Introduction
Squamous cell carcinoma of the head and neck (SCCHN)
is the sixth most common malignancy worldwide, and is a cancer with
moderately low survival and high recurrence rates. Each year,
approximately 50,000 new patients are confirmed as having SCCHN,
and patients are usually diagnosed at around 60 years of age
(1). Despite new therapeutic
modalities, long-term outcomes of patients with SCCHN remain
unsatisfactory (1). SCCHNs with
similar histology and localization receiving identical therapies
may have different clinical outcomes. Based on clinicopathological
parameters, such as T and N classifications, it is not possible to
reliably predict which patients are likely to respond successfully
to treatment or may exprerience recurrence. Differential gene and
protein expression in tumors may explain the variations in response
to the same treatment modality (2).
The Rab proteins, Ras-related small G proteins
(including the Rho-related proteins), serve a critical role in the
regulation of intracellular transport events (3,4). A
group of Rab11-interacting proteins has been described, which share
several common domains and biological properties (5–7). A
member of this family, termed Rab coupling protein (RCP), has been
shown to be important in tumorigenesis and progression of breast
cancer (8,9). However, there are few data reporting
RCP expression and its clinicopathological significance in other
types of cancer. The present study was carried out to investigate
the expression status of RCP protein in SCCHN and analyze whether
RCP expression was correlated with the clinical features and
prognosis in patients with SCCHN.
Materials and methods
Patients and tissues
A total of 95 patients with SCCHN, who underwent
partial or total laryngectomy at the Department of Otolaryngology
of Xiangya Hospital in Central South University, China, between
February 2003 and December 2005, were enrolled in this
retrospective study. The patients had no history of previous
malignancies, and no history of radiotherapy or chemotherapy.
Recurrence and metastasis were diagnosed by physical examinations,
imaging evaluation, surgical and postoperative pathological
examinations. In addition, 18 vocal nodule epithelia (obtained from
patients suffering from vocal nodules) and 16 leukoplakia epithelia
of the larynx (precancerous lesions) samples were obtained between
January 2008 and November 2010. Informed consent was obtained from
all patients prior to surgery, and this investigation was approved
by the Research Ethics Committee of Central South University,
Changsha, China.
The main clinical and pathological variables of the
patients are described in detail in Table I. There were 92 male and 3 female
patients, with a median age of 57 years (range, 32–79). According
to the TNM System of the International Union against Cancer
(10), 21 cases were supraglottic,
65 were glottic, 1 was subglottic and 8 were hypopharyngeal
carcinomas. There were 17 cases in stage I (T1N0M0), 21 cases in
stage II (T2N0M0), 27 cases in stage III (T3N0M0 13 cases, T2N1M0 6
cases, T3N1M0 8 cases) and 30 cases in stage IV (T2N2M0 3 cases,
T3N2M0 10 cases, T3N1M1 1 case, T4N0M0 4 cases, T4N1M0 7 cases and
T4N2M0 5 cases). Considering pathological grading, 58 were staged
as well-differentiated (G1), 29 as moderately differentiated (G2)
and 8 as poorly differentiated (G3). A total of 40 patients with
lymph node metastasis were validated by conventional postoperative
pathological examinations and 46 patients experienced tumor
recurrence following surgery.
 | Table IClinicopathological characteristics of
the 95 studied cases with SCCHN. |
Table I
Clinicopathological characteristics of
the 95 studied cases with SCCHN.
| Variables | No. of patients | Percentage (%) |
|---|
| Age |
| ≤57 | 48 | 50.53 |
| >57 | 47 | 49.47 |
| Gender |
| Female | 3 | 3.16 |
| Male | 92 | 96.84 |
| Alcohol intake |
| Yes | 52 | 54.74 |
| No | 43 | 45.26 |
| Smoking |
| Yes | 66 | 69.47 |
| No | 29 | 30.53 |
| Tumor site |
| Hypopharyngeal | 8 | 8.42 |
| Supraglottic | 21 | 21.11 |
| Glottic | 65 | 68.42 |
| Subglottic | 1 | 1.05 |
| Tumor grade |
| G1 | 58 | 61.05 |
| G2 | 29 | 30.53 |
| G3 | 8 | 8.42 |
| T classification |
| T1 | 17 | 17.89 |
| T2 | 30 | 31.58 |
| T3 | 32 | 33.68 |
| T4 | 16 | 16.84 |
| Clinical stage |
| I | 17 | 17.89 |
| II | 21 | 22.11 |
| III | 27 | 28.42 |
| IV | 30 | 31.58 |
| Lymph node
metastasis |
| Negative | 55 | 57.89 |
| Positive | 40 | 42.11 |
Immunohistochemistry
Immunohistochemical staining was performed according
to the manufacturer's instructions. Briefly, antigen retrieval was
carried out in 10 mmol/l citrate buffer (pH 6.0) for 15 min at
100°C in a microwave oven. Endogenous peroxidase activity was
blocked with 3% hydrogen peroxide for 10 min at room temperature.
Slides were incubated with chicken IgY anti-RCP polyclonal antibody
(Sigma, St. Louis, MO, USA) at 1:3000 dilution at 4°C overnight,
followed by the addition of HRP-labeled goat anti-chicken antibody
(KPL, Gaithersburg, MD, USA) at 1:2000 dilution for 30 min.
Immunoreactive proteins were visualized with 3,3′-diaminobenzidine
(DAB) and counterstained with Mayer's haematoxylin. Negative
control slides were probed with normal chicken serum under the same
experimental conditions. Images of stained tissues were acquired
with a Leica Qwin V3 image analysis system.
Evaluation of staining
Sections were independently evaluated and scored by
two pathologists (Xiang Li and Xueping Feng) who were blind to all
clinical data. Evaluation of staining was estimated by the pattern
of staining quantity and intensity as described by Yuan et
al (11): Quantity scores from
0 to 5 were respectively assigned if 0%, 1–10%, 11–30%, 31–50%,
51–80%, and 81–100% of the tumor cells were positive. The staining
intensity was rated on a scale of 0 to 3: 0, negative; 1, weak; 2,
moderate; and 3, strong). The multiplication of the intensity and
extent scores was used as the final staining score for RCP.
Theoretically, the scores ranged from 0 to 15. Scores above the
median (≥7) were considered as high reactivity and 0–6 as weak
reactivity.
Follow-up
A total of 95 patients with SCCHN were followed up
after surgery. The follow-up period was defined as the interval
between the date of tumor excision and that of the patient's
mortality or the last follow-up. Recurrence and metastasis were
diagnosed by clinical examination, imaging evaluation and
pathological studies. Overall survival (OS) and disease-free
survival (DFS) were calculated from the day of surgery to the date
of mortality or that of tumor relapse. The lost follow-ups and
mortality from other causes were treated as censored cases.
Statistical analyses
Statistical analyses were performed using the SPSS
statistical software version 17.0 (SPSS Inc., Chicago, IL, USA).
Statistical significance between the expression of RCP protein and
clinicopathological parameters was compared by the χ2
test. Survival analyses were undertaken using the Kaplan-Meier
method and curves were compared by the log-rank test.
Identification of relevant prognostic factors was performed by the
univariate and multivariate Cox regression analysis. Tests were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
RCP expression in squamous epithelia from
vocal nodules, leukoplakia tissues of larynx and SCCHN tissues
To investigate the protein expression profile of RCP
in SCCHN, immunohistochemistry was initially performed in 95
paraffin-embedded, archival SCCHN primary tumor samples, 18 vocal
nodules and 16 laryngeal leukoplakia specimens (precancerous
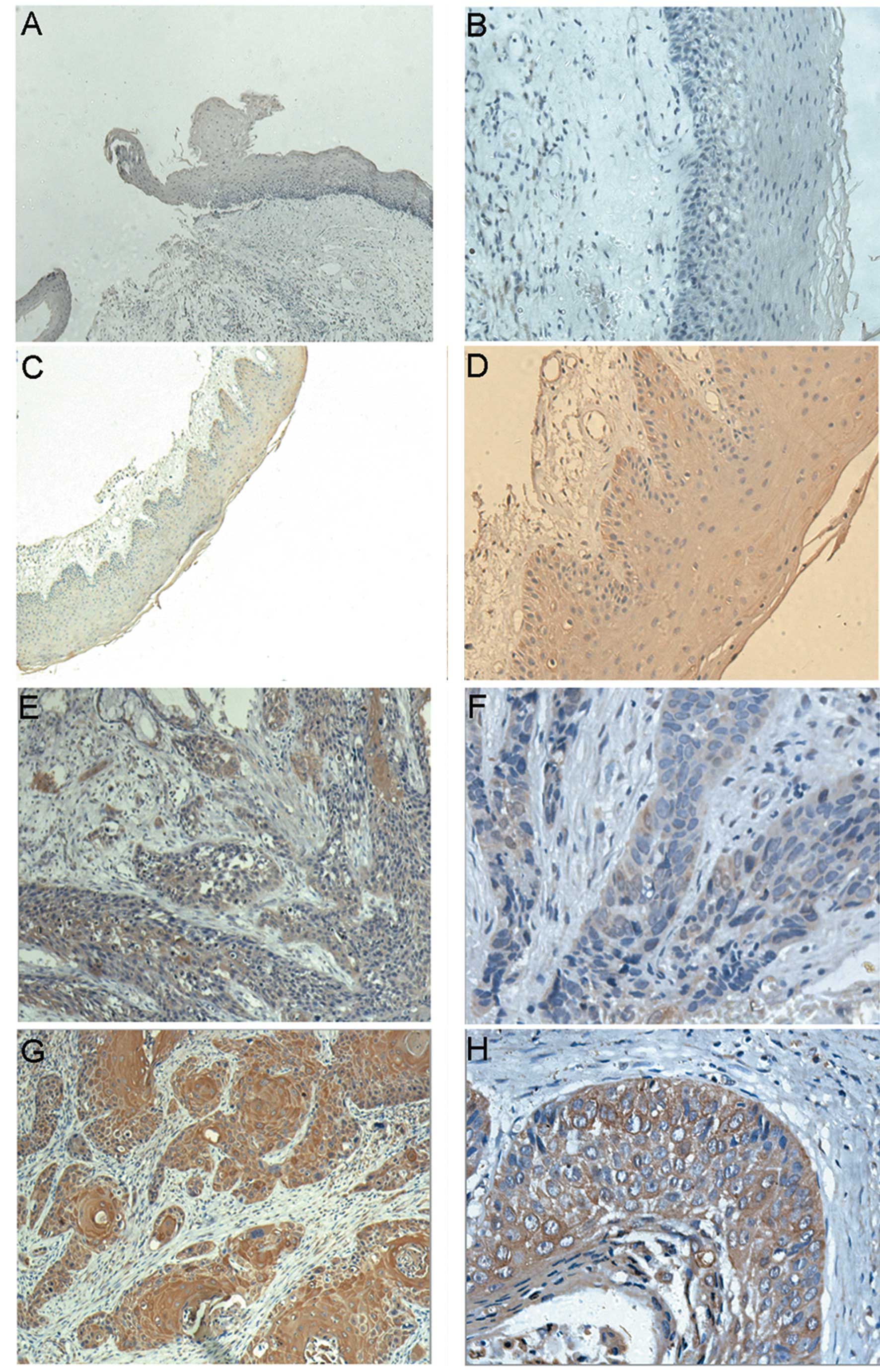
lesions). Positive immunostaining was predominantly observed in the
cytoplasm of carcinoma cells. Our data indicated that vocal nodule
epithelia, leukoplakia epithelia and SCCHN revealed a gradually
increased expression of RCP protein (P<0.05). As shown in
Table II, only 3 (16.67%) of 18
vocal nodule epithelia showed a low RCP expression (scored 1–2)
(Fig. 1A and B). Although 15 of the
leukoplakia epithelia samples had a low expression of RCP (Fig. 1C and D), their scores all ranged
from 1 to 3. While in SCCHN specimens, 65 (68.42%) cases
demonstrated a high RCP expression (scored 8–15) (Fig. 1G and H), 19 cases showed a low RCP
expression (scored 4–7) (Fig. 1E and
F), and only 11 cases (11.58 %) were scored 2–3.
 | Table IIRCP expression in squamous epithelia
from vocal nodules, leukoplakia tissues of larynx and SCCHN
tissues. |
Table II
RCP expression in squamous epithelia
from vocal nodules, leukoplakia tissues of larynx and SCCHN
tissues.
| Staining score |
|---|
|
|
|---|
| 0 | 1 | 2 | 3 | 4–7 | 8–15 |
|---|
| Squamous epithelia
from vocal cords (n=18) | 15 | 1 | 2 | - | - | - |
| Laryngeal leukoplakia
(n=16) | 1 | 2 | 11 | 2 | - | - |
| SCCHN |
| Low expression
(n=30) | - | - | 7 | 4 | 19 | - |
| High expression
(n=65) | - | - | - | - | - | 65 |
Correlation between RCP expression and
clinicopathological variables
The association between RCP protein expression and
clinicopathological characteristics of SCCHN was explored by the
χ2 test. As shown in Table
III, RCP overexpression was significantly associated with tumor
T classification (P=0.028), clinical staging (P=0.012), lymph node
metastasis (P=0.004) and recurrence (P=0.034), respectively.
However, no significant correlation was observed between RCP
protein levels and variables such as age (P=0.383), alcohol intake
(P=0.658), smoking status (P=0.811), tumor site (P=0.153) and tumor
grade (P=1.004).
 | Table IIIClinicopathological characteristics of
SCCHN and correlations with RCP expression. |
Table III
Clinicopathological characteristics of
SCCHN and correlations with RCP expression.
| Variables | Total | RCP expression | P-value |
|---|
| |
| |
|---|
| | Low (0–6) High
(7–15) | |
|---|
| Age | | | | 0.383 |
| ≤57 | 48 | 13 | 35 | |
| >57 | 47 | 17 | 30 | |
| Alcohol intake | | | | 0.658 |
| Yes | 52 | 15 | 28 | |
| No | 43 | 15 | 37 | |
| Smoking | | | | 0.811 |
| Yes | 66 | 24 | 42 | |
| No | 29 | 17 | 12 | |
| Tumor site | | | | 0.153 |
| Glottic | 65 | 24 | 41 | |
| Others | 30 | 6 | 24 | |
|
Hypopharyngeal | 8 | 0 | 8 | |
| Supraglottic | 21 | 5 | 16 | |
| Subglottic | 1 | 1 | 0 | |
| Tumor grade | | | | 1.004 |
| G1 | 58 | 18 | 40 | |
| G2+G3 | 37 | 12 | 25 | |
| T
classification | | | | 0.028 |
| T1+T2 | 47 | 20 | 27 | |
| T3+T4 | 48 | 10 | 38 | |
| Clinical stage | | | | 0.012 |
| Early stage
(I+II) | 38 | 18 | 20 | |
| Late stage
(III+IV) | 57 | 12 | 45 | |
| Lymph node
metastasis | | | | 0.004 |
| Negative | 55 | 24 | 31 | |
| Positive | 40 | 6 | 34 | |
| Recurrence
ratea | | | | 0.034 |
| No recurrence | 44 | 17 | 27 | |
| Recurrence | 46 | 8 | 38 | |
Patient follow-up and survival
analysis
In total, 95 patients with SCCHN remained in
follow-up after surgery. The median follow-up of the whole series
was 58 months (range, 2–96), and the median follow-up of the
patients alive at the last visit was 75 months (range, 51–96).
During this follow-up period, 5 cases were lost due to change of
address, 46 (48.42%) cases developed loco-regional recurrence
(median recurrence time was 17 months), among which 2 cases (2.11%)
had loco-regional recurrence twice and 1 case (1.05%) had local
recurrence and distant lung metastasis. Forty-three (43/95; 43.16%)
patients succumbed to the disease in this retrospective study, and
the main cause of death was tumor recurrence.
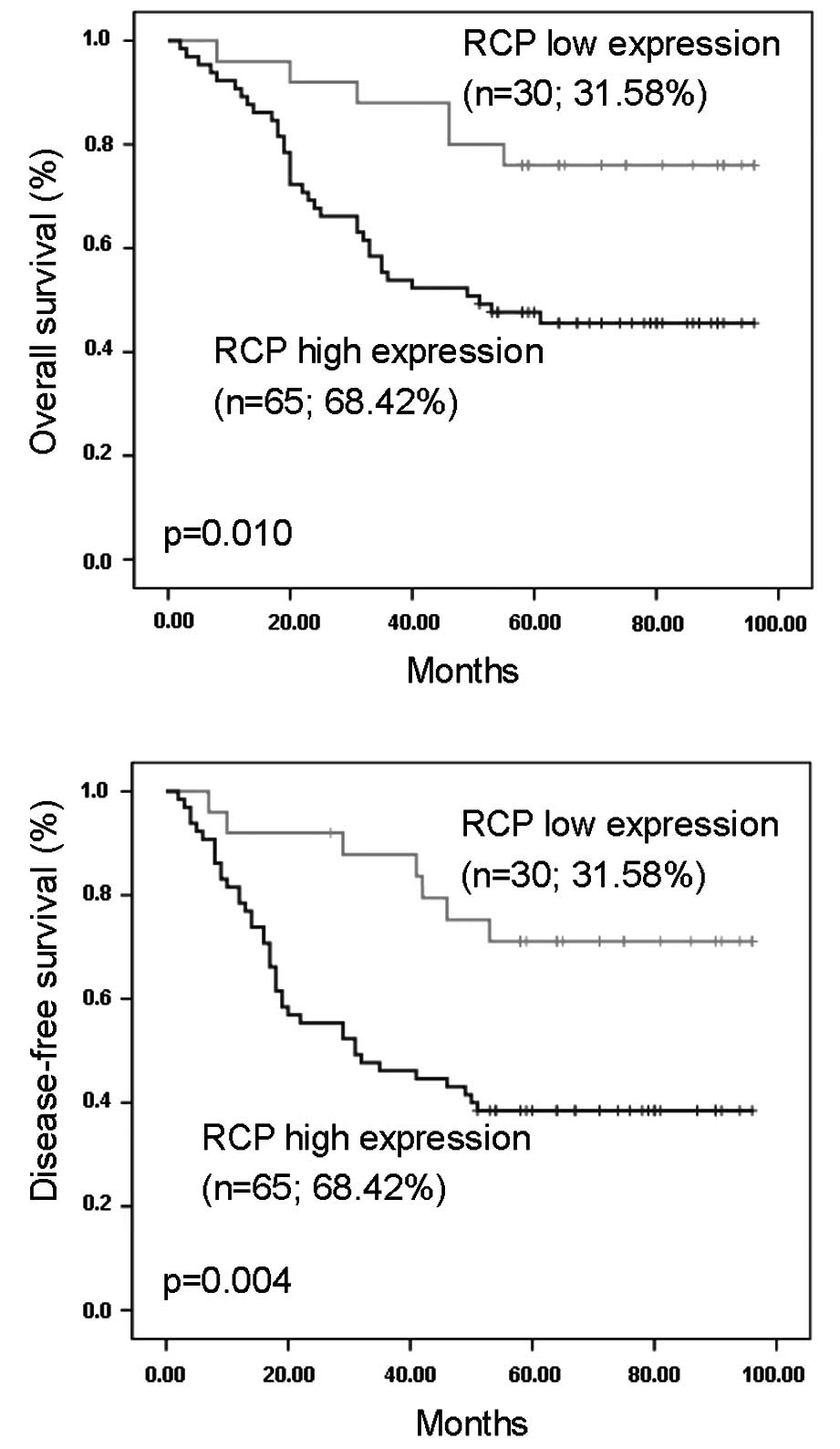
In the survival analyses by the Kaplan-Meier method,
the expression of RCP in SCCHN was significantly correlated with
disease-free survival (Fig. 2A) and
overall survival (Fig. 2B). The
log-rank test further demonstrated that the survival time was
significantly different between groups with a high and low
expression of RCP protein, indicating that a high level of RCP was
tightly correlated with a shorter survival time.
Univariate and multivariate Cox regression analyses
were performed to identify relevant prognostic factors in overall
survival using the Cox proportional hazards model (Table IV). Univariate analysis revealed
that the following variables were significantly associated with a
worse prognosis: Alcohol history (P=0.099); smoking (P=0.048); T
classification (P=0.047); clinical staging (P=0.039); lymph node
metastasis (P=0.007); recurrence (P=0.000); and RCP expression
(P=0.015). However, the multivariate analysis showed that only
recurrence had independent prognostic effects on the overall
survival of patients with SCCHN (P=0.000).
 | Table IVPrognostic factors in overall
survival by univariate and multivariate analyses (n=95). |
Table IV
Prognostic factors in overall
survival by univariate and multivariate analyses (n=95).
| Risk factors | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| HR (95 CI) | P-value | HR (95 CI) | P-value |
|---|
| Age
(≤57/>57) | 0.865
(0.469–1.597) | 0.643 | 0.991
(0.501–1.960) | 0.098 |
| Alcohol intake
(Y/N) | 1.696
(0.905–3.179) | 0.099 | 1.093
(0.546–2.192) | 0.801 |
| Smoking (Y/N) | 1.919
(1.005–3.663) | 0.048 | 1.096
(0.454–2.648) | 0.839 |
| Tumor site
(Glottic/others) | 0.629
(0.329–1.202) | 0.161 | 0.613
(0.252–1.494) | 0.282 |
| Tumor grade
(G1/G2+G3) | 0.719
(0.389–1.328) | 0.292 | 1.134
(0.517–2.486) | 0.754 |
| T classification
(T1+T2/T3+T4) | 1.909
(1.010–3.609) | 0.047 | 1.634
(0.452–5.901) | 0.454 |
| Clinical stage
(I+II/III+IV) | 2.037
(1.038–3.998) | 0.039 | 0.442
(0.104–1.879) | 0.269 |
| Metastasis
(Y/N) | 2.350
(1.265–4.366) | 0.007 | 1.804
(0.682–4.771) | 0.235 |
| Recurrence
(Y/N) | 38.221
(9.144–159.750) | 0.000 | 37.458
(8.477–165.523) | 0.000 |
| RCP expression
(High/low) | 2.938
(1.234–6.996) | 0.015 | 1.631
(0.648–4.107) | 0.299 |
Discussion
In the current study, we investigated the protein
expression of RCP in a series of 95 clinical paraffin-embedded
specimens with intact follow-up information. Immunostaining results
revealed that RCP protein levels were markedly higher in SCCHN
tissues compared with laryngeal leukoplakia (precancerous lesions),
while RCP was hardly detected in squamous epithelia from vocal
nodules. Our results were in agreement with previous studies. Zhang
et al (8) showed that RCP
had a higher expression in invasive breast ductal cancer than
normal breast epithelium, ductal carcinoma in situ
(pre-malignant), weakly aggressive mucinous and medullary
histological types. In addition, overexpression of RCP in MCF10A
normal human mammary epithelial cells resulted in the acquisition
of tumorigenic properties. For example, RCP overexpression
decreased growth factor-dependent cell growth; increased cell
survival under anoikis conditions; induced cell motility, invasion
and EMT in vitro; and increased tumor growth and progression
in vivo (8). Thus, RCP is
important in the malignant progression of SCCHN.
Subsequently, a detailed analysis for elucidating
the correlation between RCP expression and clinicopathological
variables was performed. RCP overexpression was found to be
significantly associated with tumor T classification, clinical
staging, particularly with lymph node metastasis, recurrence and a
shorter survival time. The lymph node metastasis and postoperative
tumor recurrence are important factors affecting prognosis
(1,12–13).
At present, there are few data concerning RCP expression and tumor
metastasis in other types of cancer except for breast cancer. RCP
overexpression induced cell motility, invasion and EMT in
vitro; RCP knockdown weakened tumor progression and lung
micrometastasis in vivo (8).
The mechanism by which RCP promotes metastasis may be associated
with certain downstream signals, such as EGFR and β1 integrin
(9,14–15).
Caswell et al (14) reported
that activated RCP associates with β1 integrin and acts to link
this integrin with RTKs at recycling endosomes. This consequently
drives cell proliferation and cell migration in 2D and 3D matrices.
Furthermore, when cells migrate in 3D matrices, the ability of RCP
and its binding partner RAB25 to localize integrin and EGFR
signaling to the cell front drives the extension of invasive
pseudopods. This trafficking-dependent localization of signaling
proteins such as β1 integrin and EGFR may contribute to the role of
RCP in metastasis (9,14).
The prognostic value of RCP protein in patients with
SCCHN remains to be determined. Currently, prognostic evaluation is
mainly based on traditional methods including clinical stage, tumor
site and histopathological grade. Previous studies have suggested
that other factors, such as molecular and cellular characteristics
of the primary tumors, may improve our ability to prognosticate
(16). In our present
investigation, the multivariate analysis revealed that only tumor
recurrence had independent prognostic effects on the overall
survival rate. However, the expression level of RCP protein failed
to be an independent prognostic factor in our Cox multivariate
analysis, although its expression was inversely correlated with
overall survival and disease-free survival. This failure may be
explained by the fact that the proportion of the cases diagnosed as
recurrence and lymph node metastasis in 95 patients with SCCHN was
extremely large, which may reduce the RCP significance in
multivariate analysis. Thus, the evaluation of the RCP protein may
further provide new information for patient prognosis.
In conclusion, our current study indicated that RCP
was upregulated in human SCCHN and that RCP overexpression was
significantly correlated with tumor malignant progression and poor
survival in patients with SCCHN, which suggested that RCP may serve
as a specific and a novel prognostic marker in SCCHN. However,
further studies are required to determine the molecular mechanism
of RCP involved in SCCHN progression and prognosis, which may lead
to further development of new approaches targeting RCP for
effective tumor management.
Acknowledgements
The authors would like to thank Xiang Li (Department
of Pathology, Xiangya Hospital) and Xueping Feng (Central
Laboratory of Medical Research, Xiangya Hospital) for their
evaluation of these clinical samples, and acknowledge Dr Vivek K.
Rauniyar (Department of Neurology, Xiangya Hospital) for his
critical review of this manuscript. This study was supported by
grants from the National Natural Science Foundation of China (No.
81071757, 30872852, 30901664), the Key Program of Natural Science
Foundation of Hunan Province (2010TP4012-1), the Research Fund for
the Doctoral Program of Higher Education of China (20100162110036,
20090162110065) and the Graduate Degree Thesis Innovation
Foundation of Hunan province (CX2011B059).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Fischer CA, Jung M, Zlobec I, et al:
Co-overexpression of p21 and Ki-67 in squamous cell carcinoma of
the head and neck relative to a significantly poor prognosis. Head
Neck. 33:267–273. 2010. View Article : Google Scholar
|
|
3
|
Hutagalung AH and Novick PJ: Role of Rab
GTPases in membrane traffic and cell physiology. Physiol Rev.
91:119–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jordens I, Marsman M, Kuijl C and Neefjes
J: Rab proteins, connecting transport and vesicle fusion. Traffic.
6:1070–1077. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jing J and Prekeris R: Polarized endocytic
transport: the roles of Rab11 and Rab11-FIPs in regulating cell
polarity. Histol Histopathol. 24:1171–1180. 2009.PubMed/NCBI
|
|
6
|
Hoekstra D, Tyteca D and van IJzendoorn
SC: The subapical compartment: a traffic center in membrane
polarity development. J Cell Sci. 117:2183–2192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somsel Rodman J and Wandinger-Ness A: Rab
GTPases coordinate endocytosis. J Cell Sci. 113:183–192.
2000.PubMed/NCBI
|
|
8
|
Zhang J, Liu X, Datta A, et al: RCP is a
human breast cancer-promoting gene with Ras-activating function. J
Clin Invest. 119:2171–2183. 2009.PubMed/NCBI
|
|
9
|
Mills GB, Jurisica I, Yarden Y, et al:
Genomic amplicons target vesicle recycling in breast cancer. J Clin
Invest. 119:2123–2127. 2009.PubMed/NCBI
|
|
10
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumors. Wiley-Liss; New York: 2002
|
|
11
|
Yuan P, Temam S, El-Naggar A, et al:
Over-expression of podoplanin in oral cancer and its association
with poor clinical outcome. Cancer. 107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cerezo L, Millan I, Torre A, Aragón G and
Otero J: Prognostic factors for survival and tumor control in
cervical lymph node metastases from head and neck cancer. A
multivariate study of 492 cases. Cancer. 69:1224–1234. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Xie C, Zhang X, et al: Elevated
expression of HMGB1 in squamous-cell carcinoma of the head and neck
and its clinical significance. Eur J Cancer. 46:3007–3015. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caswell PT, Chan M, Lindsay AJ, McCaffrey
MW, Boettiger D and Norman JC: Rab-coupling protein coordinates
recycling of alpha5beta1 integrin and EGFR1 to promote cell
migration in 3D microenvironments. J Cell Biol. 183:143–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morello V, Cabodi S, Sigismund S, et al:
β1 integrin controls EGFR signaling and tumorigenic properties of
lung cancer cells. Oncogene. 30:4087–4096. 2011.
|
|
16
|
Johann DJ Jr, McGuigan MD, Patel AR, et
al: Clinical proteomics and biomarker discovery. Ann N Y Acad Sci.
1022:295–305. 2004. View Article : Google Scholar : PubMed/NCBI
|