Introduction
Cellular senescence was first reported in cultured
human fibroblasts (1). Hayflick and
Moorhead observed permanent replication arrest in phase G1 of the
cell cycle following a certain number of replications, but without
loss of viability or metabolic capacity (1). This physiological limit is thought to
be a mechanism for avoiding cell immortality or preventing
prolongation of the replicative life of cells, which would increase
their susceptibility to gain successive mutations, and thus favour
their progression to malignancy (2). It was recently demonstrated that this
suppression of the proliferative capacity of cells may also be
triggered by oncogene-induced senescence (OIS) (2). Various studies of premalignant lesions
have proposed a role for OIS as an anti-oncogenic response in
vivo, describing senescent cells in premalignant lesions, but
not in tumours, and concluding that OIS may act as a barrier
against malignant transformation. Accordingly, only cells that lose
this senescent response are transformed from a benign state
(premalignant lesion) to malignancy (carcinoma). However, given the
complexity of the carcinogenic process, this does not appear to be
a cause-effect correlation, but rather a further expression of
carcinogenesis (2,3).
Three pathways have been reported to induce
senescence: the ARF/p53/p21 pathway, also involved in apoptosis;
the p16/Rb/E2F pathway, more specifically related to senescence;
and a third, less well-documented pathway via retinoic acid
receptors (4). The expression of
molecular markers of these pathways in different types of
carcinomas have been studied and correlated with prognosis. To test
the hypothesis that OIS acts as an anti-carcinogenic barrier in the
premalignant stage, we compared the expression of these markers
between premalignant and malignant oral lesions. The potential
value of findings would be to offer a basis for predicting the
malignant transformation of a premalignant lesion (e.g.,
leukoplakia or erythroplakia), and to identify senescence markers
that may serve as therapeutic targets, using senescence as a
physiological anti-tumour mechanism, by increasing their expression
in premalignant oral lesions.
Thus, the objectives of this study were to
characterize the expression of various markers involved in the main
senescence pathways in oral leukoplakia lesions, and to compare
this expression with that of the markers in oral squamous cell
carcinomas (OSCCs). We selected markers studied in other types of
carcinomas for which, however, little data are available at the
oral level, despite the fact that worldwide over half of the
500,000 new cases of head and neck cancer per year are in the oral
cavity, with a five-year survival rate of only 55% (5). Immunohistochemistry was performed on
tissue microarray (TMA) sections to study cyclin D1, maspin and Rb
for the specific senescence pathway p16-pRb; and p53 and mouse
double minute 2 (MDM2) for the apoptosis/senescence ARF-p53
pathway.
Materials and methods
Study subjects
TMA analysis was conducted in 20 samples of normal
oral mucosa (NOM), 14 of leukoplakia without dysplasia (OLD−), 11
of leukoplakia with dysplasia (OLD+) and 15 of OSCC.
Paraffin-embedded samples were provided by the Gregorio Marañon
Hospital in Madrid, the School of Dentistry of Madrid Complutense
University and the Department of Surgical Sciences of the
University of Foggia. Data were gathered from patient clinical
records with respect to their age, gender, medication, systemic
disease, tobacco use, personal history of oral cancer and on the
site from which the sample derived. Information was also collected
on the degree of dysplasia (mild/moderate/severe) in cases of OLD+,
and on the clinical/histological type and TNM classification in
OSCC cases (International Union Against Cancer criteria).
Data confidentiality was maintained by replacing all
patient identifiers with numerical codes. Informed consent was
obtained from all participating patients, and the use of biopsied
tissue was approved by the Ethics Committees of the two
institutions.
TMA construction
TMA was constructed from the anonymous samples at
the Spanish National Cancer Research Centre. The orientation of the
sample in the paraffin block was determined by hematoxylin-eosin
staining. A specialist in oral pathology identified the most
characteristic epithelial areas of oral dysplasia or carcinoma
based on optical microscope observations. The same pathologist
labelled two representative areas of each sample for the extraction
of two cylinders for the TMA. Labelling was conducted in paraffin
blocks and hematoxylin and eosin-stained slides. The pathologist
was blinded to the clinical data of the corresponding patients.
The TMA was then constructed. The array receptors
were paraffin blocks that were previously smoothed to ensure that
cylinders had the same angle of entry. Samples were introduced in
duplicate into a single TMA, and were ordered following a random
number program to minimize immunostaining bias. The TMA was
prepared with 120 cylinders corresponding to 60 patients. Samples
of pancreas, tonsil, kidney and lung were also added in duplicate
as internal controls, and an additional tonsil sample was included
for the TMA orientation. The construction was conducted using a
manual TMA apparatus (Beecher Instruments, Sun Prairie, WI, USA).
Each cylinder had a diameter of 1 mm and was placed, using digital
micrometry, 1.5 mm from its neighbors. Following completion of the
cylinder transfer, the TMA was incubated for 6 min at 64°C to
facilitate fusion of the cylinders with the paraffin. They were
subsequently cooled and cut to a thickness of 3 microns. One
section was stained with hematoxylin/eosin, and the remainder
underwent immunohistochemical analysis for each study protein.
Immunohistochemical analysis
The immunoreaction of all study proteins was
analysed. First, samples were deparaffinised and hydrated. A HIER
(Heat-Induced Epitope Retrieval) stage was conducted in a sodium
citrate buffer solution with 0.01 M trisodium citrate solution or 1
mM TE (Tris-EDTA) at pH 8.0. It was then heated for 20 min at 98°C
in a PT Link (Dako, Carpenteria, CA, USA) oven. Following heating,
sections were rinsed in cold running water for 5 min, then passed
through Tris-buffered saline (TBS) at pH 7.4. The sections were
then incubated with the corresponding antibodies, i.e., SP4 for
cyclin D1 (NeoMarkers Fremont, CA, USA), polyclonal rabbit for
maspin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), G3-245BD
for Rb (BD PharMingen, San Diego, CA, USA), DO-7 for p53
(Novocastra, Burlingame, CA, USA), IF2 for MDM2 (Calbiochem,
Darmstadt, Germany) and MIB 1 for Ki-67 (Dako, Glostrup, Denmark).
Following incubation with the primary antibodies, EnVision-FLEX
(Dako) was used in Dako Autostainer Plus Link automatic equipment
for the immunodetection of all markers with the exception of
maspin, using diaminobenzidine chromogen as the substrate. Maspin
was visualized by using the Leica Vision Bio System with a Leica
BOND MAX immunostainer. Maspin antigen retrieval was performed with
EDTA, using the same immunostainer. For the controls, incubations
were performed with omission of the specific antibodies and with
the inclusion of unrelated antibodies.
TMA analysis
Cylinder sections with no epithelium or containing
<50% of the original tissue were considered as
non-representative samples and excluded from the immunostaining
analysis. The degree of staining was determined in all cylinders
remaining intact following the immunohistochemistry by an optical
microscopy study (magnification, ×10 and ×100) of each cylinder.
The staining was conducted by two members of the team, blinded at
all times to the clinical data corresponding to the samples. A
sample was only excluded when both cylinders were
non-assessable.
Statistical analysis
Stat View v.5.0.1 (SAS Institute, Inc., NC, USA) and
Statgraphics Plus v. 5.1 (Statistical Graphics Corp., Oswego, NY,
USA) were used for the statistical analyses. The Chi-square test
and Fisher’s exact test (if N<5) were used for the the
comparison of staining among clinical stages and to establish
differences in the studied proteins among the study groups (NOM,
OLD−, OLD+ and OSCC). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological characteristics
Table I shows the
clinicopathological characteristics of the patients. No
significance was observed in patient characteristics, such as age,
gender, smoking status and alcohol consumption among the four study
groups.
 | Table IClinicopathological characteristics of
the patients by study group (NOM, OLD−, OLD+ and OSCC). |
Table I
Clinicopathological characteristics of
the patients by study group (NOM, OLD−, OLD+ and OSCC).
| NOM | OLD− | OLD+ | OSCC |
|---|
|
|
|
|
|
|---|
| Variable | No. | % | No. | % | No. | % | No. | % |
|---|
| Age |
| Mean (years) | 50.6 (15–80) | | 55.7 (26–76) | | 67.9 (42–86) | | 67.8 (48–87) | |
| Gender |
| Male | 12 | 60 | 8 | 57.1 | 4 | 36.4 | 10 | 66.7 |
| Female | 8 | 40 | 6 | 42.9 | 7 | 63.6 | 5 | 33.3 |
| Tobacco |
| Yes | 7 | 35 | 10 | 71.4 | 6 | 54.6 | 13 | 86.7 |
| No | 13 | 65 | 4 | 28.6 | 5 | 45.4 | 2 | 13.3 |
| Alcohol |
| Yes | 4 | 20 | 5 | 35.7 | 3 | 27.3 | 9 | 60 |
| No | 16 | 80 | 9 | 64.3 | 8 | 72.7 | 6 | 40 |
| Localization |
| Gingiva | 20 | 100 | 2 | 14.3 | 0 | 0 | 2 | 13.3 |
| Tongue and
floor | 0 | 0 | 11 | 78.6 | 5 | 45.5 | 8 | 53.3 |
| Other | 0 | 0 | 1 | 7.1 | 6 | 54.5 | 5 | 33.4 |
| Dysplasia |
| Mild | - | - | - | - | 6 | 54.5 | - | - |
| Moderate | - | - | - | - | 1 | 9.1 | - | - |
| Severe | - | - | - | - | 4 | 36.4 | - | - |
| T
classification |
| T1 | - | - | - | - | - | - | 7 | 46.6 |
| T2 | - | - | - | - | - | - | 6 | 40 |
| T3 | - | - | - | - | - | - | 1 | 6.7 |
| T4 | - | - | - | - | - | - | 1 | 6.7 |
| N
classification |
| N0 | - | - | - | - | - | - | 9 | 60 |
| N1 | - | - | - | - | - | - | 2 | 13.3 |
| N2 | - | - | - | - | - | - | 4 | 26.7 |
| N3 | - | - | - | - | - | - | 0 | 0 |
| Clinical stage |
| I | - | - | - | - | - | - | 5 | 33.33 |
| II | - | - | - | - | - | - | 2 | 13.33 |
| III | - | - | - | - | - | - | 3 | 20 |
| IV | - | - | - | - | - | - | 5 | 33.33 |
| Histological
type |
| G1 | - | - | - | - | - | - | 3 | 20 |
| G2 | - | - | - | - | - | - | 8 | 53.3 |
| G3 | - | - | - | - | - | - | 4 | 26.7 |
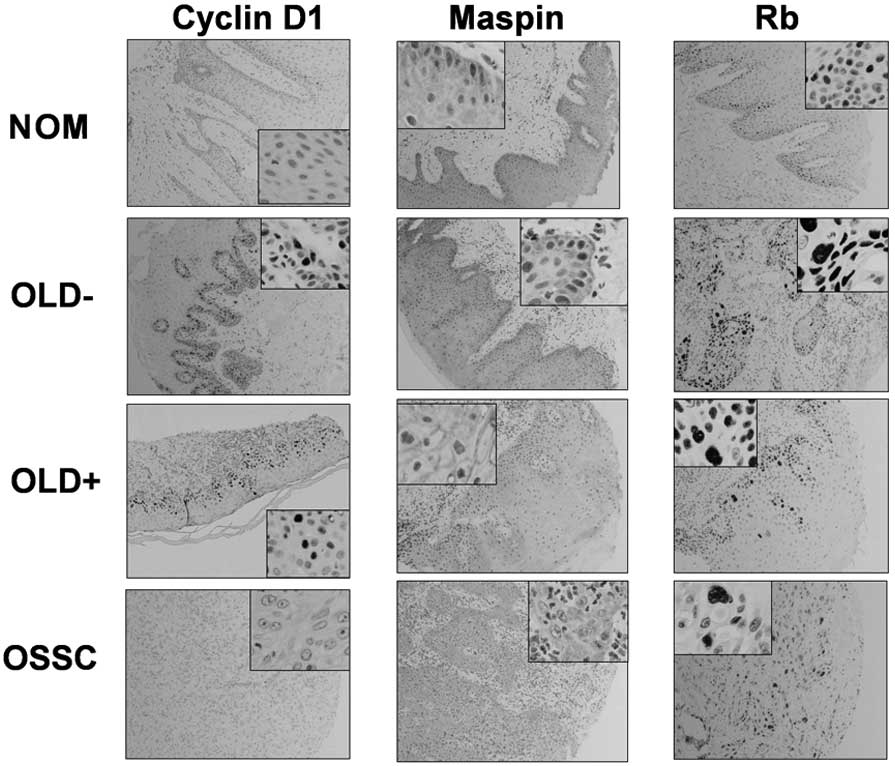
p16-pRb pathway
Fig. 1 shows
examples of the expression of the markers (cyclin D1, maspin and
Rb) of the senescence-specific pathway for each study group (NOM,
OLD−, OLD+ and OSCC). Table II
shows the immunohistochemistry results, expressed as positively or
negatively stained samples. Statistical comparisons were performed
between the following groups: NOM vs. OLD−; OLD vs. OLD+; OLD+ vs.
OSCC and NOM vs. OSCC. Expression of the three markers of this
pathway was more frequent in OLD+ vs. OSCC samples, but only the
increase in maspin was statistically significant (P=0.036). Cyclin
D1 expression was occurred more frequently in the OLD+ group
(54.5%), whereas maspin and Rb expression occurred more frequently
the OLD− group (100 and 92.8%, respectively).
 | Table IIExpression of p16-pRB senescence
pathway markers. |
Table II
Expression of p16-pRB senescence
pathway markers.
| Type of lesion | Cyclin D1 | Maspin | Rb |
|---|
|
|
|
|---|
| Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| NOM |
| (n=20) | 0 (0%) | 20 (100%) |
0.021a | 19 (95%) | 1 (5%) | 1.000a | 13 (65%) | 6 (30%) | 0.102a |
| OLD− |
| (n=14) | 4 (28.5%) | 9 (64.3%) | 0.241b | 14 (100%) | 0 (0%) | 0.440b | 13 (92.8%) | 1 (7.1%) | 1.000b |
| OLD+ |
| (n=11) | 6 (54.5%) | 4 (36.3%) | 0.697c | 10 (91%) | 0 (0%) |
0.036c | 10 (90.9%) | 0 (0%) | 0.197c |
| OSCC |
| (n=15) | 7 (46.6%) | 8 (53.3%) |
<0.001d | 7 (46.6%) | 8 (53.3%) |
0.002d | 10 (66.6%) | 5 (33.3%) | 0.919d |
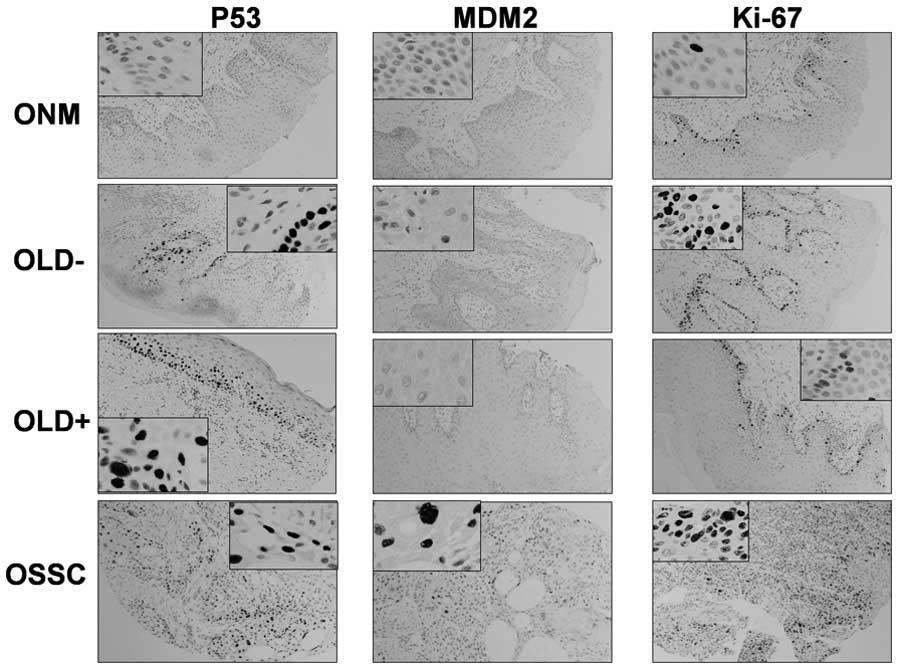
ARF-p53 pathway
Fig. 2 shows
examples of the expression of markers (p53, MDM2 and cell
proliferation marker, Ki-67) of the senescence/apoptosis pathway.
Table III shows the immuno-
histochemistry results (positively and negatively stained samples).
The same statistical comparisons were performed as for the p16-pRb
pathway. There was a tendency for a more frequent expression of p53
and MDM2 in the OLD− group (57.1 and 35.7%, respectively) and in
the OSCC group (73 and 26.6%, respectively), and for a less
frequent expression in the OLD+ group (45.4 and 0%, respectively),
than that in the NOM group (10 and 0%, respectively). The
expression of these markers was significantly more frequent in the
OLD− group than in the NOM group, as also observed for the
proliferation marker, Ki-67.
 | Table IIIExpression of ARF-p53 senescence
pathway markers. |
Table III
Expression of ARF-p53 senescence
pathway markers.
| Type of lesion | p53 | MDM2 | Ki-67 |
|---|
|
|
|
|---|
| Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| NOM |
| (n=20) | 2 (10%) | 18 (90%) |
0.006a | 0 (0%) | 20 (100%) |
0.007a | 1 (5%) | 19 (95%) | <0.001a |
| OLD− |
| (n=14) | 8 (57.1%) | 6 (42.8%) | 1.000b | 5 (35.7%) | 9 (64.3%) | 0.053b | 10 (71.4%) | 3 (21.4%) | 1.000b |
| OLD+ |
| (n=11) | 5 (45.4%) | 5 (45.4%) | 0.397c | 0 (0%) | 10 (90.9%) | 0.124c | 7 (63.6%) | 4 (27.2%) | 1.000c |
| OSCC |
| (n=15) | 11 (73%) | 4 (26.6%) |
<0.001d | 4 (26.6%) | 11 (73%) |
0.026d | 10 (66.6%) | 5 (33.3%) | <0.001d |
Discussion
Cellular senescence and apoptosis are considered
powerful tumour suppressor mechanisms through their control of the
proliferative potential of cells (3). Cellular senescence, or permanent
arrest of the replicative capacity of cells, has been demonstrated
in vitro (1) and in
vivo. Animal studies have demonstrated the presence of
senescent cells in premalignant epithelial lesions, but not in
carcinomas of the same tissues (3,6–8), thus
supporting the anti-tumour function of OIS. Various studies have
been published on senescence markers in human epithelia, but few at
the oral level, and little data are available on precancerous
lesions (9,10). The study of premalignant lesions is
of particular relevance due to the potential of applying preventive
measures.
Analysis of different proteins in the same samples,
using TMAs, is valuable to determine whether alterations have a
synergistic or cooperative effect on carcinogenesis. Alteration of
the p16-pRb pathway is regarded as an early event in dysplasia
acquisition and that the involvement of the two pathways, pRb and
p53, is associated with malignant transformation and a poor
prognosis in oral carcinoma (10).
Our detection of alterations in the two pathways in premalignant
lesions indicates that these are early events. Our results also
confirm the anti-tumour function of senescence, since the
expression of markers of the specific senescence p16-pRb pathway
was higher in the precancerous lesions than in the carcinoma ones.
Expression findings indicate an increase in the function of these
markers in premalignant lesions in comparison to healthy tissue,
and a decrease in carcinomas. Our findings for the ARF-p53 pathway
also suggest there is not only increased proliferation (Ki-67
expression) during oral carcinogenesis, but also activation of p53-
and MDM2-mediated senescence programmes.
Regarding the senescence markers studied, p53 is a
widely studied tumour suppressor oncogene (11), whose mutation or loss has been
reported in half of all human cancers. In the remaining tumours,
p53 maintains its wild-type status, but its function is inhibited
by the MDM2 oncoprotein (12).
Certain studies have correlated the overexpression of p53 to
angiogenesis and tumour recurrence (13). Results from the present study are
consistent with this correlation, since p53 expression increased
from 45.4% in OLD+ to 73% in carcinomas. MDM2 acts as a
p53-negative regulator, which maintains p53 at appropriate levels
of expression, and as in the case of p53, published findings have
been controversial (14). The
expression of the polymorphism SNP 309 has been analysed and
revealed to increase the transcription, and therefore
overexpression of MDM2, leading to the consequent suppression of
p53 (14). Few studies are
available regarding this polymorphism in oral carcinomas, and not
all of these reports have found an association with increased
cancer risk (14,15). Modulators of the p16-pRb senescence
pathway, cyclin D1 and Rb, control the progression of the cell
cycle from G to S phase, and alterations in these proteins have
been reported in oral epithelial dysplasia lesions (10). The hypophosphorylated state of pRb
is essential for progression of the cell cycle to the S phase. An
absence of pRb expression was found in a high percentage of
premalignant oral lesions (64%) and oral carcinomas (70%), which is
attributed to the loss of the cycle-regulating functions linked to
this protein (16). In the present
study, we analysed non-phosphorylated Rb and found, as predicted,
lower expression in the cancerous stage, in accordance with other
studies (17). Overexpression of
cyclin D1 has been reported in oral cancer (18) and even in premalignant lesions
(16) as being related to poor
prognosis in the majority of studies (18–20).
However, a study attempting to explain this overexpression by
analysing two polymorphisms, A870G and C1722G (21), did not yield conclusive results.
In the present study, significant differences were
only observed for maspin, thus indicating its potential role as a
prognostic marker in oral precancer. Maspin is a tumour suppressor,
which inhibits tumour-induced angiogenesis and tumour cell
motility, invasion and metastasis (22). Studies on maspin have almost
exclusively investigated established malignant lesions and have
published contradictory results. Certain studies revealed no
correlation between protein expression and prognosis (23), while other studies correlated its
overexpression with a better (22,24) or
worse (9) prognosis of carcinoma
lesions. These differences may be in part due to the fact that
maspin is primarily expressed in the cytoplasm, but may also be
observed in the nucleus, secretory vesicles and cell surface, thus
this subcellular partition may affect the function of this protein
(22). It is also possible that the
role of maspin differs according to the cell type. However, the
majority of studies of OSCC (22,24),
including the present study, indicate that maspin has a protective
role against the oncogenic process, although certain investigators
found no correlation (23) or even
reached the opposite conclusion (9).
Our observation of a higher frequency of expression
of three markers of the p16-pRb pathway (cyclin D1, Rb and maspin)
in leukoplakia lesions with dysplasia compared to OSCC lesions
appears to corroborate the tumour suppressor role of cell
senescence. However, only maspin demonstrated statistically
significant differences, supporting its value as a prognostic
marker in oral precancer. The expression of markers of the p53-ARF
senescence pathway (p53 and MDM2) was significantly higher in
leukoplakia than in healthy oral mucosa, suggesting a correlation
of these early markers with early events in oral
carcinogenesis.
In conclusion, only maspin showed differences in its
expression between precancerous and malignant lesions. However, the
results of this preliminary cross-sectional investigation should be
confirmed and expanded by studies with larger sample sizes that
consider a wider range of senescence pathway markers, including
P16, P14, P15, P21, DCR2, DEC1 and
senescence-associated-beta-galactosidase.
Acknowledgements
This study was funded by the Fundación Mutua
Madrileña, 5251146, Madrid, Spain.
References
|
1
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohtani N, Mann DJ and Hara E: Cellular
senescence: its role in tumor suppression and aging. Cancer Sci.
100:792–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collado M, Gil J, Efeyan A, Guerra C,
Schuhmacher AJ, Barradas M, et al: Tumour biology: senescence in
premalignant tumours. Nature. 436:6422005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nickoloff BJ, Lingen MW, Chang BD, Shen M,
Swift M, Curry J, et al: Tumor suppressor maspin is up-regulated
during keratinocyte senescence, exerting a paracrine antiangiogenic
activity. Cancer Res. 64:2956–2961. 2004. View Article : Google Scholar
|
|
5
|
Parkin DM, Pisani P and Ferlay J: Global
cancer statistics. CA Cancer J Clin. 49:33–64. 1999. View Article : Google Scholar
|
|
6
|
Chen Z, Trotman LC, Shaffer D, Lin HK,
Dotan ZA, Niki M, et al: Crucial role of p53-dependent cellular
senescence in suppression of Pten-deficient tumorigenesis. Nature.
436:725–730. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michaloglou C, Vredeveld LC, Soengas MS,
et al: BRAFE600-associated senescence-like cell cycle arrest of
human naevi. Nature. 436:720–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Braig M, Lee S, Loddenkemper C, Rudolph C,
Peters AH, Schlegelberger B, et al: Oncogene-induced senescence as
an initial barrier in lymphoma development. Nature. 436:660–665.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vered M, Allon I and Dayan D: Maspin, p53,
p63, and Ki-67 in epithelial lesions of the tongue: from
hyperplasia through dysplasia to carcinoma. J Oral Pathol Med.
38:314–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soni S, Kaur J, Kumar A, Chakravarti N,
Mathur M, Bahadur S, et al: Alterations of Rb pathway components
are frequent events in patients with oral epithelial dysplasia and
predict clinical outcome in patients with squamous cell carcinoma.
Oncology. 68:314–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itahana K, Dimri G and Campisi J:
Regulation of cellular senescence by p53. Eur J Biochem.
268:2784–2789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shangary S, Qin D, McEachern D, Liu M,
Miller RS, Qiu S, et al: Temporal activation of p53 by a specific
MDM2 inhibitor is selectively toxic to tumors and leads to complete
tumor growth inhibition. Proc Natl Acad Sci USA. 105:3933–3938.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lavertu P, Adelstein DJ, Myles J and Secic
M: P53 and Ki-67 as outcome predictors for advanced squamous cell
cancers of the head and neck treated with chemoradiotherapy.
Laryngoscope. 111:1878–1892. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tu HF, Chen HW, Kao SY, Lin SC, Liu CJ and
Chang KW: MDM2 SNP 309 and p53 codon 72 polymorphisms are
associated with the outcome of oral carcinoma patients receiving
postoperative irradiation. Radiother Oncol. 87:243–252. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SF, Chen IH, Liao CT, Wang HM, Liou
SH and Hsieh LL: Combined effects of MDM2 SNP 309 and p53 mutation
on oral squamous cell carcinomas associated with areca quid
chewing. Oral Oncol. 45:16–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campo-Trapero J, Cano-Sánchez J,
Palacios-Sánchez B, Sánchez-Gutiérrez JJ, González-Moles MA and
Bascones-Martínez A: Update on molecular pathology in oral cancer
and precancer. Anticancer Res. 28:1197–1205. 2008.PubMed/NCBI
|
|
17
|
El-Naggar AK, Lai S, Clayman GL, Zhou JH,
Tucker SA, Myers J, et al: Expression of p16, Rb, and cyclin D1
gene products in oral and laryngeal squamous carcinoma: biological
and clinical implications. Hum Pathol. 30:1013–1018. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu HS, Lu HH, Lui MT, Yu EH, Shen W, Chen
YP, et al: Detection of copy number amplification of cyclin D1
(CCND1) and cortactin (CTTN) in oral carcinoma and oral brushed
samples from areca chewers. Oral Oncol. 45:1032–1036. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Myo K, Uzawa N, Miyamoto R, Sonoda I, Yuki
Y and Amagasa T: Cyclin D1 gene numerical aberration is a
predictive marker for occult cervical lymph node metastasis in TNM
stage I and II squamous cell carcinoma of the oral cavity. Cancer.
104:2709–2716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Vicente JC, Herrero-Zapatero A, Fresno
MF and López-Arranz JS: Expression of cyclin D1 and Ki-67 in
squamous cell carcinoma of the oral cavity: clinicopathological and
prognostic significance. Oral Oncol. 38:301–308. 2002.PubMed/NCBI
|
|
21
|
Sathyan KM, Nalinakumari KR, Abraham T and
Kannan S: CCND1 polymorphisms (A870G and C1722G) modulate its
protein expression and survival in oral carcinoma. Oral Oncol.
44:689–697. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marioni G, Gaio E, Giacomelli L, Bertolin
A, D’Alessandro E, Stramare R, et al: Maspin subcellular
localization and expression in oral cavity squamous cell carcinoma.
Eur Arch Otorhinolaryngol. 265:S97–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho JH, Kim HS, Park CS, Kim JK, Jung KY,
Shin BK, et al: Maspin expression in early oral tongue cancer and
its relation to expression of mutant-type p53 and vascular
endothelial growth factor (VEGF). Oral Oncol. 43:272–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yasumatsu R, Nakashima T, Hirakawa N,
Kumamoto Y, Kuratomi Y, Tomita K, et al: Maspin expression in stage
I and II oral tongue squamous cell carcinoma. Head Neck.
23:962–966. 2001. View
Article : Google Scholar : PubMed/NCBI
|