Introduction
Chordomas are rare tumors that are thought to
originate from notochordal remnants. They arise from the
sacrococcygeal region in approximately 50% of cases, from the
sphenooccipital area in 35% and from the mobile spine in 15%
(1). More males than females are
affected by sacral chordoma; however, chordomas of the skull base
appear to occur with equal frequency in males and females (2). Locally, these tumors are highly
aggressive and frequently demonstrate local recurrence even
following wide resection (3).
However, there are no effective chemotherapeutic regimens available
for the treatment of chordomas (4)
and surgery remains the mainstay of chordoma management. Targeted
molecular therapy has shown promising results in the treatment of
malignancies with historically poor responses to chemotherapy.
However, the expression of molecular markers in chordomas is not
well understood. Thus, a better understanding of the molecular
pathogenesis of this disease is required.
Hypoxia is a characteristic of all solid tumors.
Under hypoxic conditions, tumor cells are deprived of oxygen due to
a limited blood supply resulting from the abnormal tumor
microvasculature (5).
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that
regulates the pathways involved in tumor cell survival,
proliferation, angiogenesis, invasion and metastasis. HIF-1α is a
significant protein that directly reacts to hypoxia (6). Hypoxia leads to a rapid increase in
HIF-1α protein levels, whereas HIF-1α is maintained at low levels
under normoxic conditions (7).
HIF-1α overexpression has been reported to correlate with tumor
progression and an unfavorable prognosis in several types of cancer
(8,9). In addition, HIF-1α directly activates
the expression of a number of pro-angiogenic factors, including
vascular endothelial growth factor (VEGF) and matrix
metalloproteinase-2 (MMP-2) (10–12).
Hypoxia-driven VEGF expression is considered to be a principal
inducer of tumor angiogenesis. HIF-1α regulates VEGF transcription
by binding to the hypoxia response element (HRE) in the VEGF
promoter region (13). HIF-1α
expression correlates with that of VEGF and microvessel density
(MVD) in several types of tumor (14,15).
Degradation and remodeling of the extracellular matrix (ECM) and
basement membranes by proteolytic enzymes are essential steps in
the processes of invasion and metastasis (16). MMP-2 has been shown to be one of the
key enzymes regulating these processes. VEGF expression has also
been found to correlate with MMP-2 expression in patients with
gastric carcinoma (17).
In a previous study, we demonstrated that HIF-1α is
expressed in chordomas and is correlated with multidrug
resistance-associated protein 1 (MRP1) (18). There are, however, no studies
available concerning the association between HIF-1α expression and
clinicopathological findings in chordomas. The angiogenic process
in sacral chordomas is also not fully understood. As a preliminary
investigation of the molecular mechanisms of this rare disease, the
aim of this study was to investigate the correlation between
HIF-1α, VEGF and MMP-2 expression and to clarify their
clinicopathological significance in chordomas. Paraffin-embedded
tissue samples from 35 sacral chordoma patients were
retrospectively obtained. We examined the expression patterns of
HIF-1α, VEGF and MMP-2 by immunohistochemistry and staining was
performed on a tissue microarray (TMA).
Materials and methods
Patients and tumor specimens
All cases of primary sacral chordoma diagnosed at
Tangdu Hospital, The Fourth Military University (Shaanxi, China),
between 1995 and 2010 were considered. The cases selected included
patients with no history of chemotherapy or radiotherapy prior to
surgery and a follow-up time of ≥12 months. A total of 35 patients
diagnosed with sacral conventional chordoma were selected from the
files. The patients ranged in age from 17 to 78 years (median, 53)
and included 28 males and 7 females. The patient records were
reviewed to collect the pathological data (tumor location and size
and status of surgical resection margins), demographic data
(patient age and gender) and follow-up information (time to local
recurrence, distant metastasis and mortality) (Table I).
 | Table IClinicopathological characteristics of
study cases. |
Table I
Clinicopathological characteristics of
study cases.
| No. | Age (years) | Gender | Sizea (cm) | Margin status | Local recurrence
(m) | Metastasis
(location/m) | Outcome | Follow-up (m) |
|---|
| 1 | 37 | Female | 10 | NE | 8 | Lung/49 | AWD | 49 |
| 2 | 50 | Male | 5 | − | No | No | NED | 21 |
| 3 | 60 | Male | 5 | NE | 12 | Lung/81 | DD | 86 |
| 4 | 56 | Male | 5 | + | No | No | NED | 18 |
| 5 | 53 | Male | 9 | NE | No | No | NED | 97 |
| 6 | 55 | Male | 8 | NE | 9 | L3, abdomen/45 | DD | 51 |
| 7 | 47 | Male | 11 | NE | 34 | No | NED | 89 |
| 8 | 56 | Male | 8 | + | No | No | NED | 13 |
| 9 | 44 | Female | 6 | − | 25 | No | NED | 37 |
| 10 | 54 | Male | 5 | NE | 111 | GM/111 | DD | 146 |
| 11 | 17 | Male | 9 | + | 20 | T11-12/20 | AWD | 20 |
| 12 | 58 | Male | 4 | NE | 24 | No | NED | 29 |
| 13 | 77 | Male | 5 | − | 38 | No | AWD | 66 |
| 14 | 67 | Male | 7 | NE | 17 | No | NED | 30 |
| 15 | 49 | Male | 8 | + | 75 | No | AWD | 75 |
| 16 | 55 | Male | 7 | − | 5 | No | NED | 12 |
| 17 | 51 | Female | 6 | + | 61 | No | DD | 69 |
| 18 | 60 | Female | 5 | NE | 34 | Abdomen/34 | DD | 81 |
| 19 | 51 | Female | 14 | NE | 41 | No | DD | 92 |
| 20 | 67 | Male | 8 | + | 7 | No | AWD | 38 |
| 21 | 47 | Male | 5 | − | No | No | NED | 43 |
| 22 | 52 | Female | 9 | NE | 23 | No | AWD | 23 |
| 23 | 27 | Male | 12 | NE | 25 | No | AWD | 25 |
| 24 | 74 | Male | 10 | − | 10 | No | DD | 43 |
| 25 | 78 | Male | 7 | NE | 6 | No | DD | 23 |
| 26 | 47 | Male | 4 | − | No | No | NED | 13 |
| 27 | 51 | Male | 8 | NE | 32 | No | AWD | 120 |
| 28 | 50 | Male | 9 | − | No | No | NED | 46 |
| 29 | 58 | Female | 8 | NE | 37 | No | NED | 65 |
| 30 | 59 | Male | 16 | + | 58 | No | NED | 63 |
| 31 | 62 | Male | 30 | − | 9 | No | DD | 112 |
| 32 | 56 | Male | 4 | − | 10 | No | DD | 39 |
| 33 | 50 | Male | 5 | − | No | No | NED | 73 |
| 34 | 53 | Male | 8 | NE | No | No | NED | 61 |
| 35 | 49 | Male | 6 | NE | No | No | NED | 45 |
TMAs were prepared using a standard protocol
(19). Archived chordoma tissues
and representative hematoxylin and eosin (H&E) slides of each
case were reviewed microscopically by a pathologist. The chordomas
were identified on the corresponding H&E slides. Two tissue
cores (2 mm in diameter) were removed from histologically
identified representative regions of each formalin-fixed
paraffin-embedded tumor. The study protocol was approved by the
ethics committee of Tangdu Hospital. Informed consent was obtained
from each patient in accordance with committee regulations.
Immunohistochemical staining for HIF-1α,
VEGF, MMP-2 and CD34
The TMA slides were investigated by
immunohistochemistry for the expression of HIF-1α (H1alpha67,
dilution 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
VEGF (VG1, dilution 1:200; Dako, Copenhagen, Denmark) and MMP-2
(polyclonal, dilution 1:150; Bioss, Beijing, China) using the
ChemMate™ Envision+HRP/DAB kit (Dako). The pre-treatment of
sections with heat-induced epitope retrieval was performed
according to the specification for each antibody. Negative controls
were prepared using phosphate-buffered solution (PBS) instead of
the primary antibody. The TMA slides were independently evaluated
by two pathologists who had no prior knowledge of the clinical data
or histological findings.
The immunohistochemical results for the HIF-1α
protein were then classified (−, no staining; +, nuclear staining
in <1% of cells; ++, nuclear staining in 1–10% of cells and/or
weak cytoplasmic staining; +++, nuclear staining in 11–50% of cells
and/or distinct cytoplasmic staining; ++++, nuclear staining in
>50% of cells and/or strong cytoplasmic staining) (20). For statistical analyses, tumors with
a final staining score of − or + with a weak or moderate/strong
immunoreactivity were classified as the low expression group and
were compared with tumors with scores of ++, +++ or ++++ that were
classified as the high expression group (20). VEGF expression was defined as
positive if distinct staining of the cytoplasm was observed in ≥10%
of tumor cells (14). Cytoplasmic
MMP-2 staining was classified as: low expression exhibited staining
in <50% of cells and high expression exhibited staining in ≥50%
of cells (21).
Evaluation of MVD
Paraffin sections (4 μm) were cut from
formalin-fixed paraffin-embedded tissue before tissue cores were
removed for CD34 staining. CD34 (QBEnd10, Ready to use, Dako)
staining was used to assess MVD by light microscopy at the site of
the highest number of capillaries and small venules. Highly
vascular areas were identified by scanning tumor sections at a low
power (x100). MVD was determined by observing the identified six
fields for each sample at a magnification of ×400, and the means
were then calculated (14). A
visible lumen was not necessary for a structure to be defined as a
vessel (22).
Statistical analysis
Statistical analysis was performed using Microsoft
Excel 2003 (Microsoft, Seattle, WA, USA) and SPSS Statistics 17.0
(SPSS, Chicago, IL, USA) for Windows. The significance of the
observed associations was determined using the Mann-Whitney U test.
The Spearman’s rank correlation test was used to determine the
association between HIF-1α, VEGF and MMP-2. The impact of HIF-1α,
VEGF and MMP-2 expression on patient survival was assessed using
the Kaplan-Meier method and compared using the log-rank test.
P<0.05 was considered to indicate a statistically significant
result.
Results
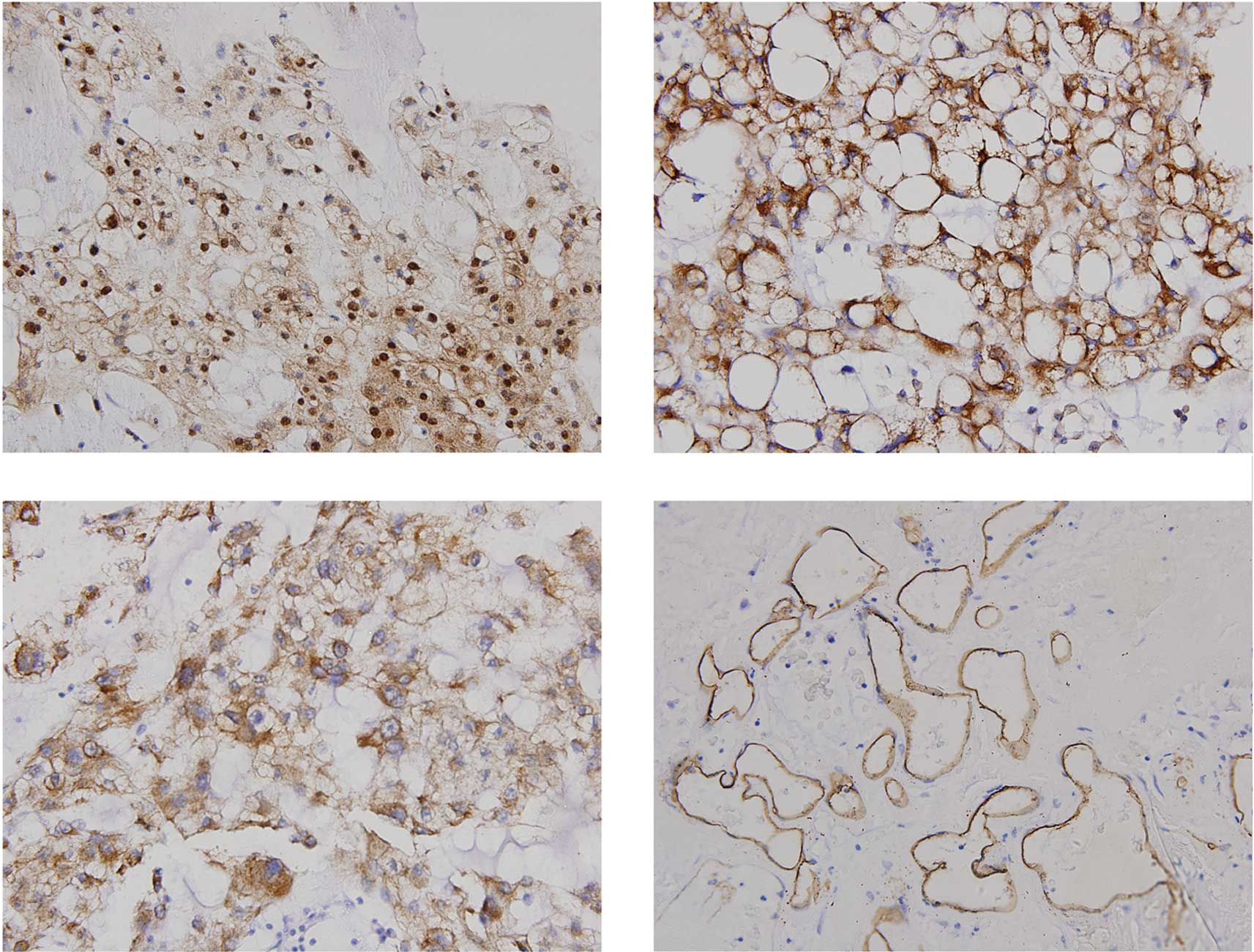
Immunohistochemical staining of HIF-1α,
VEGF and MMP-2
HIF-1α, VEGF and MMP-2 immunostaining was assessed
and the samples were grouped into high-or low-grade categories.
HIF-1α expression showed a nuclear and/or cytoplasmic staining
pattern with diverse intensities (Fig.
1). Of the 35 chordomas, 26 (74.3%) exhibited strongly positive
HIF-1α staining (score ++, +++ or ++++) and 9 (25.7%) showed weakly
positive staining (score − or +). Immunohistochemical staining of
VEGF and MMP-2 was observed predominantly in the cytoplasm of the
tumor cells. Of the 35 chordoma cases, 25 (71.4%) exhibited high
VEGF expression levels. MMP-2 expression was classified as high in
24 (68.6%) of the chordoma cases. The immunohistochemical staining
of each protein and their correlation with patient age, gender,
tumor size, local recurrence and distant metastases are shown in
Table II. No correlation was found
between any of the three factors and clinicopathological
characteristics (patient age, gender, tumor size, local recurrence
and distant metastases) for any patient (P>0.05).
 | Table IICorrelation of HIF-1α, VEGF and MMP-2
expression with clinicopathological characteristics in chordoma
cases. |
Table II
Correlation of HIF-1α, VEGF and MMP-2
expression with clinicopathological characteristics in chordoma
cases.
| | HIF-1α | | VEGF | | MMP-2 | |
|---|
| |
| |
| |
| |
|---|
|
Characteristics | Case number | High | Low | P-valuea | High | Low | P-value | High | Low | P-value |
|---|
| Age (years) |
| >53b | 18 | 13 | 5 | 0.810 | 13 | 5 | 0.928 | 10 | 8 | 0.152 |
| ≤53 | 17 | 13 | 4 | | 12 | 5 | | 14 | 3 | |
| Gender |
| Female | 7 | 7 | 0 | 0.239 | 6 | 1 | 0.529 | 5 | 2 | 0.903 |
| Male | 28 | 19 | 9 | | 19 | 9 | | 19 | 9 | |
| Size of tumor
(cm) |
| <8b | 16 | 11 | 5 | 0.565 | 12 | 4 | 0.733 | 10 | 6 | 0.563 |
| ≥8 | 19 | 15 | 4 | | 13 | 6 | | 14 | 5 | |
| Recurrence |
| Yes | 25 | 18 | 7 | 0.725 | 16 | 9 | 0.240 | 16 | 9 | 0.494 |
| No | 10 | 8 | 2 | | 9 | 1 | | 8 | 2 | |
| Metastasis |
| Yes | 6 | 4 | 2 | 0.781 | 4 | 2 | 0.872 | 4 | 2 | 0.958 |
| No | 29 | 22 | 7 | | 21 | 8 | | 20 | 9 | |
Correlation between HIF-1α, VEGF and
MMP-2 expression
Of the 35 sacral chordoma samples, 26 (74.3%) showed
intense HIF-1α immunoreactivity in the nucleus and/or cytoplasm of
the tumor cells. In the 26 sacral chordomas classified as having a
high level of expression of HIF-1α, the VEGF-positive and
VEGF-negative expression frequency was 22/26 (84.6%) and 4/26
(15.4%), respectively. By contrast, of the 9 chordomas classified
as exhibiting a low level of expression of HIF-1α, 3/9 (33.3%) were
VEGF-positive and 6/9 (66.7%) were VEGF-negative. The correlation
between the HIF-1α and VEGF expression levels was significant
(P=0.002). In addition, of the 26 sacral chordomas classified as
showing a high HIF-1α expression, the frequencies of high and low
MMP-2 expression were 20/26 (76.9%) and 6/26 (23.1%), respectively.
There tended to be an association between the expression levels of
HIF-1α and MMP-2, but the correlation was not significant
(P=0.074). The Spearman’s rank correlation analysis indicated that
the expression of VEGF was positively correlated with MMP-2
expression (P=0.001).
Correlation between the protein
expression and MVD
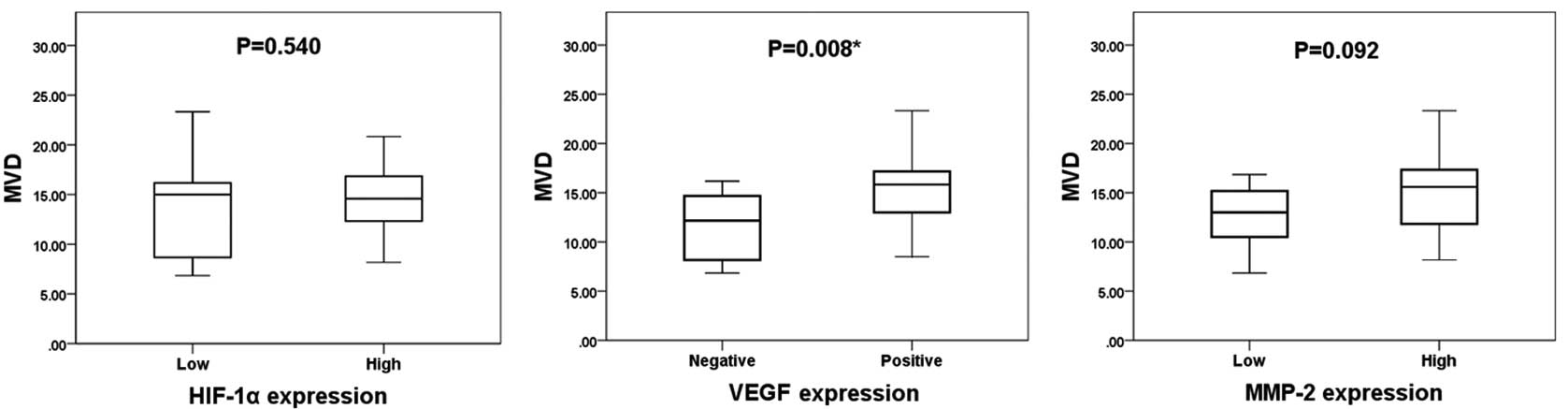
The mean MVD value was 14.2±4.0. The expression
levels of HIF-1α, VEGF and MMP-2 compared with MVD are shown in
Fig. 2. The mean MVD (in
microvessels per field) in the high and low HIF-1α expression
groups was 14.4±3.4 and 13.5±5.5, respectively. The mean MVD in the
VEGF-positive and VEGF-negative groups was 15.3±3.7 and 11.5±3.6,
respectively, and the mean MVD in the high and low MMP-2 expression
groups was 14.9±4.0 and 12.6±3.6, respectively. VEGF expression was
significantly associated with MVD (P=0.008), whereas the expression
levels of HIF-1α and MMP-2 were not significantly associated with
MVD, as determined by the Mann-Whitney U test (P=0.540 and P=0.092,
respectively).
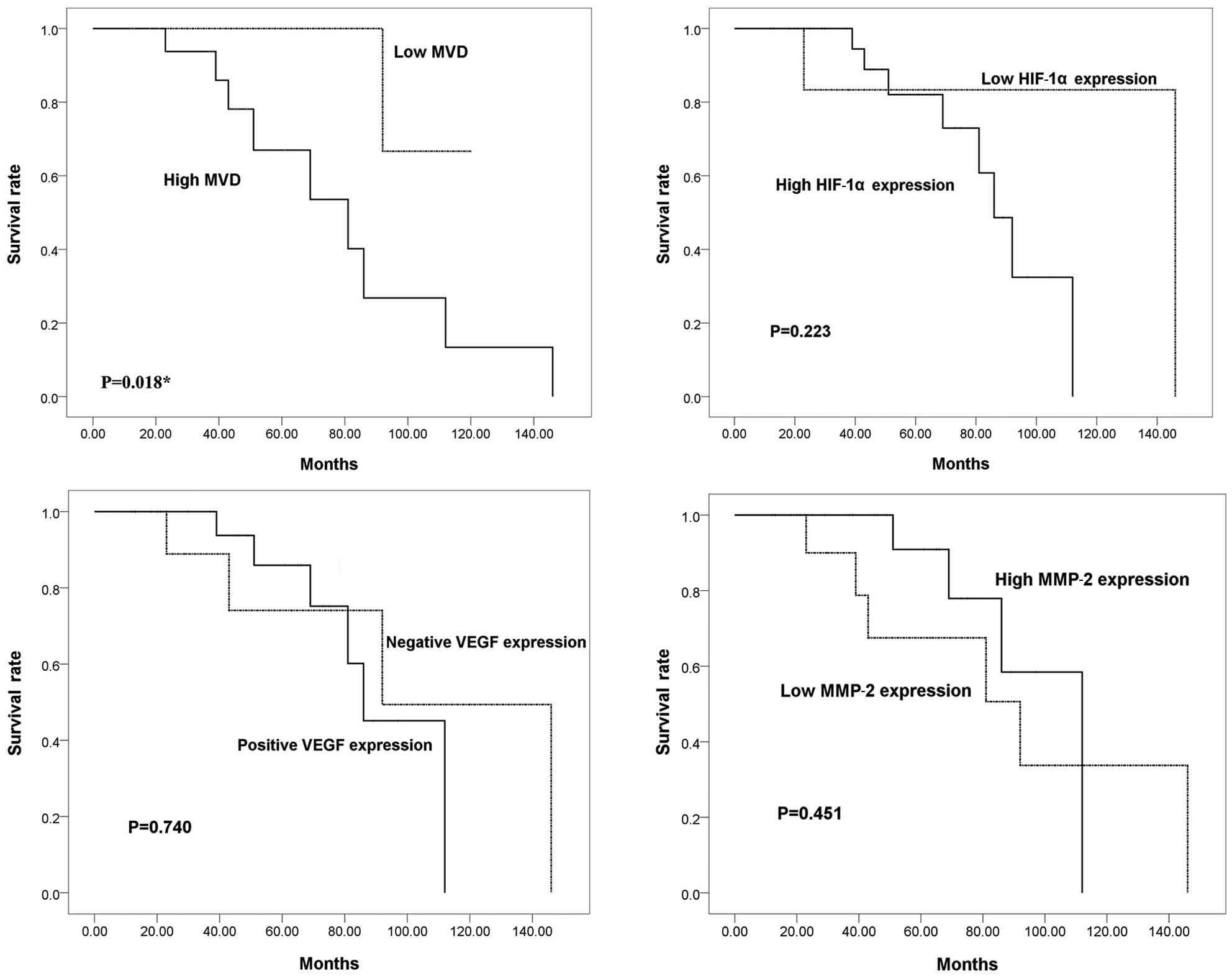
Correlation between patient survival and
MVD and HIF-1α, VEGF and MMP-2 expression
To investigate whether HIF-1α, VEGF and MMP-2
expression levels are associated with the outcome of patients with
sacral chordoma, Kaplan-Meier analyses were performed. The mean
duration of follow-up was 54.7 months (range, 12–146). Local
recurrence occurred in 25 (71.4%) of the 35 chordoma patients
during follow-up, with an average time to first local recurrence of
29.2 months (range, 5–111). Six patients presented with metastatic
disease when the disease progressed to a more advanced stage
(median time, 47 months; range, 20–111). The overall survival rates
at 5 and 10 years were 82.3 and 29.9%, respectively. Kaplan-Meier
analyses, however, indicated that there were no significant
differences in the survival rates between the high and low HIF-1α
expression groups, the VEGF-positive and VEGF-negative groups or
the high and low MMP-2 expression groups (P=0.223, P=0.740 and
P=0.451, respectively; Fig. 3). The
survival of patients with a high MVD value was significantly
shorter than that of patients with a low MVD value (P=0.018).
Discussion
Angiogenesis is an early event in carcinogenesis and
one of the hallmark characteristics of tumors (23). Neovascularization is critical for
tumor progression as the supply of oxygen and nutrients becomes
limited in tumor cells located more than 100 μm away from a blood
vessel (24). Angiogenesis is a
multistep process including basement membrane degradation,
endothelial cell migration, sprouting into the interstitial space,
endothelial cell proliferation, lumen formation and new basement
membrane and anastomosis formation (25). There are several
angiogenesis-related and -promoting factors that are thought to be
essential for this process during tumor development. A compelling
body of evidence indicates that VEGF is one of the most significant
pro-angiogenic factors involved in tumor growth and metastasis. It
is widely accepted that VEGF expression is mediated by HIF-1α under
hypoxic conditions (11,14,15,17).
During hypoxia, HIF-1α is protected from ubiquitination and
proteasomal degradation and activates the expression of a number of
pro-angiogenic factors, including VEGF, by binding to the HRE in
the VEGF promoter region. Furthermore, HIF-1α and VEGF expression
levels have been analysed in a number of types of human cancer and
their intracellular expression levels have been found to correlate
with tumorigenesis and angiogenesis (14,15).
Multiple proteinases, including MMPs, are also considered to be
involved in the degradation of the vascular basement membrane and
remodeling of the ECM during angiogenesis. Although the mechanisms
underlying the HIF-1α-mediated regulation of MMP-2 protein
synthesis are currently unclear, it has been demonstrated that the
overexpression of HIF-1α may lead to an increase in the secretion
and activation of MMP-2 (12).
MMP-2 expression has also been reported to correlate with VEGF
levels in patients diagnosed with gastric carcinoma (17), indicating that MMP-2 expression is
closely linked with angiogenesis. However, the correlation between
angiogenesis and chordomas is not well understood and has not been
extensively studied. The present study was performed to
retrospectively evaluate HIF-1α, VEGF and MMP-2 immunohistochemical
reactivity in sacral chordoma patients and to explore the
correlation of the expression of these proteins with
clinicopathological characteristics and prognosis. MVD was assessed
using CD34 to determine its correlation with patient outcome.
In this study, we investigated HIF-1α, VEGF and
MMP-2 expression levels in human sacral chordoma using
immunohistochemistry. Staining of HIF-1α was observed in the
cytoplasm and/or nuclei of tumor cells. However, we found that
differences in clinicopathological characteristics, including
patient age, gender, tumor size, local recurrence and distant
metastasis, did not correlate with the expression levels of HIF-1α,
VEGF or MMP-2. Our results demonstrate that there is a significant
correlation between HIF-1α and VEGF expression levels. This study
is the first to demonstrate such a correlation in human chordomas.
It is also the first to explore the correlation between VEGF and
MMP-2 immunoreactivity in chordomas. In addition, we have
determined that the expression levels of MMP-2 are correlated with
the expression of HIF-1α, although this correlation was not
significant. Previous studies have indicated that the three
pro-angiogenic factors are associated with patient prognosis for
several malignancies (17,20,21).
In our study, 35 cases with follow-up data were examined to
determine whether the expression levels of HIF-1α, VEGF and MMP-2
were of prognostic value. Chordoma patient outcome, however, was
not significantly associated with the expression of the three
proteins. We quantified tumor angiogenesis based on MVD using CD34
labeling. As a result, a significant correlation was found between
the expression of VEGF and MVD. Furthermore, MVD was found to be
associated with sacral chordoma patient prognosis. Chordomas are
rare and the number of specimens was limited; there were only 35
samples in this study. As the group of low HIF-1α expression had
only 9 patients, it is likely that the number of samples was
insufficient for significance to be determined. However, the
10-year survival rate in the high HIF-1α expression group versus
the low expression group was 0 versus 83.3% (Fig. 2B). As a result, a larger number of
samples is required to confirm whether or not these protein
expression levels correlate with prognosis.
Chen et al found that VEGF expression
correlated with MMP-9 expression and was significantly higher in
sacral chordoma tissue compared with adjacent normal tissue,
although the difference in the disease-free survival rates between
the VEGF-positive and VEGF-negative groups was not significant
(26). The present study and that
by Chen et al found no association between VEGF expression
and disease-free survival or the survival rate; however, the number
of patients in the two studies may not be sufficient to obtain
prognostic information. Naka et al found that in skull base
chordoma, MMP-9 was rarely expressed, but MMP-2 expression was
frequently observed (27). These
authors also found that MMP-2 expression was of prognostic value in
non-skull base chordoma, but not in skull base chordoma (27,28).
Although different results were observed in the skull base and
non-skull base chordomas, together, these data indicate that the
expression of VEGF and MMPs may be significant in chordoma
progression.
At present, the effective treatment of chordomas is
challenging. Even following wide surgical resection, the tumor
frequently recurs. Chordomas have long been known to be resistant
to chemotherapeutic drugs, presenting an obstacle that has to be
overcome for the effective treatment of this disease. Due to its
rare occurrence and a limited number of cell lines, little is known
concerning the molecular basis of this disease. The results of our
previous study suggest that both chordoma tissue and cell lines
express HIF-1α and that the expression of HIF-1α is correlated with
MRP1, which is known to be involved in hypoxia-induced
chemoresistance (18). Taken
together, these data indicate that HIF-1α is a significant
contributor to the process of angiogenesis and hypoxia-induced
chemoresistance in chordoma cells. As shown in this study,
angiogenesis is a significant process in sacral chordoma. The
process of angiogenesis may be a potential therapeutic target in
the treatment of chordomas.
Acknowledgements
The authors wish to thank the pathologists Dr Yang
Lianjia and Wen Yanhua for their assistance with the
immunohistochemical analysis. We sincerely appreciate the
assistance of Liu Yunyan and Ma Qiong with our experiments. We are
also grateful to Wang Yuanshan for her invaluable assistance in
editing the manuscript.
References
|
1
|
McMaster ML, Goldstein AM, Bromley CM,
Ishibe N and Parry DM: Chordoma: incidence and survival patterns in
the United States, 1973–1995. Cancer Causes Control. 12:1–11.
2001.
|
|
2
|
Anson KM, Byrne PO, Robertson ID, Gullan
RW and Montgomery AC: Radical excision of sacrococcygeal tumours.
Br J Surg. 81:460–461. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tzortzidis F, Elahi F, Wright D, Natarajan
SK and Sekhar LN: Patient outcome at long-term follow-up after
aggressive microsurgical resection of cranial base chordomas.
Neurosurgery. 59:230–237. 2006. View Article : Google Scholar
|
|
4
|
Chugh R, Tawbi H, Lucas DR, Biermann JS,
Schuetze SM and Baker LH: Chordoma: the nonsarcoma primary bone
tumor. Oncologist. 12:1344–1350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaupel P, Hockel M and Mayer A: Detection
and characterization of tumor hypoxia using pO2
histography. Antioxid Redox Signal. 9:1221–1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor 1alpha is mediated by an
O2-dependent degradation domain via the
ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 95:7987–7992.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Welsh SJ, Koh MY and Powis G: The hypoxic
inducible stress response as a target for cancer drug discovery.
Semin Oncol. 33:486–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semenza GL: Evaluation of HIF-1 inhibitors
as anticancer agents. Drug Discov Today. 12:853–859. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai Y, Bae K and Siemann DW: Impact of
hypoxia on the metastatic potential of human prostate cancer cells.
Int J Radiat Oncol Biol Phys. 81:521–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenz GL: Activation of vascular endothelial
growth factor gene transcription by hypoxia-inducible factor 1. Mol
Cell Biol. 16:4604–4613. 1996.PubMed/NCBI
|
|
12
|
Shyu KG, Hsu FL, Wang MJ, Wang BW and Lin
S: Hypoxia-inducible factor 1alpha regulates lung adenocarcinoma
cell invasion. Exp Cell Res. 313:1181–1191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryan HE, Poloni M, McNulty W, Elson D,
Gassmann M, Arbeit JM and Johnson RS: Hypoxia-inducible
factor-1alpha is a positive factor in solid tumor growth. Cancer
Res. 60:4010–4015. 2000.PubMed/NCBI
|
|
14
|
Kuwai T, Kitadai Y, Tanaka S, Elson D,
Gassmann M, Arbeit JM and Johnson RS: Expression of
hypoxia-inducible factor-1alpha is associated with tumor
vascularization in human colorectal carcinoma. Int J Cancer.
105:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura S, Kitadai Y, Tanaka S, Kuwai T,
Hihara J, Yoshida K, Toge T and Chayama K: Expression of
hypoxia-inducible factor (HIF)-1alpha is associated with vascular
endothelial growth factor expression and tumour angiogenesis in
human oesophageal squamous cell carcinoma. Eur J Cancer.
40:1904–1912. 2004. View Article : Google Scholar
|
|
16
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res.
26:3579–3583. 2006.PubMed/NCBI
|
|
18
|
Ji Z, Long H, Hu Y, Qiu X, Chen X, Li Z,
Fan D, Ma B and Fan Q: Expression of MDR1, HIF-1alpha and MRP1 in
sacral chordoma and chordoma cell line CM-319. J Exp Clin Cancer
Res. 29:1582010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schweizer MS, Schumacher L and Rubin MA:
Constructing tissue microarrays for research use. Curr Protoc Hum
Genet. Chapter 10(Unit 10)7–Feb. 2004, PubMed/NCBI
|
|
20
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
21
|
Xiang ZL, Zeng ZC, Fan J, Tang ZY, Zeng HY
and Gao DM: Expression profiling of fixed tissues identified
hypoxia-inducible factor-1 alpha, VEGF, and matrix
metalloproteinase-2 as biomarkers of lymph node metastasis in
hepatocellular carcinoma. Clin Cancer Res. 17:5463–5472. 2011.
View Article : Google Scholar
|
|
22
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bussolino F, Mantovani A and Persico G:
Molecular mechanisms of blood vessel formation. Trends Biochem Sci.
22:251–256. 1997. View Article : Google Scholar
|
|
26
|
Chen KW, Yang HL, Lu J, Wang GL, Ji YM, Wu
GZ, Zhu LF, Liu JY, Chen XQ and Gu YP: Expression of vascular
endothelial growth factor and matrix metalloproteinase-9 in sacral
chordoma. J Neurooncol. 101:357–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Naka T, Kuester D, Boltze C, Schulz TO,
Samii A, Herold C, Ostertag H and Roessner A: Expression of matrix
metallo- proteinases-1, -2, and -9; tissue inhibitors of matrix
metalloproteinases-1 and -2; cathepsin B; urokinase plasminogen
activator; and plasminogen activator inhibitor, type I in skull
base chordoma. Hum Pathol. 39:217–223. 2008. View Article : Google Scholar
|
|
28
|
Naka T, Boltze C, Kuester D, Schulz TO,
Samii A, Herold C, Ostertag H and Roessner A: Expression of matrix
metalloproteinase (MMP)-1, MMP-2, MMP-9, cathepsin B, and urokinase
plasminogen activator in non-skull base chordoma. Am J Clin Pathol.
122:926–930. 2004. View Article : Google Scholar : PubMed/NCBI
|