Introduction
Endometrial cancer is the most frequent
gynecological malignancy in the world (1). Endometrial adenocarcinoma is the most
common type of diagnosed endometrial cancer, which originates in
glandular tissue and is characterized via a sequence of
hyperplastic changes in the endometrium; each with increasing
malignant potential. Endometrial adenocarcinoma is graded
histologically according to the International Federation of
Gynecology and Obstetrics. In this system, grade 1 designates a
well-differentiated tumor with a <5% solid growth pattern, grade
2 designates a moderately differentiated tumor of 5–50% solid
growth, and grade 3 designates a poorly differentiated tumor with
>50% solid tumor. Peroxiredoxins (Prxs), synonymous with
thioredoxin peroxidases, are a family of proteins, whose members
were initially identified as thiol-specific antioxidant enzymes
(2–4). Prxs are involved in the enzymatic
degradation of hydrogen peroxide, organic hydroperoxides and
peroxynitrite (5–7). They are found in a wide range of
organisms, including bacteria, plants and mammals, and are
classified into three major subclasses: typical 2-cysteine Prxs
(Prx I-IV), atypical 2-cysteine Prxs (Prx V) and 1-cysteine Prxs
(Prx VI). A number of studies have reported Prx overexpression in
various types of malignant cancer cells (8,9).
Therefore, we investigated the Prx isoforms (Prx I-IV) to determine
whether their expression is associated with cancer progression in
endometrial cancer.
Patients and methods
Patient follow-up and tissue array
material
Patient samples for tissue array were retrieved from
the files of the Department of Gynecologic Oncology at the
Kyung-Hee Medical Center. A total of 240 patients, who were
diagnosed with endometrial cancer by immunohistochemistry and
underwent surgery at the Kyung-Hee Medical Center for preinvasive
and invasive cervical cancer, were enrolled in this study. Each
sample was classified into 3 groups: normal endometrium (61
patients), endometrial hyperplasia (56 patients) and endometrial
cancer (123 patients) (Table
I).
 | Table IPatient samples analyzed by
immunohistochemistry. |
Table I
Patient samples analyzed by
immunohistochemistry.
| Histopathological
diagnosis | No. of cases |
|---|
| Normala | 61 |
| Hyperb | 56 |
| ECc | 123 |
| Total | 240 |
The study was performed with the informed consent of
the patients and with the approval of the local Ethics Committee of
the Kyung-Hee Medical Center in Korea.
Immunohistochemistry
The expression levels of the Prx isoforms were
determined by immunohistochemistry. The paraffin-embedded specimens
were fixed in 4% buffered formalin. The tissues were cut into 4 μm
sections, and attached on a glass slide. They were then
deparaffinized in xylene and rehydrated through ethanol and
distilled water, and the endogenous peroxidase was blocked with
0.3% hydrogen peroxide for 10 min. The tissues were then immersed
in 10 mM citric acid monohydrate (pH 6.0) for 8 min, and boiled in
a microwave oven at 850 W. The specimens were chilled on ice for 20
min. The samples were blocked by protein block, serum-free media
for 20 min. The specimens were incubated overnight at 4°C with a
monoclonal antibody against Prx I, II, III, IV, V or VI (Ab
Frontier, Korea) at a dilution of 1:500. The immunostained sections
were visualized using an EnVision Detection kit (Dako,
Denmark).
Statistical analysis
Statistical analysis was performed with SPSS for
Windows 11.5. The associations were determined using a two-tailed
t-test, Fisher’s exact probability test and Pearson’s correlation
test. Agreement of the double evaluation was calculated by Cohen’s
K-correlation analysis. Cumulative survival curves were plotted
according to the Kaplan-Meier method and the generalized log-rank
test was applied to compare the survival curves. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Prx I
To determine whether the expression level of Prx I
is associated with endometrial cancer development, we examined the
immunohistochemical expression of Prx I in endometrial cancer
tissue. For this assay, we used 42 tissue samples from patients
(normal sample, 11; endometrial hyperplasia, 11; endometrial
cancer, 20). The normal tissues were expressed as negative (45.5%)
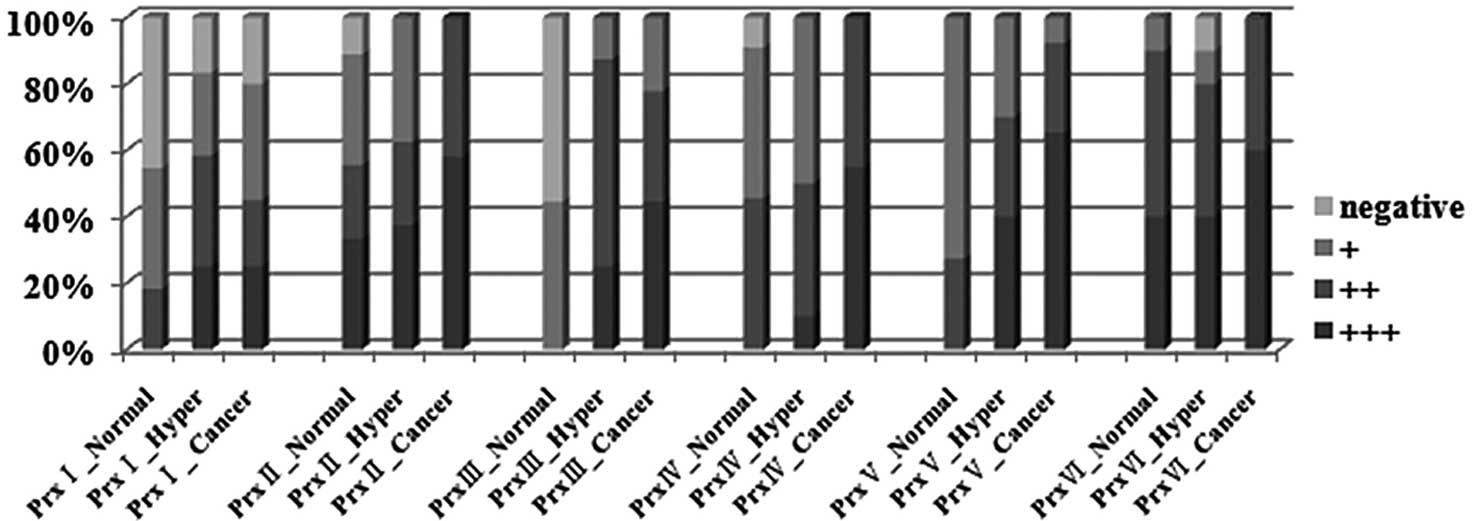
or weakly positive (36.4%) for Prx I (Figs. 1 and 2 and Table
II). In the endometrial hyperplasia samples, Prx I expression
levels were moderately positive (36.4%) or strongly positive
(27.3%). In the cancer samples, Prx I expression was strongly
positive (25%) or moderately positive (20%). However, 20 and 30% of
the endometrial cancer samples were negative and weakly positive
for Prx I, respectively (Figs. 1
and 2, Table II). Therefore, we did not find any
direct association between Prx I and endometrial cancer
progression.
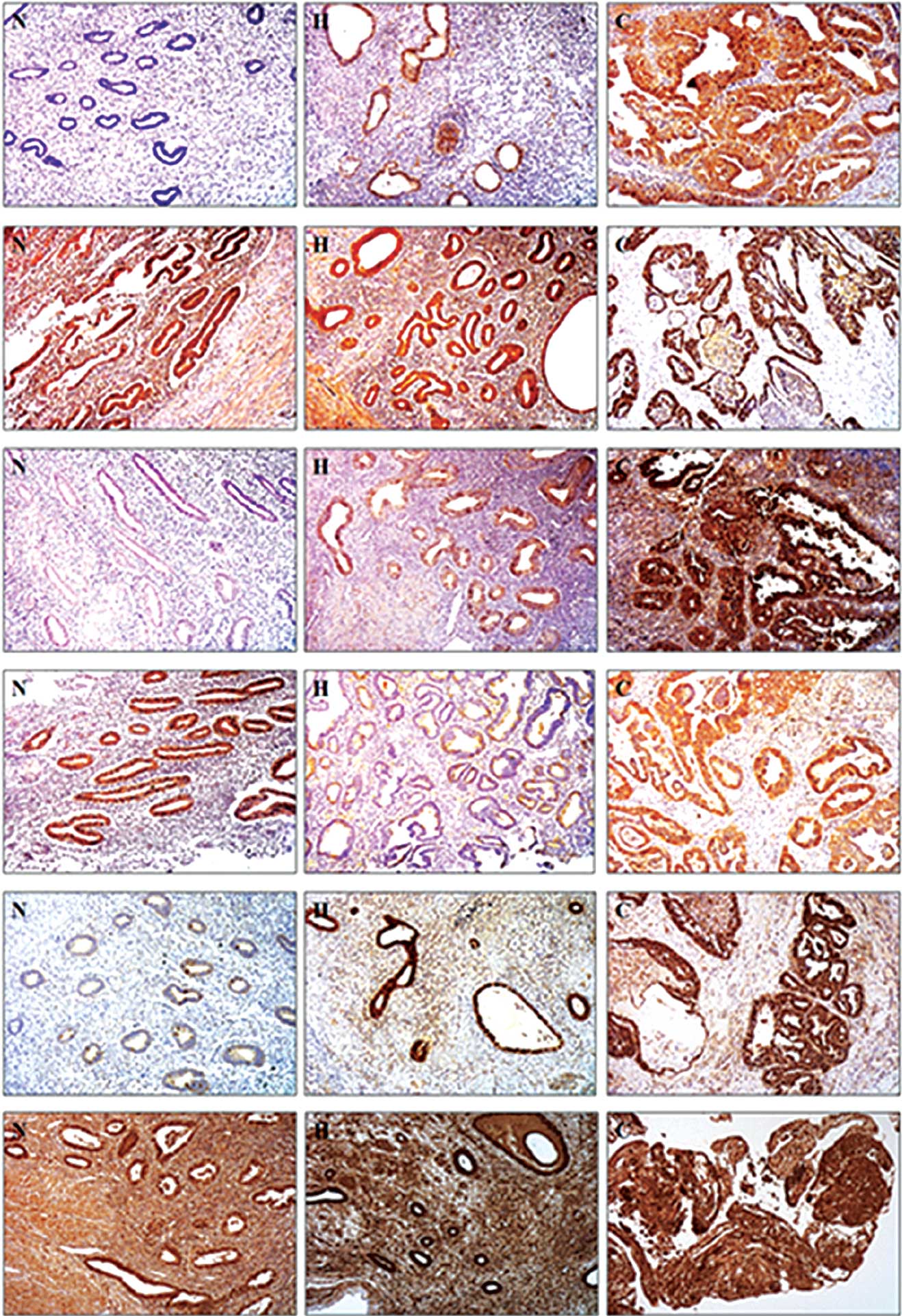 | Figure 1Immunohistochemical staining of Prx
isoforms in human endometrial tissues. (A) Prx I, (B) Prx II, (C)
Prx III, (D) Prx IV, (E) Prx V and (F) Prx VI. Each Prx isoform (A,
B, C, D, E and F) was immunostained in (N) normal endometrium, (H)
endometrial hyperplasia and (C) endometrial cancer samples. Prx,
peroxiredoxin. |
 | Table IIIntensity of Prx immunostaining in
normal, hyperplasia and endometrial cancer samples (%). |
Table II
Intensity of Prx immunostaining in
normal, hyperplasia and endometrial cancer samples (%).
| Prx isoform | Sample | − | + | ++ | +++ |
|---|
| I | Normala | 5/11 (45.5) | 4/11 (36.4) | 2/11 (18.2) | 0/11 (0.0) |
| Hyperb | 2/11 (18.2) | 3/11 (27.3) | 4/11 (36.4) | 3/11 (27.3) |
| Cancerc | 4/20 (20.0) | 7/20 (35.0) | 4/20 (20.0) | 5/20 (25.0) |
| II | Normala | 1/9 (11.1) | 3/9 (33.3) | 2/9 (22.2) | 3/9 (33.3) |
| Hyperb | 0/8 (0.0) | 3/8 (37.5) | 2/8 (25.0) | 3/8 (37.5) |
| Cancerc | 0/19 (0.0) | 0/19 (0.0) | 8/19 (42.1) | 11/19 (57.9) |
| III | Normala | 5/9 (55.6) | 4/9 (44.4) | 0/9 (0.0) | 0/9 (0.0) |
| Hyperb | 0/8 (0.0) | 1/8 (12.5) | 5/8 (62.5) | 2/8 (25.0) |
| Cancerc | 0/18 (0.0) | 4/18 (22.2) | 6/18 (33.3) | 8/18 (44.4) |
| IV | Normala | 1/11 (9.1) | 5/11 (45.5) | 5/11 (45.5) | 0/11 (0.0) |
| Hyperb | 0/10 (0.0) | 5/10 (50.0) | 4/10 (40.0) | 1/10 (10.0) |
| Cancerc | 0/20 (0.0) | 0/20 (0.0) | 9/20 (45.0) | 11/20 (55.0) |
| V | Normala | 0/11 (0.0) | 8/11 (72.7) | 3/11 (27.3) | 0/11 (0.0) |
| Hyperb | 0/10 (0.0) | 3/10 (30.0) | 3/10 (30.0) | 4/10 (40.0) |
| Cancerc | 0/26 (0.0) | 2/26 (7.7) | 7/26 (27) | 12/21 (65.3) |
| VI | Normala | 0/10 (0.0) | 1/10 (10.0) | 5/10 (50.0) | 4/10 (40.0) |
| Hyperb | 1/10 (10.0) | 1/10 (10.0) | 4/10 (40.0) | 4/10 (40.0) |
| Cancerc | 0/20 (0.0) | 0/20 (0.0) | 8/20 (40.0) | 12/20 (60.0) |
Prx II
Immunohistochemisty with Prx II antibody (normal
sample, 9; endometrial hyperplasia, 8; endometrial cancer, 19) was
performed. The endometrial cancer samples were strongly positive
(57.9%) or moderately positive (42.1%) for Prx II (Figs. 1 and 2 and Table
II). However, 33.3 and 22.2% of the normal samples also
demonstrated strongly positive and weakly positive expression
levels of Prx II, respectively (Figs.
1 and 2, Table II). Therefore, we concluded that
Prx II expression levels are not directly associated with
endometrial cancer development.
Prx III
We performed immunohistochemistry using the Prx III
antibody (normal sample, 9; endometrial hyperplasia, 8; endometrial
cancer, 18). In the normal tissues, 55.6 (p=0.004) and 44.4%
(p=0.001) of the samples demonstrated a negative and weak
expression of Prx III, respectively (Figs. 1 and 2 and Table
II). However, 62.5 (p=0.004) and 44.4% (p=0.0005) of the
samples were moderately positive for Prx III in endometrial
hyperplasia and endometrial cancer samples, respectively.
Additionally, 33% (p=0.001) of the endometrial cancer samples
demonstrated a strong Prx III expression (Figs. 1 and 2, Table
II). These results suggest that Prx III is at least associated
with endometrial cancer development.
Prx IV
Immunohistochemistry using the Prx IV antibody
(normal sample, 11; endometrial hyperplasia, 10; endometrial
cancer, 20) was performed. A total of 55% (p=0.001) of the
endometrial cancer samples were strongly positive, and 50%
(p=0.003) of the endometrial hyperplasia samples were weakly
positive for Prx IV (Figs. 1 and
2 and Table II). However, a moderately positive
(45.5%) and weakly positive (45.5%) expression of Prx IV was also
observed in the normal samples, suggesting that Prx IV is not
directly associated with endometrial cancer development.
Prx V
We performed immunohistochemistry using the Prx V
antibody (normal sample, 11; endometrial hyperplasia, 10;
endometrial cancer, 21). A total of 65.3% (p=0.002) of the
endometrial cancer samples were strongly positive for Prx V,
whereas 72.7% of the normal tissues were weakly positive (Figs. 1 and 2 and Table
II). In the endometrial hyperplasia samples, 40.0% were
strongly positive for Prx V (p=0.001) (Figs. 1 and 2, Table
II). These results suggest that Prx V is upregulated during
endometrial cancer development.
Prx VI
We performed immunohistochemistry using the Prx VI
antibody (normal sample, 10; endometrial hyperplasia, 10;
endometrial cancer, 20). The majority of samples, including normal
and endometrial cancer, were moderately or strongly positive for
Prx VI, indicating that there is no notable difference in Prx VI
expression between the normal and endometrial cancer samples
(Fig. 1 and Table II).
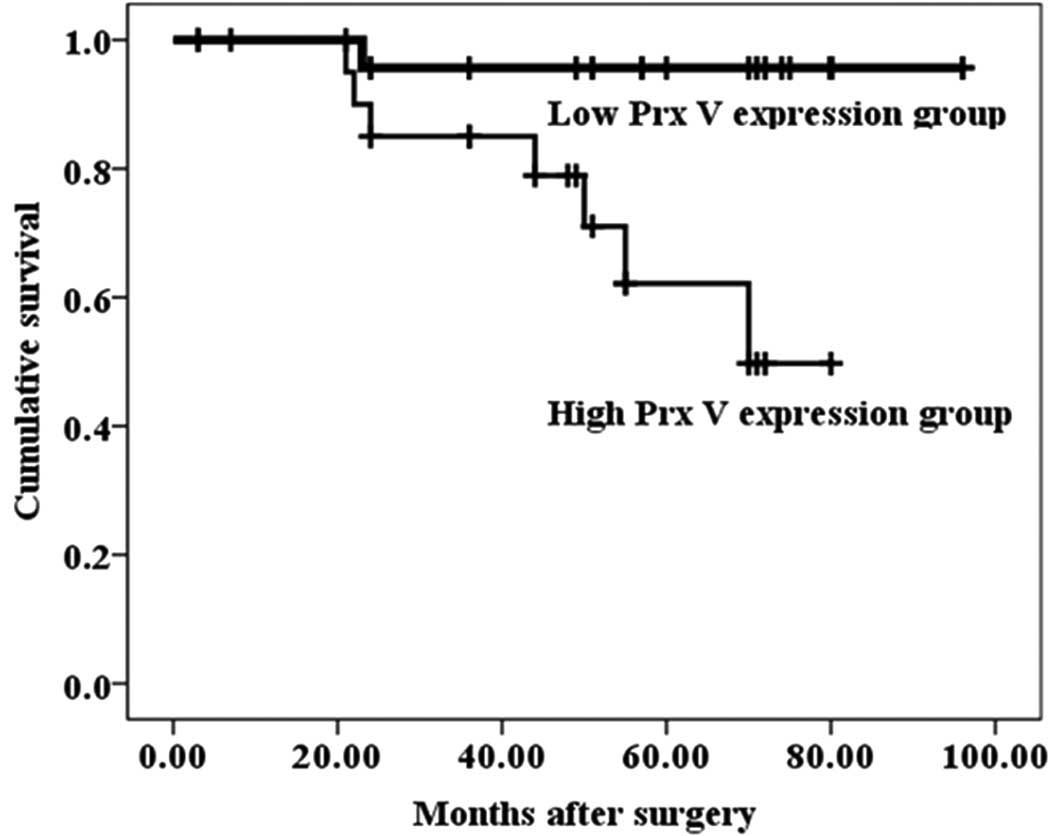
Survival analysis
We investigated whether the expression of the Prx
isoforms served as a prognostic biomarker for endometrial cancer by
using the follow-up data of surgically treated endometrial cancer
patients. The patients were followed up for a period of 96 months.
The Prx V expression group was divided into the low Prx V
expression group, which included the patients with a weak or
moderate Prx V expression, and the high Prx V expression group,
which included the patients with a strong Prx V expression, in
endometrial cancer samples. The survival rate and mean survival
length for the low Prx V expression group were 96.2% and 92.8
months, respectively. However, the survival rate and mean survival
length for the high Prx V expression group were 66.7% and 63.3
months, respectively. The Kaplan-Meier survival analysis
demonstrated that Prx V expression in endometrial cancer is
significantly associated with the survival rate (Fig. 3). When evaluated with the log-rank
test, the other Prx isoforms did not demonstrate a significant
association.
Discussion
To the best of our knowledge, few studies have been
conducted on all six Prx isoforms in various types of human cancer
(9,10). Prx III, IV and V are known to be
upregulated in breast malignancy, while in colorectal neoplasms,
Prx I, II, III and V are elevated (11), and recently, it was reported that
Prx III and IV are consistently upregulated in prostate cancer
(12). These studies suggest the
induction of Prx isoforms in response to increased free radicals in
cancer tissue.
For the first time, we assessed the expression
levels of Prx isoform members in endometrial cancer samples. Our
data have shown that Prx III and V were clearly elevated in the
majority of endometrial cancer cells as assessed by
immunohistochemistry (Figs. 1 and
2, Table II). Notably, Prx III and V were
highly expressed during endometrial cancer development, suggesting
that they may be used as tumor markers to predict the progression
of endometrial cancer. In accordance with our data, Prx III has
been known to be associated with the formation and development of
hepatocellular carcinoma (13).
Additionally, the preferential overexpression of Prx III was
reported in breast, colorectal and prostate cancer. Previously, we
reported the upregulated expression of Prx III in cervical cancer
development (14). In the present
study, we report that Prx V is upregulated in response to
endometrial cancer development, and that Prx V expression in
endometrial cancer is significantly associated with the patient
survival rate. Few studies on Prx V and cancer development are
currently available. There is, however, indirect evidence that Prx
V protects cells from oxidative stress. Prx V is known to prevent
the p53-dependent generation of reactive oxidative species (ROS)
and p53-induced apoptosis in the mouse cell line (15). Prx V was recently reported to be
highly expressed in immunostimulated macrophages (16). Although the protective role of Prx V
against ROS in endometrial cancer cells is assumed, more studies
are required to understand the pathophysiological meaning of
preferentially expressed Prx V over other types of Prx in
endometrial cancer, and its association with the survival rate.
In conclusion, we have demonstrated that Prx III and
V are preferentially overexpressed in human endometrial carcinoma,
and the expression levels are associated with tumor grade. In
addition, a high expression of Prx V correlates with a worse
survival rate. Based on these observations, we suggest that Prx V
is a potential prognosis marker for endometrial cancer.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Education, Science and Technology
(Nos. 2009-0072431 and 20090063274), and a grant from the
Next-Generation BioGreen 21 Program (No. PJ008086), Rural
Development Administration, Republic of Korea.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics. CA Cancer J Clin. 57:43–66.
2007.
|
|
2
|
Chae HZ, Kim IH, Kim K and Rhee SG:
Cloning, sequencing, and mutation of thiol-specific antioxidant
gene of Saccharomyces cerevisiae. J Biol Chem.
268:16815–16821. 1993.PubMed/NCBI
|
|
3
|
Chae HZ, Kim IH, Kim K and Rhee SG:
Cloning and sequencing of thiol-specific antioxidant from mammalian
brain: alkyl hydroperoxide reductase and thiol-specific antioxidant
define a large family of antioxidant enzymes. Proc Natl Acad Sci
USA. 91:7017–7021. 1994. View Article : Google Scholar
|
|
4
|
Chae HZ, Uhm TB and Rhee SG: Dimerization
of thiol-specific antioxidant and the essential role of cysteine
47. Proc Natl Acad Sci USA. 91:7022–7026. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofmann B, Hecht HJ and Flohe L:
Peroxiredoxins. Biol Chem. 383:347–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rhee SG, Kang SW, Netto LE, Seo MS and
Stadtman ER: A family of novel peroxidases, peroxiredoxins.
Biofactors. 10:207–209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wood ZA, Schröder E, Robin HJ and Poole
LB: Structure, mechanism and regulation of peroxiredoxins. Trends
Biochem Sci. 28:32–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang JW, Lee SH, Jeong JY, Chae HZ, Kim
YC, Park ZY and Yoo YJ: Peroxiredoxin-I is an autoimmunogenic tumor
antigen in non-small cell lung cancer. FEBS Lett. 579:2873–2877.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karihtala P, Mäntyniemi A, Kang SW,
Kinnula VL and Soini Y: Peroxiredoxins in breast carcinoma. Clin
Cancer Res. 9:3418–3424. 2003.PubMed/NCBI
|
|
10
|
Noh DY, Ahn SJ, Lee RA, Kim SW, Park IA
and Chae HZ: Overexpression of peroxiredoxin in human breast
cancer. Anticancer Res. 21:2085–2090. 2001.PubMed/NCBI
|
|
11
|
Wu XY, Fu ZX and Wang XH: Peroxiredoxins
in colorectal neoplasms. Histol Histopathol. 25:1297–1303.
2010.PubMed/NCBI
|
|
12
|
Basu A, Banerjee H, Rojas H, Martinez SR,
Roy S, Jia Z, Lilly MB, De León M and Casiano CA: Differential
expression of peroxiredoxins in prostate cancer: consistent
upregulation of PRDX3 and PRDX4. Prostate. 71:755–765. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi JH, Kim TN, Kim S, Baek SH, Kim JH,
Lee SR and Kim JR: Overexpression of mitochondrial thioredoxin
reductase and peroxiredoxin III in hepatocellular carcinomas.
Anticancer Res. 22:3331–3335. 2002.PubMed/NCBI
|
|
14
|
Kim KY, Yu MR, Han SH, Oh IK, Choi YJ, Kim
SS, Yoon KS, Jung MH and Choe W: Expression of human peroxiredoxin
isoforms in response to cervical carcinogenesis. Oncol Rep.
21:1391–1396. 2009.PubMed/NCBI
|
|
15
|
Zhou Y, Kok KH, Chun AC, Wong CM, Wu HW,
Lin MC, Fung PC, Kung H and Jin DY: Mouse peroxiredoxin V is a
thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochem
Biophys Res Commun. 268:921–927. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abbas K, Breton J, Picot CR, Quesniaux V,
Bouton C and Drapier JC: Signaling events leading to peroxiredoxin
5 up-regulation in immunostimulated macrophages. Free Radic Biol
Med. 47:794–802. 2009. View Article : Google Scholar : PubMed/NCBI
|