Introduction
Reprogramming of somatic cells into induced
pluripotent stem cells was achieved by gene transfection of 4
transcription factors, c-Myc, Sox2, Oct3/4, and Klf4 (1–3). These
4 transcription factors and Nanog are overexpressed in embryonic
stem (ES) cells, known to be regulators of identity of ES cells,
and are responsible for the maintenance of ES cells. Recent
attention has focused on functions of these genes, i.e., how they
act as stemness factors. Besides reprogramming to pluripotent cells
and maintaining their pluripotency and self-renewing nature, there
has been increasing evidence in terms of such stemness factors as
involved in malignancies. Microarray analysis by Ben-Porath et
al showed that aggressive breast cancer preferentially
overexpressed genes normally enriched in ES cells, and this ES-like
genetic signature in breast cancer is associated with poor clinical
outcome (4). It is known that c-Myc
is an oncogene, Sox2 expression is involved in invasion and
metastasis of pancreatic intraepithelial neoplasia (5), Oct3/4 expression is involved in
tumorigenesis via the activation of its downstream genes in breast
cancer-initiating cells (6),
upregulation of Klf4 in esophageal epithelial cells induces
inflammation-mediated esophageal squamous cell cancer (7,8), and
Nanog is expressed in various types of tumor, including carcinoma
of the breast, cervix and ovary (9–11). On
the basis of these findings, stemness factors may contribute to
carcinogenesis, invasion and cancer metastasis.
The tumor suppressor gene p53 is a signifcant
negative regulator of carcinogenesis (12). It is activated in response to DNA
damage stress signals and then binds to the promoter of Nanog and
suppresses Nanog expression (13,14).
Cancer development involves the accumulation of genetic changes
that lead to malignant transformation of normal cells (15,16).
It is supposed that stemness factors and p53 may form a network and
regulate the downstream gene expression during carcinogenesis prior
to morphological changes (17). If
the early genetic alteration of such genes in cancer development
were revealed, it would be possible to achieve the early detection
of malignancy and optimal oncological therapy, and also implement a
risk evaluation of precancerous lesions prior to cancer
development, enabling the rapid application of chemoprevention
therapies targeted at these genes.
However, there have been few reports showing such
genetic alterations in cancer development, particularly in the
early stage of cancer or in the case of precancerous lesions
(18). This lack of evidence may be
due to the difficulty in modeling longitudinal genetic alterations
in the normal epithelial-dysplasia carcinoma sequence in humans
(19). To examine this notion, we
used the carcinogen-induced carcinogenesis model of mice, which
uses N-methylbenzylnitrosoamine (NMBA) administration to induce
squamous cell carcinoma in the mouse forestomach.
Materials and methods
Mice
C57BL/6J mice p53+/+ (CLEA Japan, Inc., Tokyo,
Japan) were crossed with C57BL/6J p53+/- mice (RIKEN Japan,
Saitama, Japan). The p53 offspring were differentiated by the
genotyping of tail DNA by quantitative real-time PCR (qPCR). The
animals were maintained in hanging polycarbonate cages and were
provided with food, at a controlled temperature (23±1°C) and a 12-h
light/dark cycle, in accordance with the NIH Guide for Care and Use
of Laboratory Animals.
Chemical carcinogen-induced
carcinogenesis
The animal studies were approved by the
Institutional Animal Care and Use Committee at Osaka University,
Japan. Six-week-old p53+/+ mice (n=26) and p53+/- mice (n=11) were
administered 6 intraesophageal doses of NMBA (2 mg/kg weight) twice
weekly, via a thin tube over 3 weeks (20); the NMBA caused squamous cell
carcinoma in the mouse forestomach. P53(+/+) and p53(+/-) C57BL/6J
mice (n=5 each; age, 26 weeks) not treated with NMBA were used for
evaluating sporadic forestomach tumor development. P53+/+ mice were
sacrificed at weeks 4 (n=5), 8 (n=5), 12 (n=5), 20 (n=6) and 30
(n=5) following NMBA administration, and p53 +/- mice were
sacrificed at weeks 4 (n=4), 8 (n=2), 12 (n=3) and 20 (n=2)
following NMBA administration. The mice were analyzed for tumor
incidence by longitudinally opening the stomach and counting the
number of mice-bearing tumors in the forestomach. The opened
stomach was divided into 2 sections: one section was fixed in 10%
buffered formalin, embedded in paraffin, and stained with H&E
for histopathology or immunohistochemistry (IHC), and the other
section was used for RNA extraction for performing qPCR and
microarray analyses.
IHC
IHC was performed using the Histofine Mousestain
kit. Paraffin-embedded sections (5 μm) were deparaffinized in
xylene and dehydrated through graded ethanol. The sections were
immersed in antigen retrieval buffer (pH 6.0, citrate buffer) and
heated for 10 min at 121°C in an autoclave. Continuous sections
were incubated with 3% H2O2 to block
endogenous peroxidase activity. Slides were then incubated with
goat serum for 20 min to reduce non-specific background staining.
Blocked sections were incubated at a 1:50 dilution of a primary
antibody (rabbit polyclonal anti-p53 antibody; SC-6243; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) and incubated overnight at
4°C. The subsequent reaction was performed using the EnVision kit
(Dako, Carpinteria, CA, USA) according to the manufacturer’s
instructions, followed by DAB development. The slides were then
counterstained with hematoxylin. IHC analysis was performed by
evaluating the percentage of p53-positive nuclei in the mouse
forestomach epithelium.
qPCR
Total RNA was extracted from the forestomach of the
sacrificed mice using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Reverse transcription was performed using a
SuperScript III reverse transcription kit (Invitrogen). qPCR was
performed using the LightCycler TaqMan Master kit (Roche
Diagnostics, Tokyo) for cDNA amplification of specific target
genes. Purified cDNA from mouse ES cells was used as a positive
control for the target genes. The expression of mRNA copies was
normalized to GAPDH mRNA expression. Primers for Oct3/4, c-Myc,
Klf-4, Sox2 and Nanog were used.
Statistical analysis
Continuous values were expressed as the mean ±
standard error. The correlation between the target gene expression
and tumor incidence differences was analyzed by the χ2
test or Fisher’s exact test. Statistical analyses were performed
using JMP v8.0 software (SAS Institute, Cary, NC, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
NMBA-induced carcinogenesis
A total of 37 mice (26 C57BL/6J p53+/+ and 11
C57BL/6J p53+/-) were administered intraesophagus doses of NMBA.
C57BL/6J p53+/+ mice were sacrificed at weeks 4 (n=5), 8 (n=5), 12
(n=5), 20 (n=6) and 30 (n=5) following NMBA administration, and
C57BL/6J p53+/-mice were sacrificed at weeks 4 (n=4), 8 (n=2), 12
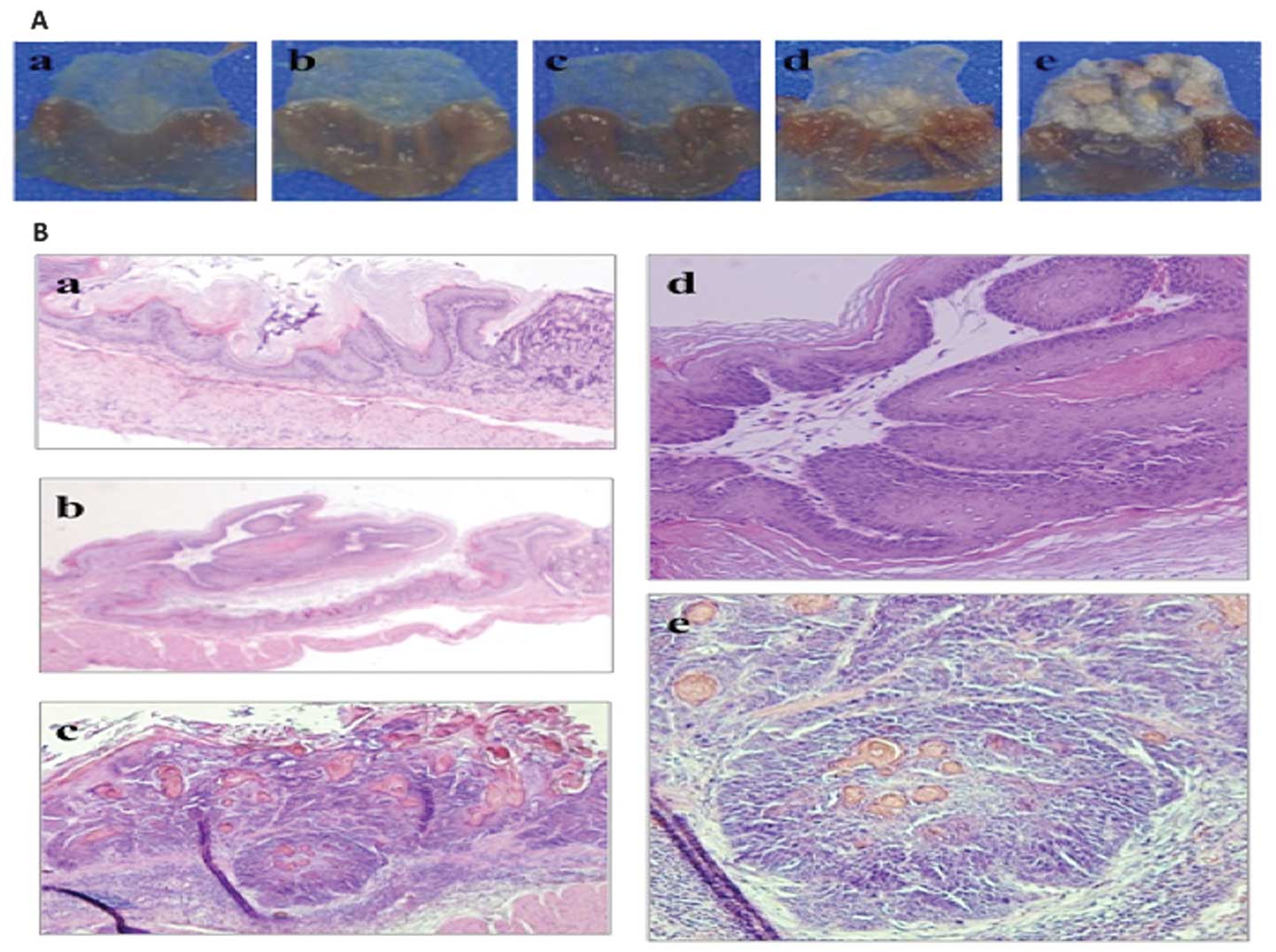
(n=3) and 20 (n=2) following NMBA administration. Macroscopic
findings revealed that in the p53+/+ mice, despite evident changes
not being observed until 12 weeks after NMBA administration,
elevated tumors and thickened forestomach were detected in 2 of 6
mice 20 weeks following NMBA administration and in 5 of 5 mice 30
weeks following NMBA administration. Thirty weeks after NMBA
administration, histological examination revealed an evident
squamous cell carcinoma in the forestomach that had metastasized
into submucosal layer. In the p53(+/-) mice, elevated tumors and
thickened forestomachs were already evident in 2 of 4 mice at 4
weeks, in 2 of 2 mice at 8 weeks, in 3 of 3 mice at 12 weeks and in
2 of 2 mice at 20 weeks following NMBA administration. In the
p53(+/+) and p53(+/-) mice not treated with NMBA (n=5 in each; age,
26 weeks), there was no evidence of sporadic forestomach tumors
(Fig. 1).
p53 IHC
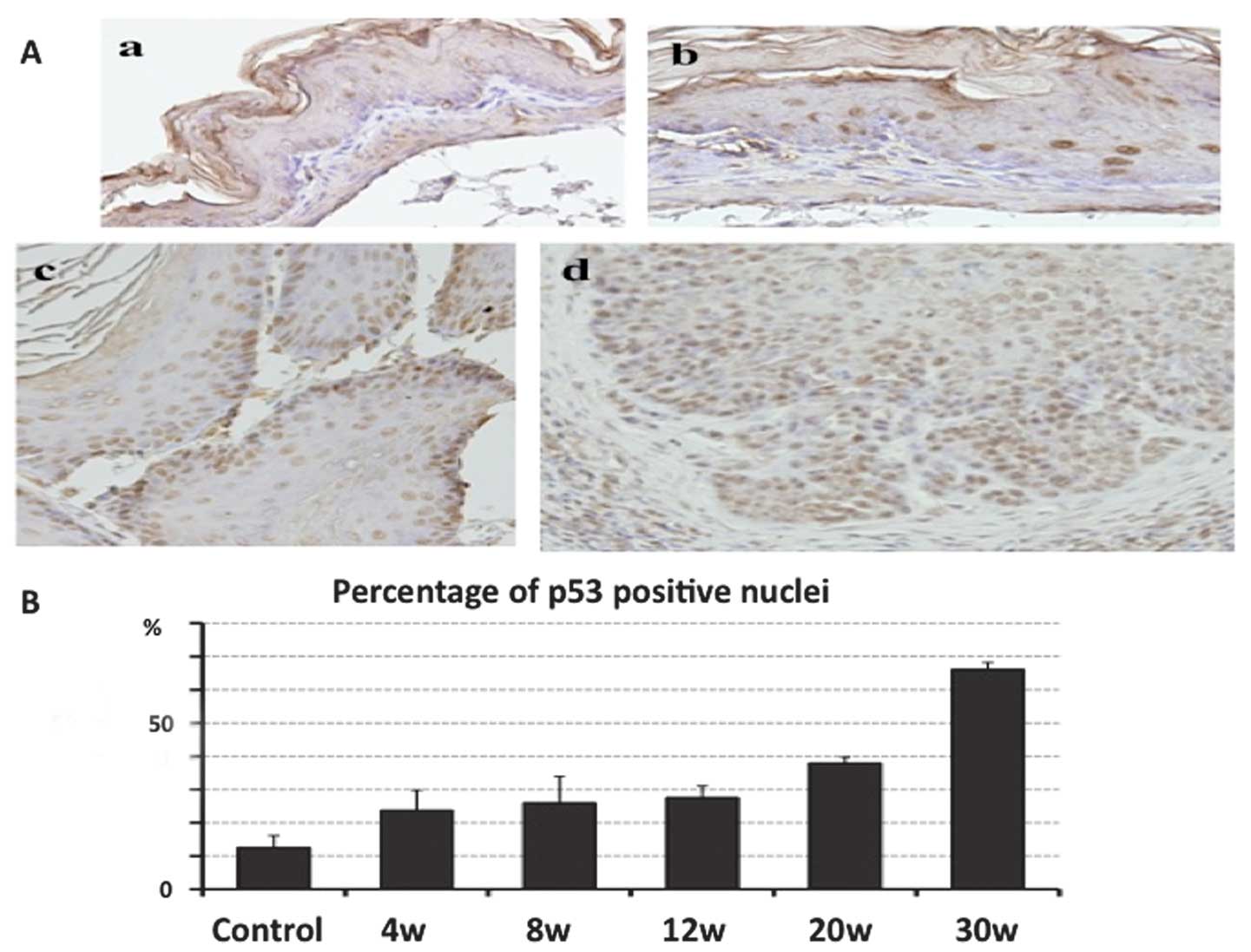
IHC analysis of p53 was performed for each p53(+/+)
mouse tissue sample, and the percentage of p53-positive nuclei in
the forestomach epithelium was determined. In the control mice, p53
was rarely expressed in the forestomach epithelium, with only 12.6%
p53-positive nuclei. The expression of p53 gradually increased at
4, 8, 12, 20 and 30 weeks following NMBA administration. Notably,
the percentage of p53- positive nuclei at 30 weeks following NMBA
administration was 66.2%, and p53 was overexpressed in the squamous
cell carcinoma lesions (Fig.
2).
Expression pattern of stemness
factors
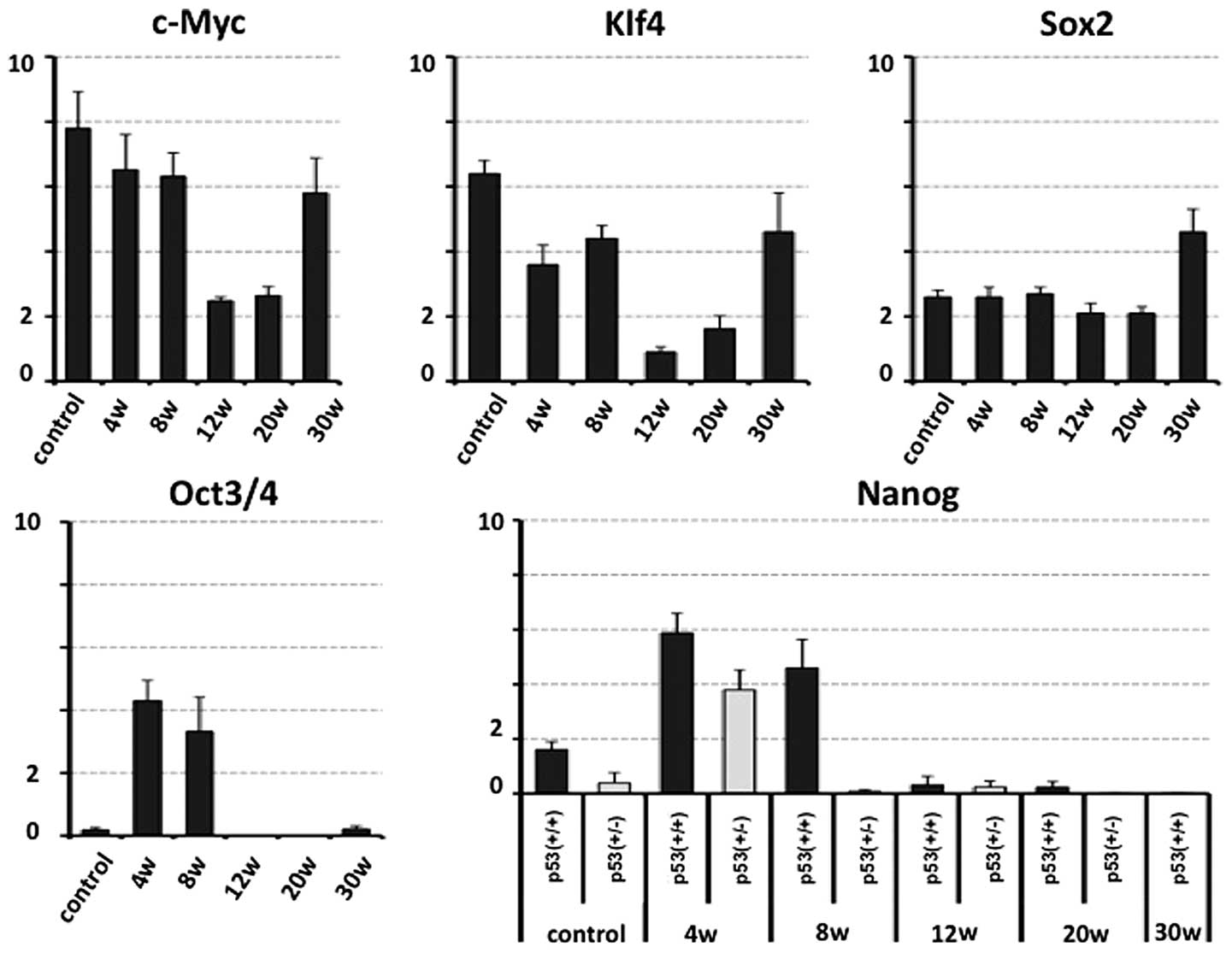
Bulk RNA was extracted from the forestomachs of the
sacrificed mice and qPCR analysis of stemness factors relative to
GAPDH was performed. It was predicted that the expression of the
stemness factors in the p53+/+ mice would be upregulated in the
early phase of carcinogenesis. The qPCR analysis demonstrated that
the c-Myc and Klf4 expression decreased at 12 and 20 weeks
following administration of the treatment (before evident
morphological changes were observed), and increased at 30 weeks
following administration. The Sox2 expression in the mouse ES cell
line (positive control) and in mice 20 weeks following NMBA
administration were compared and a significant increase was
observed at 30 weeks following NMBA administration. There was
little Oct3/4 and Nanog expression in control tissue samples
compared with the positive control for qPCR detection (Fig. 3). The expression of Oct3/4 and Nanog
increased at the early stages following NMBA administration, and
Nanog expression was not positively affected by the deficiency of
p53.
Discussion
This study was designed to examine the genetic
alteration of stemness factors in the early initiation phase of
carcinogenesis using chemical carcinogen-induced carcinogenesis
in vivo. NMBA has been widely used to induce tumors in the
rodent esophagus and forestomach by producing an electrophilic
methylating agent that produces the mutagenic adduct
O6-methylguanine in DNA (21,22).
Previous studies that examined the gene expression associated with
cancer were mainly horizontal comparison analyses that compared
normal epithelia to cancer lesions or multiple surgically resected
specimens. Few studies, however, have reported longitudinal or
serial changes during carcinogenesis (23,24). A
plausible carcinogenesis model may make significant assessments
possible before evident morphological changes have been
established.
We hypothesized that the early detection of
increased gene expression in stemness factors in several types of
cancer, where stemness factors are overexpressed compared with
those in the normal epithelium, is useful in the detection of
cancer in high-risk groups, or for adopting interventional therapy
to prevent cancer development (chemoprevention). Nanog and Oct3/4
were expressed in the early stages of carcinogenesis, and the
expression of c-Myc and Klf4 was decreased once during the
precancerous stage and increased when apparent tumors containing
squamous cell carcinoma developed. The Sox2 expression level
remained unchanged before evident tumors appeared, but
significantly increased when tumors evidently developed.
Furthermore, we examined the correlation between Nanog and p53
using p53(+/-) mice; however, the unexpectedly heterozygous
deletion of p53 did not upregulate Nanog expression.
In the present study, we investigated the molecular
events, i.e., any alterations of expression of immature-related
genes, including c-Myc, Klf4, Nanog, Oct3/4 and Sox2 (reprogramming
factors), before apparent tumors were established in the mouse
forestomach. The data demonstrated that, although further study is
required for Nanog, the expression of Oct3/4 was increased even in
‘normal’ epithelial cells, suggesting that Oct3/4 is involved in
the progression of carcinogenesis from normal epithelial cells at
early stages. Thus, the study indicates a use for this gene as a
biomarker in forestomach tumor formation.
Acknowledgements
This study was partly supported by a grant from the
Core Research for Evolutional Science and Technology (CREST) (H.I.,
N.H., M.M.), a Grant-in-Aid for Scientific Research on Priority
Areas (D.Y., M.M.), a Grant-in-Aid for Scientific Research from the
Ministry of Education, Culture, Sports, Science and Technology
(H.I., D.Y., M.M.), a Grant-in-Aid for the 3rd Comprehensive
10-year Strategy for Cancer Control Ministry of Health, Labour and
Welfare (H.I., M.M.), a grant from the Tokyo Biochemical Research
Foundation (M.M.) and a grant from the Princess Takamatsu Cancer
Research Fund, Japan (H.I.).
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar
|
|
3
|
Yamanaka S: Elite and stochastic models
for induced pluripotent stem cell generation. Nature. 460:49–52.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lengerke C, Fehm T, Kurth R, Neubauer H,
Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L,
Fend F, Perner S, Bareiss PM and Staebler A: Expression of the
embryonic stem cell marker SOX2 in early-stage breast carcinoma.
BMC Cancer. 11:422011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tetreault MP, Wang ML, Yang Y, Travis J,
Yu QC, Klein-Szanto AJ and Katz JP: Klf4 overexpression activates
epithelial cytokines and inflammation-mediated esophageal squamous
cell cancer in mice. Gastroenterology. 139:2124–2134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H
and Liu Z: MicroRNA-10b promotes migration and invasion through
KLF4 in human esophageal cancer cell lines. J Biol Chem.
285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeter CR, Badeaux M, Choy G, Chandra D,
Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ and Tang
DG: Functional evidence that the self-renewal gene NANOG regulates
human tumor development. Stem Cells. 27:993–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glinsky GV: ‘Stemness’ genomics law
governs clinical behavior of human cancer: implications for
decision making in disease management. J Clin Oncol. 26:2846–2853.
2008.
|
|
11
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS and Wu CW:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hickman ES, Moroni MC and Helin K: The
role of p53 and pRB in apoptosis and cancer. Curr Opin Genet Dev.
12:60–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan G and Thomson JA: Nanog and
transcriptional networks in embryonic stem cell pluripotency. Cell
Res. 17:42–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin T, Chao C, Saito S, Mazur SJ, Murphy
ME, Appella E and Xu Y: p53 induces differentiation of mouse
embryonic stem cells by suppressing Nanog expression. Nat Cell
Biol. 7:165–171. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartkova J, Horejsi Z, Koed K, Kramer A,
Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C,
Orntoft T, Lukas J and Bartek J: DNA damage response as a candidate
anti-cancer barrier in early human tumorigenesis. Nature.
434:864–870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gorgoulis VG, Vassiliou LV, Karakaidos P,
Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr,
Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C and
Halazonetis TD: Activation of the DNA damage checkpoint and genomic
instability in human precancerous lesions. Nature. 434:907–913.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rich AM, Kerdpon D and Reade PC: p53
expression in oral precancer and cancer. Aust Dent J. 44:103–105.
1999. View Article : Google Scholar
|
|
18
|
Pontén J: Cell biology of precancer. Eur J
Cancer. 37:S97–S113. 2001.
|
|
19
|
Ozols R: Esophageal cancer. Curr Problems
Cancer. 18:191–246. 1994.
|
|
20
|
Fong LYY, Ishii H, Nguyen VT, Vecchione A,
Farber JT, Croce CM and Huebner K: p53 deficiency accelerates
induction and progression of esophageal and forestomach tumors in
zinc-deficient mice. Cancer Res. 63:186–195. 2003.PubMed/NCBI
|
|
21
|
Carlton PS, Kresty LA, Siglin JC, Morse
MA, Lu J, Morgan C and Stoner GD: Inhibition of
N-nitrosomethylbenzylamine-induced tumorigenesis in the rat
esophagus by dietary freeze-dried strawberries. Carcinogenesis.
22:441–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dumon KR, Ishii H, Fong LYY, Zanesi N,
Fidanza V, Mancini R, Vecchione A, Baffa R, Trapasso F, During MJ,
Huebner K and Croce CM: FHIT gene therapy prevents tumor
development in Fhit-deficient mice. Proc Natl Acad Sci USA.
98:3346–3351. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coia LR: The esophagus. Moss’ Radiation
Oncology: Rationale, Techniques, Results. Cox J: Mosby-Year Book;
St. Louis: pp. 4091994
|
|
24
|
Blot WJ: Esophageal cancer trends and risk
factors. Semin Oncol. 21:403–410. 1994.PubMed/NCBI
|