. This is clinically relevant as one of the
most important prognostic factors for EAC is the depth of invasion
and the presence or absence of lymphovascular invasion (18). A previous report from our group
revealed that the presence of metastatic lymph nodes was
significantly associated with poor survival (19), and another study has shown the
5-year survival rate of patients with lymph node metastasis to be
only 33% (20). EMT is a critical
process during the early stage of metastasis, where cancer cells
lose their epithelial characteristics and convert to a motile
mesenchymal phenotype. Numerous cancer cells were demonstrated to
undergo EMT during the progression towards metastatic competence.
The loss of E-cadherin expression has been considered to increase
tumor metastasis in gastrointestinal cancers including esophageal
and gastric cancers, colorectal and hepatocellular carcinomas and
pancreatic cancers (21–24). The major proteins involved in the
transcriptional repression of E-cadherin are the zinc finger
proteins Snail and Slug, and a basic helix-loop-helix protein
Twist. Snail and Slug were shown to be capable of binding directly
to E-box motifs on target gene promoters to downregulate E-cadherin
expression, while Snail has been proposed to be a strong repressor
of the transcription of the E-cadherin gene (25,26).
An increased expression of Twist has also been found to correlate
with tumor invasion and metastasis (27), although the mechanism by which Twist
downregulates E-cadherin expression remains to be determined. It
appears that Twist is able to bind to E-box sites as a homo-dimer,
but it is thought to function as a transcriptional activator in
this context (28).
Following elucidation of the role of these
transcriptional factors in cell culture, the expression of
transcriptional factors in surgical specimens from advanced
esophageal cancers was assessed. Natsugoe et al evaluated
the correlation of Snail expression with the prognosis of 194
advanced esophageal squamous cell carcinoma (SCC) patients who
underwent esophagectomy with lymph node dissection (29). In that study, tumors positive for
Snail expression invaded deeper (P=0.0385), had more distant lymph
node metastases (P=0.0051) and had more advanced stages (P=0.0044)
than those that were negative for Snail expression. Patients with a
reduced E-cadherin expression or positive Snail expression had poor
clinical outcomes. In the preserved E-cadherin group, the overall
survival rate was better in patients with a negative Snail
expression than in those with a positive Snail expression
(P=0.035). A comparison of the proportion of Snail expression in
E-cadherin-preserved tumor specimens and E-cadherin-reduced
specimens showed no significant difference between the two groups
(P=0.096).
The result may be explained by Snail playing a key
role in EMT transition, however, Snail was not the only trigger for
initiating EMT. Yuen et al studied the Twist levels in an
esophagectomy specimen of SCC and examined the correlation with the
occurrence of distant metastasis (30). These authors demonstrated that a
high level of Twist expression was significantly associated with a
greater risk of developing distant metastasis within one year of
esophagectomy (OR, 3.46; 95% CI 1.20–9.98; P=0.022). They also
reported that the Twist protein level was significantly increased
in esophageal SCC specimens compared with non-neoplastic esophageal
epithelium (P<0.001) and that the staining patterns observed in
SCC specimens demonstrated a diffuse cytoplasmic pattern. In their
study, Jethwa et al demonstrated that Snail and Slug were
significantly overexpressed (P<0.05) in surgically treated EAC
specimens compared to non-neoplastic BE specimens, and only Slug
was significantly overexpressed (P<0.05) in EAC specimens
compared to metaplastic BE specimens (15). This is the only study assessing the
expression profile of the transcriptional factors in EAC specimens,
and the result is comparable to those studies in advanced SCC.
Although it appears that Snail, Slug and Twist have a role in EMT
transition, the timeframe during which each transcriptional factor
is involved in the transition has not yet been elucidated. As
Jethwa et al did not identify any definite pathological
stages of the specimens and used metaplastic BE specimens as a
comparison to surgically treated EAC specimens, the results
indicated that Slug may be involved in the late metastatic process.
However, whether or not only Slug is associated with the initiation
of the metastatic process in EAC remains to be determined.
Our hypothesis was that the expression of Snail,
Slug and Twist in BE-related EAC was variable and might be
dependent on the progression status. No studies have assessed the
expression of transcriptional factors in early esophageal cancers.
We selected surgically treated specimens from patients with early
EAC who were initially considered for curative endoscopic therapy
based on the endoscopic findings. Even in our early EAC cohort
(p-T1N0M0, n=7; P-T1N1M0, n=2; p-T2N1M0, n=1), Snail, Slug and
Twist were all abundantly expressed in the mucosa with uniform
staining. Unlike the previous studies of advanced staged esophageal
cancers which demonstrated the overexpression of each specific
transcriptional protein, our results indicated that early stage
cancers predominantly comprise cells with metastatic potential.
The expression of CD133, the most representative
marker of CSCs (11), was
evalutated in the same cohort. Metastasis and tumor dormancy
characterize numerous solid tumors and the CSC model has been
proposed to account for inherent differences in tumor-regenerating
capacity (10,31,32).
Hermann et al have found that CD133 was exclusively
expressed in the tissue samples derived from pancreatic cancer
patients (33). In the invasive
front of pancreatic tumors, a distinct subpopulation of
CD133-positive CSCs was identified and it determined the metastatic
phenotype of the tumor. The EMT transition that enables potentially
invasive cancer cells to disseminate from a primary tumor has also
been thought to promote their self-renewal (34). Mani et al identified a direct
link between EMT and the gain of CSC properties (35). In their study, the induction of EMT
in human mammary epithelial cells resulted in the expression of
stem cell markers, and stem-like cells isolated from mouse or human
mammary carcinomas expressed EMT markers as well. The present study
is in concordance with these previous findings as we found abundant
expressions of CD133 and the transcriptional factor proteins,
Snail, Slug and Twist in the invading front of early, but
potentially metastatic, EAC specimens. Our results emphasize the
need to completely remove these early cancers.
Our findings that the markers were abundantly
expressed not only partially in the actual invasive front but also
in a uniform manner in the entire cancer tissue may contradict the
basic theory that a subset of cancer cells within a tumor has the
ability to self-renew and differentiate. This observation could be
explained by a number of factors. Firstly, a higher percentage of
CSCs in the fraction of cells may be identified by the antibody
profile as compared with those cells that do not fit the respective
profile. This may indicate that not every cell within the published
profiles has CSC characteristics. There may be markers in each
respective system that have yet to be identified that may help
define each cancer stem cell population more precisely. Secondly,
an investigation into CSCs with potential for clonal evolution in
the late tumor progression stage, where more aggressive CSCs become
dominant in the cancer specimen, should be conducted. Finally, it
is possible that, unlike late stage cancers, early stage cancers
predominantly comprise cells with metastatic potential.
In conclusion, we presented the first study to
characterize the transcriptional factors for E-cadherin, Snail,
Slug, Twist and the representative cancer cell marker, CD133 in the
specimens of early EAC in BE. The invading edges of the tumor were
found to abundantly express Snail, Slug, Twist and CD133,
suggesting that early stage cancers predominantly constitute cells
with metastatic potential.
References
|
1
|
Pohl H and Welch HG: The role of
overdiagnosis and reclassification in the marked increase of
esophageal adenocarcinoma incidence. J Natl Cancer Inst.
19:142–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Surveillance Epidemiology and End Results.
http://seer.cancer.gov/csr/1975_2008/index.html
Updated November 2011.
|
|
3
|
Birkmeyer JD, Siewers AE, Finlayson EV, et
al: Hospital volume and surgical mortality in the United States. N
Engl J Med. 11:1128–1137. 2002.
|
|
4
|
Liu L, Hofstetter W, Rashid A, et al:
Significance of the depth of tumor invasion and lymph node
metastasis in superficially invasive (T1) esophageal
adenocarcinoma. Am J Surg Pathol. 29:1079–1085. 2005.PubMed/NCBI
|
|
5
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spaderna S, Schmalhofer O, Hlubek F, et
al: A transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Usami Y, Satake S, Nakayama F, et al:
Snail-associated epithelial-mesenchymal transition promotes
oesophageal squamous cell carcinoma motility and progression. J
Pathol. 215:330–339. 2008. View Article : Google Scholar
|
|
9
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumors: accumulating evidence and unsolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizrak D, Brittan M and Alison MR: CD133:
molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin S, Li J, Hu C, et al: CD133 positive
hepatocellular carcinoma cells possess high capacity for
tumorigenicity. Int J Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad GA, Wu TT, Wigle DA, et al:
Endoscopic and surgical treatment of mucosal (T1a) esophageal
adenocarcinoma in Barrett’s esophagus. Gastroenterology.
137:815–823. 2009.
|
|
15
|
Jethwa P, Naqvi M, Hardy RG, et al:
Overexpression of Slug is associated with malignant progression of
esophageal adenocarcinoma. World J Gastroenterol. 14:1044–1052.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
17
|
Lagarde SM, ten Kate FJ, Reitsma JB, et
al: Prognostic factors in adenocarcinoma of the esophagus or
gastroesophageal junction. J Clin Oncol. 24:4347–4355. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hahn HP, Shahsafaei A and Odze RD:
Vascular and lymphatic properties of the superficial and deep
lamina propria in Barrett esophagus. Am J Surg Pathol.
32:1454–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badreddine RJ, Prasad GA, Lewis JT, et al:
Depth of submucosal invasion does not predict lymph node metastasis
and survival of patients with esophageal carcinoma. Clin
Gastroenterol Hepatol. 8:248–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Westerterp M, Koppert LB, Buskens CJ, et
al: Outcome of surgical treatment for early adenocarcinoma of the
esophagus or gastro-esophageal junction. Virchows Arch.
446:497–504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Washington K, Chiappori A, Hamilton K, et
al: Expression of beta-catenin, alpha-catenin, and E-cadherin in
Barrett’s esophagus and esophageal adenocarcinomas. Mod Pathol.
11:805–813. 1998.
|
|
22
|
Oda T, Kanai Y, Oyama T, et al: E-cadherin
gene mutations in human gastric carcinoma cell lines. Proc Natl
Acad Sci USA. 91:1858–1862. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang MH, Chen CL, Chau GY, et al:
Comprehensive analysis of the independent effect of Twist and Snail
in promoting metastasis of hepatocellular carcinoma. Hepatology.
50:1464–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lowy AM, Knight J and Groden J:
Restoration of E-cadherin/beta-catenin expression in pancreatic
cancer cells inhibits growth by induction of apoptosis. Surgery.
132:141–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cano A, Pérez-Moreno MA, Rodrigo I, et al:
The transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Batlle E, Sancho E, Francí C, et al: The
transcription factor Snail is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vernon AE and LaBonne C: Tumor metastasis:
a new twist on epithelial - mesenchymal transitions. Curr Biol.
14:719–721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castanon I and Baylies MK: A Twist in
fate: evolutionary comparison of Twist structure and function.
Gene. 287:11–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Natsugoe S, Uchikado Y, Okumura H, et al:
Snail plays a key role in E-cadherin-preserved esophageal squamous
cell carcinoma. Oncol Rep. 17:517–523. 2007.PubMed/NCBI
|
|
30
|
Yuen HF, Chan YP, Wong ML, et al:
Upregulation of Twist in oesophageal squamous cell carcinoma is
associated with neoplastic transformation and distant metastasis. J
Clin Pathol. 60:510–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reya T, Morrison SJ, Clarke MF, et al:
Stem cells, cancer, and cancer stem cells. Nature. 414:105–111.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nowell PC: The clonal evolution of tumor
cell populations. Science. 194:23–28. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brabletz T, Jung A, Spaderna S, et al:
Opinion: migrating cancer stem cells - an integrated concept of
malignant tumour progression. Nat Rev Cancer. 5:744–749. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|