Introduction
The B-type erythropoietin-producing hepatocellular
(EphB) receptor tyrosine kinases and their ephrin ligands
(ephrin-Bs) are involved in crucial aspects of the embryologic
development and differentiation of the nervous system (1). However, the activities of EphB
receptors and ephrin-Bs are not restricted to the neural system.
Evidence suggests that certain members of the EphB family and their
ligands are important in angiogenesis and oncogenesis (2–5). EphB2
and EphB3/ephrin-B signalling regulates cell sorting and cell
migration through contact-mediated cell repulsion (6,7).
Overexpression of EphB2, EphB4 and ephrin-B1 has been described in
gastric, colon and breast cancers (8–12).
Although the ephrin-Eph system plays a number of roles in malignant
tumors, to the best of our knowledge, little is known regarding its
expression and role in pancreatic cancer. In the present study, we
investigated the expression patterns of ephrin-B and EphB mRNA in a
series of human pancreatic cancers and assessed the clinical
significance of the gene expression to clinicopathological
characteristics and patient prognosis.
Patients and methods
Patients
Cancer specimens from 46 patients with pancreatic
cancer were obtained following partial duodenopancreatectomy
(Whipple resection) at The First Affiliated Hospital of Xi’an
Jiaotong University (Shaanxi, China), after obtaining informed
consent from patients and approval by the ethics committee of the
university. Specimens were divided into two sections. One was fixed
in neutral-buffered formaldehyde and processed for
histopathological evaluation and the other section was immediately
frozen in liquid nitrogen and stored at −80°C until use for the
EphB and ephrin-B mRNA extraction and subsequent real-time
quantitative reverse transcription PCR (qRT-PCR). Two normal
pancreases, one pancreatic pseudocyst and one serous cystadenoma
were used as non-malignant controls.
Between Feburary 2000 and September 2004, 46
patients at The First Affiliated Hospital were consecutively
diagnosed with pancreatic cancer and underwent potentially curative
resection of the pathologically confirmed adenocarcinoma of the
pancreas with negative resection margins (R0). The patients
received gemcitabine at 1,000 mg/m2 intravenously (IV)
over 100 min every 2 weeks for 6 cycles. All 46 patients had a
Karnofsky performance status of ≥60 and adequate hematologic, renal
and hepatic function as defined by a standard protocol in our
department. Follow-up occurred at 3-month intervals for 1 year,
then 6-month intervals for 3 years and yearly thereafter. The last
date of patient follow-up was October 2007. Follow-up consisted of
physical examination, complete blood cell count, liver function
testing, chest X-ray and CT scan as clinically indicated. Tumor
stages were classified according to the American Joint Committee on
Cancer (AJCC) classification (http://www.cancerstaging.org/). Pain was assessed in
all patients prior to surgery using a standardized questionnaire as
previously described (13).
Quantitative RT-PCR analysis of the EphB
and ephrin-B expression
Total RNA was extracted from frozen samples with
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). DNaseI-treated
total RNA (10 μg) was used for reverse transcription with
Superscript II (Invitrogen). The resulting cDNA products were
amplified using a LightCycler instrument. The total 10 μl of
reaction mixture consisted of a master mixture (LightCycler DNA
master hybridization probes; Roche, Mannheim, Germany), 4.0 mM
MgCl2, 0.25 μl of EphB, ephrin-B or β-actin
primers, 0.4 μM of each probe and 1 μl of template
cDNA in a LightCycler capillary. The primers were designed using
published sequences and are shown in Table I.
 | Table IPrimers used in quantitative reverse
transcription PCR. |
Table I
Primers used in quantitative reverse
transcription PCR.
| Gene (Acc. No.) | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| EphB2
(NM_004442) |
GAAGGAGCTCAGTGAGTACAACG |
GCACCTGGAAGACATAGATGG |
| Ephrin-B2
(NM_004093) |
GCAAGTTCTGCTGGATCAAC |
AGGATGTTGTTCCCCGAATG |
| β-actin
(NM_001101) |
TCACCCACACTGTGCCCATCTACGA |
CGGAACCGCTCATTCGCCAATGG |
For EphB and ephrin-B amplification, 95°C (90 sec)
for the hot start was followed by 42 rounds of amplification at
95°C (0 sec) for denaturation, 50°C (10 sec) for annealing and 72°C
(10 sec) for extension, with a temperature slope of 20°C/sec,
performed in the LightCycler. The same temperature profile was used
for all experiments, except for an annealing temperature of 55°C
for β-actin. External standards for EphB and ephrin-B mRNA and
β-actin were known concentrations of MCF7 cell line (human breast
cancer cell line) RNA prepared by 10-fold serial dilutions. Each
run consisted of five external standards, a negative control
without a template and the patient samples. Relative mRNA in each
sample was then automatically quantitated by reference to the
standard curve constructed each time according to the LightCycler
software.
Statistical analysis
Statistical analysis was performed using the SPSS-PC
package (version 13.0; SPSS, Chicago, IL, USA). The χ2
and Fisher’s exact tests were used to analyze the association
between the expression of the EphB and ephrin-B genes, measured by
qRT-PCR, and the clinicopathological features of pancreatic cancer.
The thresholds (upper quartiles) were defined as positive or
overexpression of the genes. Disease-free and overall survival
endpoints were analyzed. Survival time was calculated from the date
of diagnosis until mortality (for overall survival) or, if the
patient was still alive, until the last follow-up visit
(disease-free and overall survival). Local, regional or distant
tumor progression was taken into account as adverse events for
disease-free survival. Mortality from any cause was considered for
overall survival. The Kaplan-Meier method with the log-rank test
was used to calculate survival rates and differences in survival
curves. The Cox proportional hazards regression model with a
stepwise procedure was used to analyze the simultaneous effect of
prognostic factors. P<0.05 was considered to indicate a
statistically significant result.
Results
Gene expression
Initially, the expression of 7 EphB and ephrin-B
genes (EphB1-4,6 and ephrin-B1,2) were screened by real-time
qRT-PCR on two normal pancreases, one pancreatic pseudocyst, one
serous cystadenoma, as well as five pancreatic cancer specimens.
The expression levels of EphB1, B3, B4, B6 and ephrin-B1 were
relatively low in the normal and tumour samples and these genes
were not considered further. Thus, EphB2 and ephrin-B2 were
selected for further analysis.
Distribution of the EphB and ephrin-B
genes in a series of 46 human pancreatic cancers
The distribution of pancreatic cancer specimens
according to their EphB2 and ephrin-B2 mRNA expression is shown in
Fig. 1. The median concentration of
EphB2 was 0.21 (range, 0.007–4.26; lower quartile, 0.090; upper
quartile, 0.40). The median concentration of ephrin-B2 was 0.093
(range, 0.002–2.41; lower quartile, 0.017; upper quartile,
0.15).
Correlation between EphB2 and ephrin-B2
expression status and clinicopathological characteristics
Table II shows the
results of the χ2 and Fisher’s exact tests of EphB2 and
ephrin-B2 expression and various clinicopathological
parameters.
 | Table IICorrelation between EphB2 and
ephrin-B2 expression and clinicopathological characteristics of 46
patients with pancreatic cancer. |
Table II
Correlation between EphB2 and
ephrin-B2 expression and clinicopathological characteristics of 46
patients with pancreatic cancer.
| EphB2 | | | Ephrin-B2 | | |
|---|
|
| | |
| | |
|---|
| Characteristics | EphB2 − <Upper
quartile | EphB2 + ≥Upper
quartile | χ2 | P-value | Ephrin-B2 − <Upper
quartile | Ephrin-B2 + ≥Upper
quartile | χ2 | P-value |
|---|
| Age (years) |
| <60 | 12 | 10 | 0.067 | 0.796 | 13 | 9 | 0.113 | 0.736 |
| ≥60 | 14 | 10 | | | 13 | 11 | | |
| Gender |
| Male | 12 | 9 | 0.006 | 0.938 | 13 | 8 | 0.456 | 0.500 |
| Female | 14 | 11 | | | 13 | 12 | | |
| Histological
differentiation |
| Poorly | 11 | 6 | 1.645 | 0.439 | 11 | 6 | 1.645 | 0.439 |
| Moderately | 12 | 9 | | | 12 | 9 | | |
| Well | 3 | 5 | | | 3 | 5 | | |
| Stage (AJCC) |
| I | 17 | 15 | 2.044 | 0.360 | 16 | 16 | 2.425 | 0.297 |
| II | 4 | 4 | | | 5 | 3 | | |
| III | 5 | 1 | | | 5 | 1 | | |
| Pain |
| 0 | 8 | 1 | 18.592 | 0.000 | 7 | 2 | | |
| 1 | 10 | 1 | | | 10 | 1 | | |
| 2 | 5 | 5 | | | 5 | 5 | | |
| 3 | 3 | 13 | | | 4 | 12 | 13.590 | 0.004 |
| T (pTNM) |
| T1 | 16 | 5 | 16.626 | 0.001 | 15 | 6 | 9.084 | 0.028 |
| T2 | 4 | 0 | | | 3 | 1 | | |
| T3 | 1 | 10 | | | 2 | 9 | | |
| T4 | 5 | 5 | | | 6 | 4 | | |
| Lymph node
metastasis |
| Absent | 17 | 5 | 7.389 | 0.007 | 16 | 6 | 4.506 | 0.034 |
| Present | 9 | 15 | | | 10 | 14 | | |
No significant differences were found between the
overexpressed and non-overexpressed EphB2 and ephrin-B2 groups with
regard to age, gender or histological differentiation.
Overexpression of EphB2 and ephrin-B2 in pancreatic
cancer specimens was more common in the the presence of lymph node
metastasis (75.0 vs. 34.6% and 70.0 vs. 38.4%, respectively;
P<0.05), in the classification of T factor (T1 plus T2 vs. T3
plus T4: 75.0 vs. 23.1% and 65.0 vs. 30.7%, respectively;
P<0.05) and in the degree of pain (90.0 vs. 30.7% and 85.0 vs.
34.6%, respectively; P<0.05; Table
II).
Uni-and multivariate analyses of
prognostic factors in pancreatic cancer patients
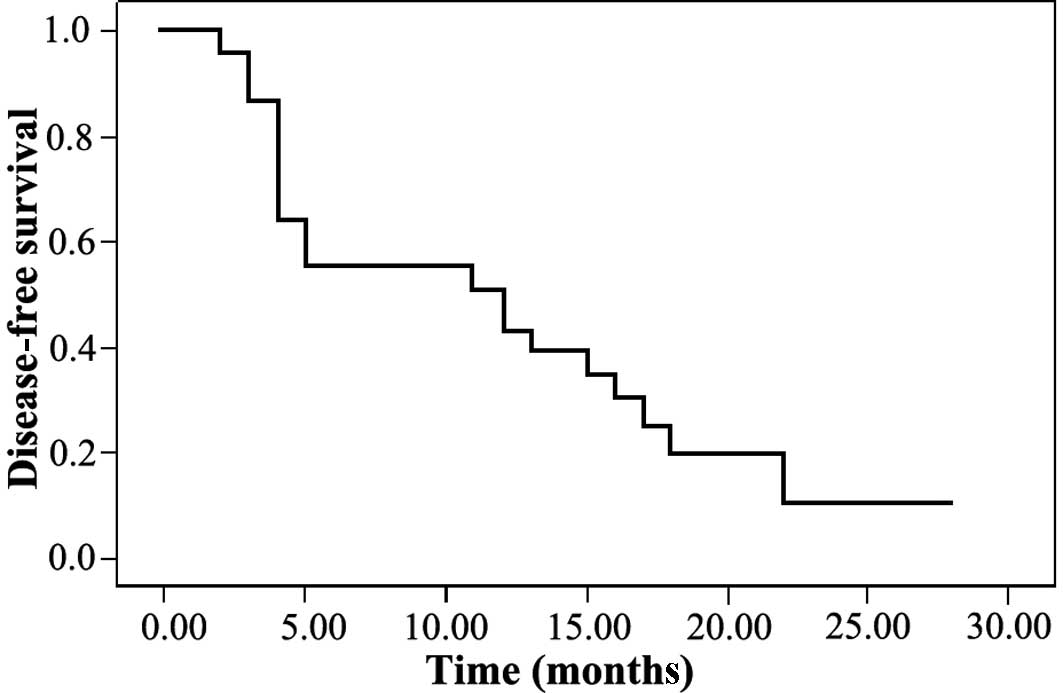
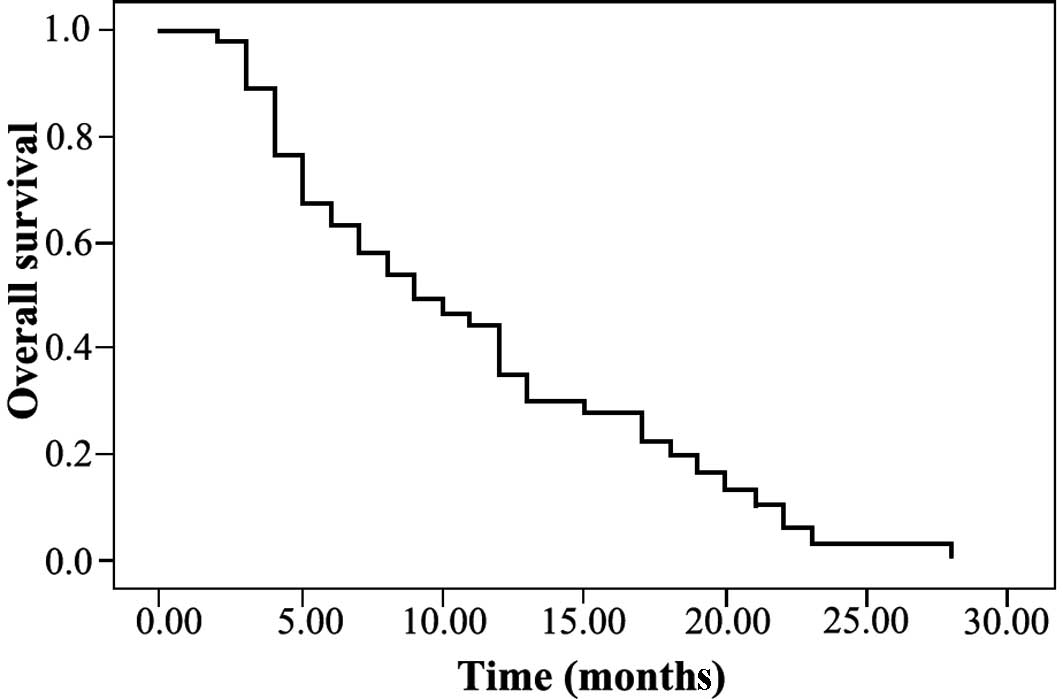
One-year disease-free and overall survival rates
were 39.0 and 30.0%, respectively (Figs. 1 and 2). Forty-one patients had a tumor when
they died and 38 patients experienced recurrence of a tumor: 21 in
the liver, 10 in the lymph node, 3 in the peritoneum and 4 in other
organs.
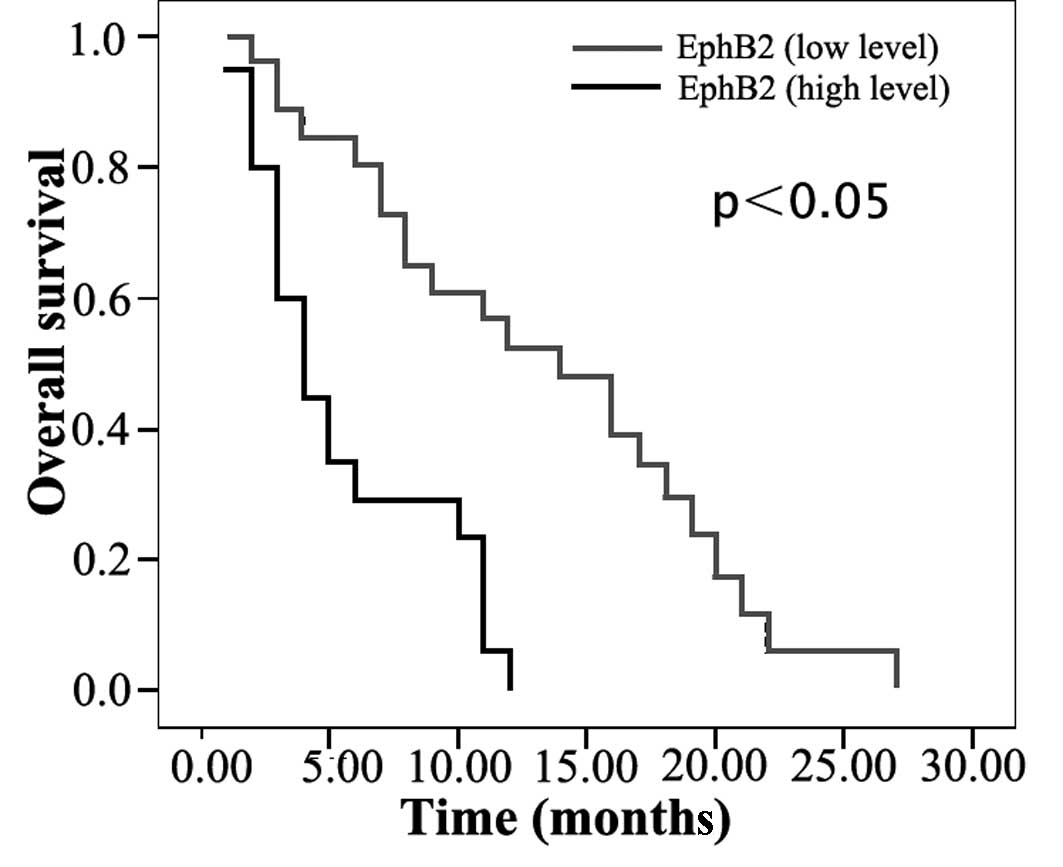
The prognostic significance of the expression of
EphB2 and ephrin-B2 was analyzed for the disease-free and overall
survival rates. Patients with a low EphB2 and ephrin-B2 expression
had better 1-year survival rates than those with a high expression
(disease-free, 55 vs. 15% and 43 vs. 35%; overall, 52 vs. 0% and 44
vs. 11%; all P<0.05; Table
III). Univariate analysis revealed that the EphB2 and ephrin-B2
expression level, presence of lymph node metastasis, histological
differentiation, the degree of pain and T stage of pathological
tumor-node-metastasis (pTNM) staging system were significant
prognosticators for disease-free and overall survival (Table III; Figs. 3 and 4).
 | Table IIIUnivariate analysis of
clinicopathological characteristics for disease-free and overall
survival of 46 patients with pancreatic cancer. |
Table III
Univariate analysis of
clinicopathological characteristics for disease-free and overall
survival of 46 patients with pancreatic cancer.
|
Characteristics | No. of
patients | 1-year disease-free
survival rate % | P-value | 1-year overall
survival rate % | P-value |
|---|
| Age (years) |
| <60 | 22 | 20 | 0.431 | 20 | 0.534 |
| ≥60 | 24 | 53 | | 39 | |
| Gender |
| Male | 21 | 27 | 0.116 | 32 | 0.357 |
| Female | 25 | 49 | | 29 | |
| Histological
differentiation |
| Poorly | 17 | 50 | 0.095 | 47 | 0.124 |
| Moderately | 21 | 42 | | 27 | |
| Well | 8 | 0 | | 0 | |
| Stage (AJCC) |
| I | 32 | 38 | 0.251 | 29 | 0.659 |
| II | 8 | 19 | | 17 | |
| III | 6 | 67 | | 50 | |
| Pain |
| 0 | 9 | 74 | 0.002 | 64 | 0.004 |
| 1 | 11 | 41 | | 41 | |
| 2 | 10 | 37 | | 20 | |
| 3 | 16 | 14 | | 7 | |
| T (pTNM) |
| T1 | 21 | 60 | 0.000 | 56 | 0.000 |
| T2 | 4 | 75 | | 50 | |
| T3 | 11 | 0 | | 0 | |
| T4 | 10 | 0 | | 0 | |
| Lymph node
metastasis |
| Absent | 22 | 58 | 0.000 | 61 | 0.000 |
| Present | 24 | 0 | | 0 | |
| EphB2
expression |
| <Upper
quartile | 26 | 55 | 0.002 | 52 | 0.000 |
| ≥Upper
quartile | 20 | 15 | | 0 | |
| ephrin-B2 |
| <Upper
quartile | 26 | 43 | 0.520 | 44 | 0.042 |
| ≥Upper
quartile | 20 | 35 | | 11 | |
Multivariate analysis with factors proven to be
significant in the univariate analysis revealed that the EphB2
expression level and T stage of the pTNM staging system were
independent prognostic factors for disease-free and overall
survival. The presence of lymph node metastasis is an independent
prognostic factor for overall survival only (Table IV).
 | Table IVMultivariate analysis of
clinicopathological characteristics for disease-free and overall
survival of patients with pancreatic cancer. |
Table IV
Multivariate analysis of
clinicopathological characteristics for disease-free and overall
survival of patients with pancreatic cancer.
|
Characteristics | Odds ratio | 95% CI | χ2
value | P-value |
|---|
| Disease-free
survival |
| EphB2
expression | 4.480 | 1.206–16.640 | 5.017 | 0.025 |
| Lymph node
metastasis | 1.568 | 0.405–6.073 | 0.424 | 0.515 |
| Histologic
differentiation | 1.041 | 0.537–2.019 | 0.014 | 0.905 |
| T (pTNM) | 1.757 | 1.012–3.048 | 4.014 | 0.045 |
| Pain | 1.579 | 0.995–2.504 | 3.767 | 0.052 |
| Overall
survival |
| EphB2
expression | 5.109 | 1.633–15.983 | 7.855 | 0.005 |
| Lymph node
metastasis | 5.901 | 1.881–18.513 | 9.257 | 0.002 |
| Histologic
differentiation | 1.017 | 0.557–1.856 | 0.003 | 0.957 |
| T (pTNM) | 2.046 | 1.273–3.286 | 8.757 | 0.003 |
| Pain | 1.070 | 0.702–1.631 | 0.098 | 0.754 |
Discussion
Eph receptors are the largest known family of
receptor protein tyrosine kinases and are activated by interactions
with cell-surface ligands termed ephrins, which are anchored on
plasma membranes through either a glycosyl phosphatidylinositol
linkage (ephrin-A) or a transmembrane domain (ephrin-B). The Ephs
(receptors) and their ligands (ephrins) may be divided into two
subclasses, A and B, on the basis of sequence homology, structure
and binding affinity (1–4). The Eph family of receptor kinases and
their ligands are well known to be involved in fundamental
developmental processes of the nervous system, including axon
guidance, axon fasciculation, neural crest cell migration,
acquisition of brain subregional identity and neuronal cell
survival. Moreover, in recent years, ephrins and Ephs have been
found to be expressed during the development of numerous human
tumors, including melanoma, glioblastoma, lung carcinoma and breast
carcinoma (2–5). However, information concerning the
expression of ephrins and Ephs in pancreatic cancer is sparse. In
particular, to the best of our knowledge, little is known regarding
the expression and role of the different EphBs and ephrin-Bs in
pancreatic cancer. In the current study, we investigated the
expression patterns of ephrin-B and EphB mRNA in a series of human
pancreatic cancers and demonstrated that the overexpression of
EphB2 is correlated with progression and pain in human pancreatic
cancer. Our observations indicate that EphB1, B3, B4, B6 and
ephrin-B1 are coordinately silenced in the majority of pancreatic
cancers and non-tumorous samples. The mechanism of the silencing of
EphB expression in pancreatic cancer and non-tumorous samples
remains uncharacterized, but in the great majority of cancers,
downregulation of EphB receptors occurs at the mRNA level (4,7).
Our results clearly demonstrate that the
overexpression of EphB2 and ephrin-B2 had a significantly higher
incidence with lymph node metastasis, advanced classification of T
factor and a higher degree of pain. Patients with low EphB2 and
ephrin-B2 expression yielded better 1-year survival rates than
those with a high expression. The proportion of individuals
surviving pancreatic cancer is extremely low and the length of time
between diagnosis and mortality is typically short, usually less
than six months (13).
Approximately 2 out of 10 individuals with pancreatic cancer live
for at least one year after their cancer is found. Fewer than 4%
are likely to be alive after 5 years (http://www.cancerstaging.org/) (13). Thus, 1-year rates were used as the
standard way of looking at a patient’s outlook in this study.
Multivariate analysis identified the level of EphB2 expression as
an independent prognostic factor for disease-free and overall
survival (P<0.01, P<0.0001, respectively). Our results are
consistent with those of previous studies, which indicated that an
increased expression of EphB2 in breast cancer was associated with
poor overall survival and that there was a clear trend that high
levels of the EphB2 protein were negatively associated with
disease-free survival. However, this is contrary to previous data
presented in colorectal cancer that suggest that EphB2 expression
is a good prognostic factor. The discrepancy may be explained by
fundamental differences in the role of EphB2 in cancers of the
solid and hollow organs. The Eph receptor families and their
ligands are well known to signal complicated cell processes, which
may result in distinct or occasionally even opposing effects on
cell behaviour depending on context (14).
Pain affects cancer prognosis and has been shown to
be an independent prognostic factor in pancreatic cancers (13). Overexpression of EphB2 is correlated
with a higher degree of pain, the mechanism for which is not yet
apparent. However, the ephrin-B-EphB receptor signalling has been
shown to contribute to neuropathic pain by regulating excitability
of nociceptive-related neurons in dorsal root ganglion and dorsa
horn and the synaptic plasticity at the spinal level (15). Moreover, ephrin-B2 siRNA may be a
potential therapeutic agent for neuropathic pain (16).
In conlusion, high levels of EphB2 expression are
associated with shortened survival in patients with pancreatic
cancer and multivariate analyses suggest that EphB2 is an
independent prognostic factor. The Eph family of receptor tyrosine
kinases is an attractive candidate for therapeutic targets.
Previous studies have developed a monoclonal antibody, peptide or
small molecules against EphB2 in an attempt to block its activation
(17–19), which may be applied as a potential
remedy in pancreatic cancer as there are few treatment options
available for patients with a disease with such a high mortality
rate, resulting in poor outcomes.
References
|
1
|
Coulthard MG, Duffy S, Down M, Evans B,
Power M, Smith F, Stylianou C, Kleikamp S, Oates A, Lackmann M,
Burns GF and Boyd AW: The role of the Eph-ephrin signalling system
in the regulation of developmental patterning. Int J Dev Biol.
46:375–384. 2002.PubMed/NCBI
|
|
2
|
Klein R: Bidirectional signals establish
boundaries. Curr Biol. 9:R691–R694. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gale NW, Holland SJ, Valenzuela DM,
Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H,
Wilkinson DJ, Pawson T, Davis S and Yancoupoulos GD: Eph receptors
and ligands comprise two major specificity subclasses and are
reciprocally compartmentalised during embryogenesis. Neuron.
17:9–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyd AW and Lackmann M: Signals from Eph
and ephrin proteins: a developmental tool kit. Sci STKE.
112:re202001.PubMed/NCBI
|
|
5
|
Hirai H, Maru Y, Hagiwara K, Nishida J and
Takaku F: A novel putative tyrosine kinase receptor encoded by the
eph gene. Science. 238:1717–1720. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Batlle E, Henderson JT, Beghtel H, van den
Bron MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering
M, Pawson T and Clevers H: Beta-Catenin and TCF mediate cell
positioning in the intestinal epithelium by controlling the
expression of EphB/ephrin B. Cell. 111:251–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Batlle E, Bacani J, Begthel H, Jonkheer S,
Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T,
Gallinger S, Pals S and Clevers H: EphB receptor activity
suppresses colorectal cancer progression. Nature. 435:1126–1130.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kataoka H, Tanaka M, Kanamori M, Yoshii S,
Ihara M, Wang YJ, Song JP, Li ZY, Arai H, Otsuki Y, Kobayashi T,
Konno H, Hanai H and Sugimura H: Expression profile of EFNB1,
EFNB2, two ligands of EPHB2 in human gastric cancer. J Cancer Res
Clin Oncol. 28:343–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stephenson SA, Slomka S, Douglas EL,
Hewett PJ and Hardingham JE: Receptor protein tyrosine kinase EphB4
is up-regulated in colon cancer. Int J Dev Biol. 46:375–384.
2002.
|
|
10
|
Berclaz G, Flutsch B, Altermatt HJ,
Rohrback H, Djonov V, Ziemiecki A, Dreher E and Andres AC: Loss of
EphB4 receptor tyrosine kinase protein expression during
carcinogenesis of the human breast. Pain. 139:168–180. 2008.
|
|
11
|
Nikolova Z, Djonov V, Zuercher G, Andres
AC and Ziemiecki A: Cell-type specific and estrogen dependent
expression of the receptor tyrosine kinase EphB4 and its ligand
ephrin B2 during mammary gland morphogenesis. J Cell Sci.
111:2741–2751. 1998.PubMed/NCBI
|
|
12
|
Liu W, Ahmad SA, Jung YD, Reinmuth N, Fan
F, Bucana CD and Ellis LM: Co-expression of ephrin-Bs and their
receptors in colon carcinoma. Cancer. 94:934–939. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Dang C, Ma Q and Shimahara Y:
Expression of nerve growth factor receptors and their prognostic
value in human pancreatic cancer. Oncol Rep. 14:161–171.
2005.PubMed/NCBI
|
|
14
|
Holmberg J and Frisen J: Ephrins are not
only unattractive. Trends Neurosci. 25:239–243. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi H, Kitamura T, Sekiguchi M,
Homma MK, Kabuyama Y, Konno S, Kikuchi S and Homma Y: Involvement
of EphB1 receptor/EphrinB2 ligand in neuropathic pain. Spine (Phila
Pa 1976). 32:1592–1598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song XJ, Zheng JH, Cao JL, Liu WT, Song XS
and Huang ZJ: EphrinB-EphB receptor signaling contributes to
neuropathic pain by regulating neural excitability and spinal
synaptic plasticity in rats. Pain. 139:168–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao W, Luis E, Ross S, Silva J, Tan C,
Crowley C, Chui C, Franz G, Senter P, Koeppen H and Polakis P:
EphB2 as a therapeutic antibody drug target for the treatment of
colorectal cancer. Cancer Res. 64:781–788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toledo-Sherman L, Deretey E,
Slon-Usakiewicz JJ, Ng W, Dai JR, Foster JE, Redden PR, Uger MD,
Liao LC, Pasternak A and Reid N: Frontal affinity chromatography
with MS detection of EphB2 tyrosine kinase receptor. 2
Identification of small-molecule inhibitors via coupling with
virtual screening. J Med Chem. 48:3221–3230. 2005. View Article : Google Scholar
|
|
19
|
Koolpe M, Burgess R, Dail M and Pasquale
EB: EphB receptor-binding peptides identified by phage display
enable design of an antagonist with ephrin-like affinity. J Biol
Chem. 280:17301–17311. 2005. View Article : Google Scholar : PubMed/NCBI
|