Introduction
Cervical cancer is the third most common type of
cancer in females, and the seventh most common type overall, with
an estimated 529,000 new cases in 2008 (1,2).
Epigenetic changes are as equally responsible as genetic changes in
the development and progression of various types of cancer,
including cervical cancer. These epigenetic changes include DNA
methylation and histone deacetylation.
Aberrant promoter hypermethylation of tumor
suppressor genes has been shown to be involved in human neoplasia
(3). The genomic organization at
9p21 houses two members of the INK4 family of cyclin-dependent
kinase inhibitors (CDKIs), p15 and p16, and an
unrelated gene p14ARF. Notably,
p14ARF utilizes two of the same exons as
p16, but is translated in an alternative reading frame.
p16 is the most commonly altered gene in human malignancies
(4).
p15 is located 25 kb from the p16 gene
and its protein shares significant areas of homologous amino acid
sequences with p16 (5).
p16 is a major target in carcinogenesis, rivaled in
frequency only by the p53 tumor suppressor gene (6). The major mechanism of p15 gene
inactivation in acute myelogenous leukemia (AML) is methylation of
the 5′ promoter region of the gene, which leads to transcription
silencing (7). The p15 gene
is also aberrantly methylated in several human neoplasms,
particularly among hematopoietic malignancies (8).
Hypermethylation of the p16 promoter region
has been detected in various types of human cancer (9,10). In
their study, Feng et al reported similar promoter
methylation patterns in genes from exfoliated cell samples and
corresponding biopsy specimens. Furthermore, the frequency of
hypermethylation increased statistically significantly with the
increasing severity of neoplasia present in the cervical biopsy
(11). p16 hypermethylation
is an early event in cervical carcinogenesis, but no study has
examined its association with risk factors, including passive
smoking, oral contraceptive (OC) use and age at first sexual
intercourse (AFSI). Additionally, no study has examined the
significant hypermethylation of p14 and p15 genes in
cervical cancer.
Materials and methods
Sample collection
A total of 125 biopsy samples were collected with
informed consent from patients diagnosed with cervical cancer.
Ethics approval was provided by the Mohan Dai Oswal Cancer
Treatment and Research Foundation, Ludhiana, India. One hundred
blood samples from the healthy females (control) were collected in
tubes with anticoagulant (heparin). Twenty-five biopsy samples,
obtained from females in which hysterectomy had been carried out,
but the cervix was normal, were used as controls.
DNA extraction
Cells obtained from tissue biopsies and blood
samples were lysed in digestion buffer (10 mM Tris-HCl, pH 8.0, 10
mM EDTA, 150 mM NaCl and 2% SDS) containing proteinase K (0.2
mg/ml). DNA was then purified using the standard phenol-chloroform
extraction and ethanol precipitation.
Human papillomavirus (HPV) infection and
HPV-16 typing
The HPV consensus primers, MY09 and MY11, were used
in the PCR assay for HPV infection (12). A total of100 ng of HPV-16 viral
genome cloned into pBR322 was used as the positive control. The
HPV-16 pBR322 plasmid DNA was a gift from E.M. DeVilliers of
Deutsches Krebsforschungszentrum, Heidelberg, Germany. For typing
of HPV-16, the primer sets and the methodology used was performed
as previously described (13).
Methylation-specific PCR (MS-PCR)
DNA isolated from the biopsy and blood samples was
modified with sodium bisulphite and MS-PCR was carried out using
specific primers for methylation and unmethylation for the
p16INK4a, p14ARF
and p15INK4b genes (14–16).
The amplified products were run on a 2% agarose gel.
Bisulfite sequencing
For sequencing, the MS-PCR product was purified
using a gel purification kit (Sigma-Aldrich, St. Louis, MO, USA)
according to the manufacturer’s instructions. The sequencing was
carried out by a 3100 ABI sequencer and the DNA sequence was then
collected using a chromatogram.
Reverse transcription PCR (RT-PCR)
RNA was isolated from the biopsy and blood samples
using TRIzol reagent. An equal amount of RNA was used to synthesize
cDNA using the RevertAid first-strand cDNA synthesis kit
(Fermentas, Glen Burnie, MD, USA). RT-PCR was carried out to
determine the alteration in the level of mRNA expression due to
promoter hypermethylation using specific primers (15–17).
β-actin was used as the internal control.
Statistical analysis
The association between hypermethylation of the
genes and risk of cervical cancer was estimated by computing odds
ratios (ORs) and 95% confidence intervals (CI) using the Chi-square
test, Fisher’s exact test and multivariate logistic regression
analysis, which included several potential confounding variables.
The reported OR may be interpreted as age-adjusted estimates of the
relative risk of developing cervical cancer with the methylation of
studied genes. The amount of mRNA expression was obtained by
grading a ratio between the densitometry results of the gene and
β-actin. Statistical analysis was performed using SPSS version 11.5
and Epi Info version 3.2. The γ-coefficient was calculated to
correlate the hypermethylation of tumor suppressor genes with an
increase in the stage of cervical cancer. P<0.05 was considered
to indicate a statistically significant difference.
Results
Epidemiological characteristics
The cases and the controls were well-matched with
respect to age, gender and residence. The mean age ± SD of the
cases and controls was 48.33±10.23 and 46.71±11.85 years,
respectively. A total of 50.4% of patients were in the ≤45 years
age-group, while 49.6% of patients were in the >45 years
age-group (data not shown).
HPV infection and HPV-16 typing
It was observed that 116 out of 125 (92.8%) cervical
cancer patients were HPV-positive. Typing was carried out for
HPV-16, which demonstrated that out of the HPV-infected patients,
69.8% were HPV-16 (Table I).
 | Table IHPV infection and HPV-16 typing in
cervical cancer patients and controls. |
Table I
HPV infection and HPV-16 typing in
cervical cancer patients and controls.
| Patients (%) | Control (%) |
|---|
| HPV-positive |
| (+) ve | 116 (92.8) | 5 (5) |
| (−) ve | 9 (7.2) | 95 (95) |
| HPV-16 typing |
| (+) ve | 81 (69.8) | 3 (60) |
| (−) ve | 35 (30.1) | 2 (40) |
Status of the promoter hypermethylation
of p14ARF and INK family
No significant difference in the methylation of
p14ARF (P>0.05) between patients and
controls was observed, but a statistically significant difference
in the methylation of p15INK4b (P<0.05)
between patients and controls was observed. Methylation of
p15INK4b was found to marginally increase
the risk of cervical cancer (OR=1.54; 95% CI=1.20–1.99).
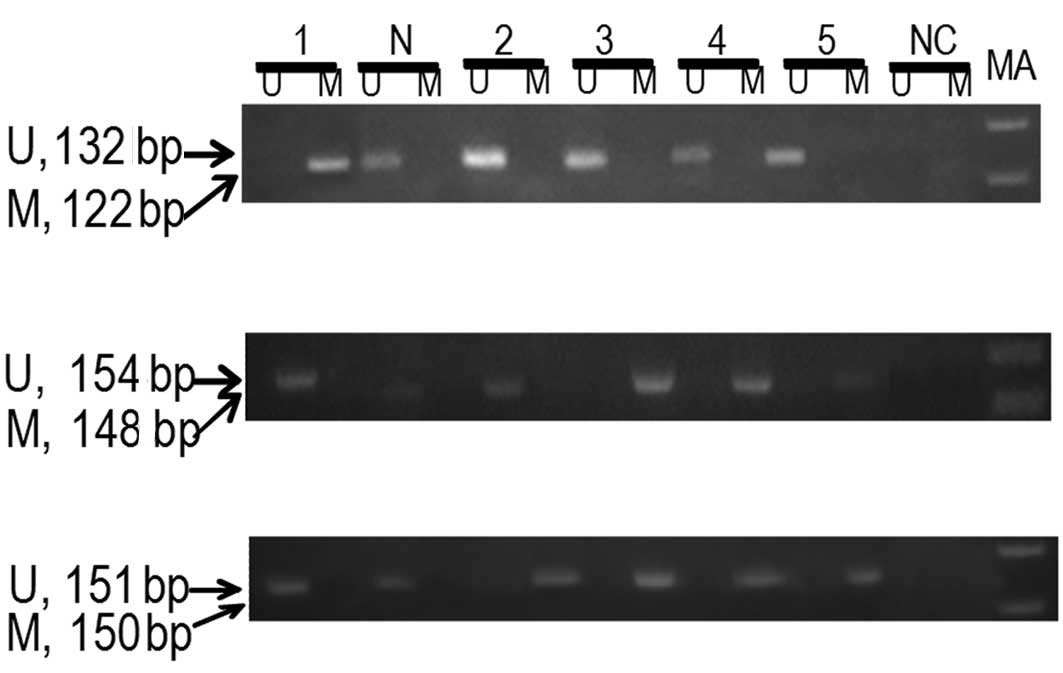
Hypermethylation of p16INK4a was observed
in 36% of cervical cancer patients (Fig. 1). A statistically significant
difference in the methylation of p16INK4a
(P<0.001) between patients and controls was observed. The
hypermethylation of p16INK4a in cervical
cancer patients was associated with an approximately 2.19-fold
increase in the risk of cervical cancer (OR=2.19; 95% CI=1.85–2.59)
(Table II). No statistically
significant correlation was observed between
p14ARF, p15INK4b
and p16INK4a methylation and HPV infection
in cervical cancer patients (P>0.05) (data not shown).
 | Table IIFrequency of methylation of
p14ARF, p15INK4b
and p16INK4a with relative risk in
cervical cancer patients and controls. |
Table II
Frequency of methylation of
p14ARF, p15INK4b
and p16INK4a with relative risk in
cervical cancer patients and controls.
| Methylation | Patient n=125
(%) | Control n=100
(%) | OR (95% CI) | P-value |
|---|
|
p14ARF | 11 (8.8) | 2 (2) | 1.57
(1.21–2.05) | 0.059 |
|
p15INK4b | 14 (11.2) | 3 (3) | 1.54
(1.20–1.99) | 0.03 |
|
p16INK4a | 45 (36) | 1 (1) | 2.19
(1.85–2.59) | 0.000 |
Methylation of p16INK4a was
found to be statistically significant in patients of the ≤45 and
>45 years age groups (P<0.05). The risk of cervical cancer
was increased 2.15-fold in the ≤45-year age group (OR=2.15; 95%
CI=1.69–2.74), and 2.22-fold in the >45-year age group (OR=2.22;
95% CI=1.75–2.81). p16INK4a
hypermethylation was found to be statistically significant in the
passive smokers (P<0.05). The risk of cervical cancer increased
2.20-fold (OR=2.20; 95% CI=1.39–3.48) and 2.16-fold (OR=2.16; 95%
CI=1.67–2.80) in association with p16INK4a
methylation in the passive smokers and OC users, respectively
(Tables III and IV).
 | Table IIIFrequency of methylation of
p14ARF, p15INK4b
and p16INK4a compared to passive smoking
in cancer patients and controls. |
Table III
Frequency of methylation of
p14ARF, p15INK4b
and p16INK4a compared to passive smoking
in cancer patients and controls.
| Methylation | Patient n=26
(%) | Control n=12
(%) | OR (95% CI) | P-value |
|---|
|
p14ARF | 7 (26.9) | 2 (16.6) | 1.19
(0.77–1.84) | 0.68 |
|
p15INK4b | 8 (30.7) | 1 (8.3) | 1.43
(0.99–2.07) | 0.22 |
|
p16INK4a | 16 (61.5) | 0 (0) | 2.20
(1.39–3.48) | 0.001 |
 | Table IVFrequency of methylation of
p14ARF, p15INK4b
and p16INK4a compared to use of oral
contraceptives in cancer patients and controls. |
Table IV
Frequency of methylation of
p14ARF, p15INK4b
and p16INK4a compared to use of oral
contraceptives in cancer patients and controls.
| Methylation | Patient n=62
(%) | Control n=43
(%) | OR (95% CI) | P-value |
|---|
|
p14ARF | 4 (6.4) | 1 (2.3) | 1.38
(0.86–2.20) | 0.64 |
|
p15INK4b | 9 (14.5) | 3 (6.9) | 1.32
(0.91–1.91) | 0.35 |
|
p16INK4a | 28 (45.1) | 1 (2.3) | 2.16
(1.67–2.80) | 0.000 |
A significant association between the methylation of
p16INK4a and risk of cervical cancer was
observed in patients with AFSI ≤20 and >20 years (P<0.001).
In addition, the hypermethylation of
p16INK4a was associated with a 2.25-fold
increased risk of cervical cancer in patients with AFSI ≤20 years
and 2.11-fold in patients with AFSI >20 years.
Bisulfite sequencing
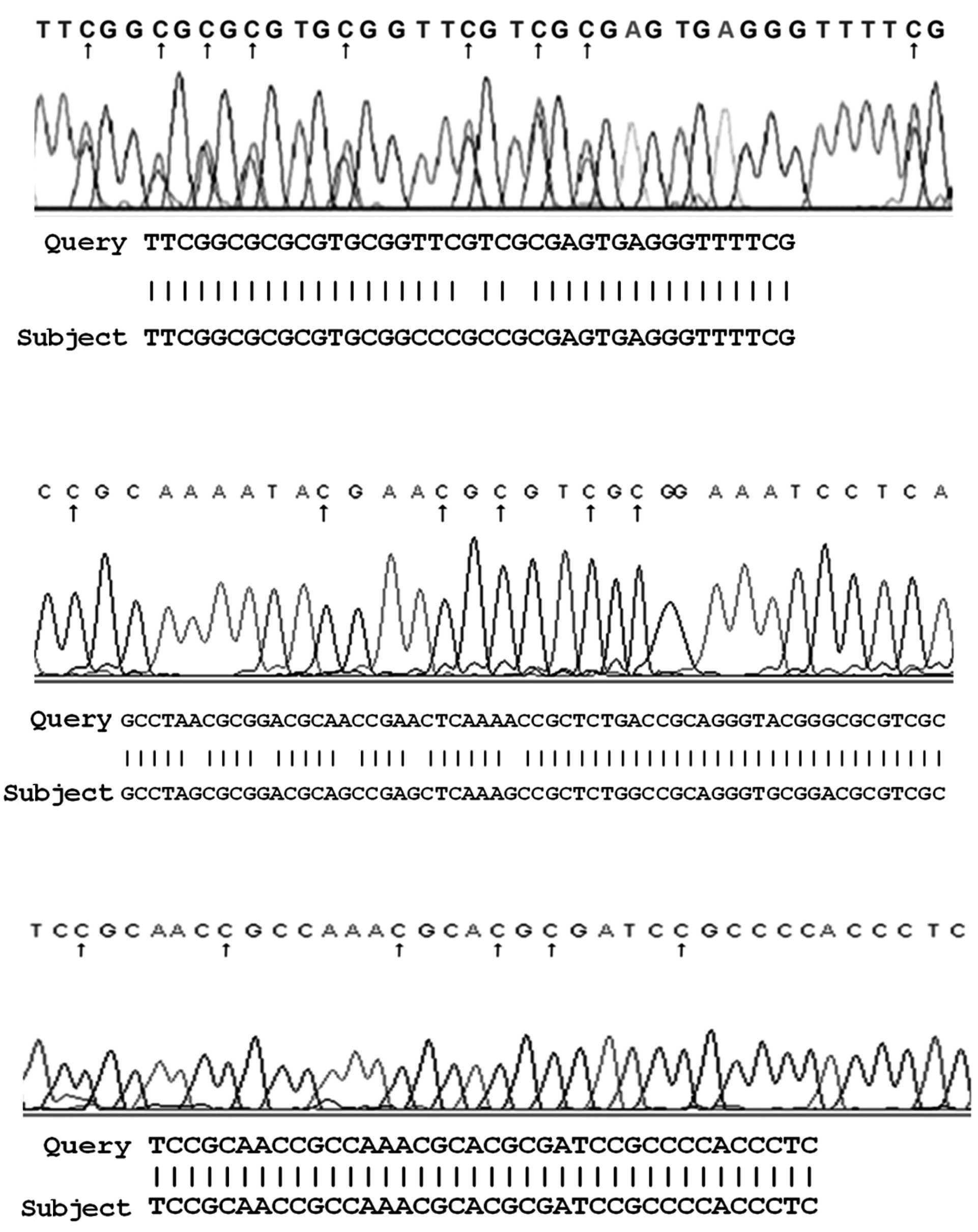
The bisulfite sequencing of samples hypermethylated
in the promoter region of the three studied genes revealed a
conversion of unmethylated cytosine, but not methylated cytosine
(Fig. 2).
Correlation of methylation status with
stage of cervical cancer
There was no increase in the percentage of
methylation of p14ARF and
p15INK4b with stage (data not shown). By
contrast, an increase in the percentage of methylation of
p16INK4a was observed with the increase in
stage of cervical cancer. The methylation of
p16INK4a was found to be higher in the
advanced stages. However, to calculate the significance of
methylation with the stage of disease more samples should be
studied. The γ-coefficient was also not found to be significant for
the three genes; p16 (γ=0.200, P=0.115), p14
(γ=0.314, P=0.166) and p15 (0.348, P=0.079).
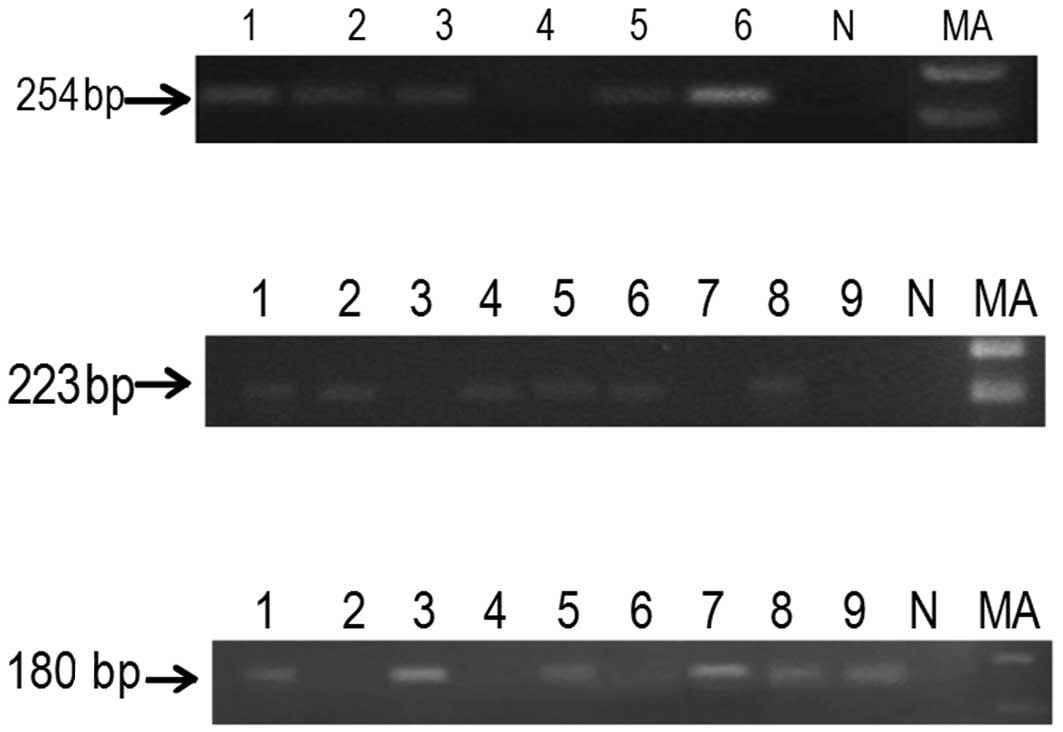
Semi-quantitative RT-PCR
The mRNA expression of
p14ARF and
p15INK4b was significantly reduced in
patients with a hypermethylated promoter compared to the patients
with an unmethylated promoter (Fig.
3). The mRNA expression was significantly reduced in patients
with a methylated promoter (P<0.001) of
p16INK4a compared to patients with an
unmethylated promoter. Downregulation at the transcriptional level
was approximately 85.3% due to the hypermethylation of
p16INK4a (Table V).
 | Table VmRNA expression in methylated and
unmethylated p14ARF,
p15INK4b and
p16INK4a in cervical cancer patients. |
Table V
mRNA expression in methylated and
unmethylated p14ARF,
p15INK4b and
p16INK4a in cervical cancer patients.
| Gene | Mean (±SE)
| Downregulated
expression of genes (%) | P-value |
|---|
| Unmethylated | Methylated |
|---|
|
p14ARF | 0.858 (±0.02) | 0.594 (±0.03) | 31.0 | 0.00 |
|
p15INK4b | 0.795 (±0.02) | 0.634 (±0.01) | 20.2 | 0.03 |
|
p16INK4a | 0.940 (±0.03) | 0.118 (±0.03) | 85.3 | 0.000 |
Discussion
Cervical cancer is one of the most common types of
cancer that affects female reproductive organs. It is the seventh
most common type of cancer overall and the third most common among
females (1). In developing
countries, cervical cancer is often the most common cancer in
females and constitutes up to 25% of all female cancers (2). The risk factors of cervical cancer
include HPV infection and cofactors including age, smoking, oral
contraceptives, low age at first sexual intercourse, deficient diet
and a family history of cervical cancer.
HPV infection is the cause of almost all cases of
cervical cancer (18). HPV 16 is
the most commonly occurring subtype in cervical neoplasia, however,
HPV 18 is associated with more advanced cervical neoplasia.
The incidence of cervical cancer has been reported
to increase with age. The results of several case-control and
cross-sectional studies indicate that females married to smokers
experience a higher risk of cervical neoplasia than females married
to non-smokers (19).
Several studies have reported that prolonged use of
oral contraceptives increases the risk of cervical cancer. Early
AFSI has been associated with an increased risk of high-risk HPV
infection, a sexually transmitted infection, that in susceptible
females is responsible for virtually all cases of invasive cervical
cancer (ICC) (20).
The majority of recent studies have focused on the
study of epigenetic changes resulting in many types of neoplasia
(21). DNA methylation was the
first epigenetic alteration to be observed in cancer cells
(22). Recent studies have found
that certain genes are hypermethylated in preinvasive lesions,
raising the possibility that testing for methylation of these genes
may prove to be a useful screening tool (23), particularly in cervical cancer as it
evolves through a series of well-defined stages.
Methylation status of the
p14ARF gene promoter has been found to be
a useful biomarker for pathological and clinical outcome and
prognosis of patients with colon, oral squamous cell carcinoma
(24) and non-small cell lung
cancer. However, of a panel of 16 genes selected for a study in
cervical cancer, p14ARF did not
demonstrate promoter methylation (23).
Hypermethylation of p14ARF
was present in 8.8% of patients, and in the present population it
was not found to be significant. Additionally, the risk factors did
not have any significant impact on the methylation pattern of
p14ARF.
In this study, due to a low frequency of
p14ARF and no significant correlation with
risk factors, p14ARF does not appear to be
a significant biomarker for the diagnosis of cervical cancer.
p15 promoter methylation was approximately
11.1% (5/45) in non-small cell lung cancer (25). However, hypermethylation of
p15INK4b has not yet been reported in
cervical cancer.
In this study, p15INK4b
hypermethylation was observed in 11.2% of patients.
p15INK4b methylation was found to be
significant in cervical cancer patients in the north Indian
population, and its methylation was found to marginally increase
the risk of cervical cancer (P<0.05). This is the first study
demonstrating significant hypermethylation of this gene in cervical
cancer.
A significant trend towards an increase in the risk
of cervical cancer was not observed with methylation of the
p15INK4b gene in association with risk
factors in the patients compared to the healthy controls.
The methylation profile and alteration in
transcription suggests that the downregulated expression of
p15INK4b by aberrant promoter methylation
is an inactivating event in cervical cancer. However, this study
requires extension to a larger population size in order to verify
whether p15 can be used as a significant or reliable marker
in cervical cancer in the north Indian population.
p16 is the most commonly altered gene in
human malignancies (4).
Hypermethylation of the p16 tumor suppressor gene and its
effect on transcriptional downregulation or silencing is one of the
major mechanisms of p16INK4a gene
inactivation in various types of cancer, including cervical
carcinoma (26).
p16INK4a methylation was
found to be significant in cervical cancer patients in the north
Indian population and its methylation was found to increase the
risk of cervical cancer (P<0.001). The present result is
consistent with other reports on cervical cancer (23,26) on
other population groups.
Previous studies have not identified an association
between p16 alterations, including mutation or promoter
hypermethylation, and HPV infection (27). In this study, a significant trend
towards an increased risk of cervical cancer in association with
HPV infection was not observed with methylation of the
p16INK4a gene in patient samples compared
to healthy controls.
Findings of a recent study showed hypermethylation
of the p16INK4a gene promoter to be
unchanged according to the patient age (28). Similar observations were also
obtained in the present study, suggesting that age is not
associated with the increase in the risk of cervical cancer with
respect to p16 hypermethylation.
Aberrant p16 methylation has been reported to
be associated with active tobacco use in patients with squamous
cell carcinoma and high-grade dysplasia (29). Methylation of
p16INK4a was found to be statistically
significant even in the case of passive smokers, which increased
the risk of cervical cancer. It also significantly increased the
risk of cervical cancer among OC users (P<0.001). AFSI did not
have a significant impact on the methylation of
p16INK4a in the present study.
Results of a previous study showed that high-stage
cancers exhibited an increased promoter methylation frequency for
p16 (22). In the present
study, an increasing trend of methylation of p16 was
observed with increasing pathological change, confirming p16
as a biomarker.
The results from the present study have shown that
downregulation of the mRNA expression of
p16INK4a by its aberrant promoter
methylation is a significant inactivating event in cervical cancer.
This gene may be used as a significant and reliable biomarker in
cervical cancer in the north Indian population. Hypermethylation of
p16 was also found to be significantly associated with
passive smoking and OC use. Hypermethylation of p15 was also
observed to be significant although at a low frequency.
Although the present findings require extension to a
larger series, this study suggests that the pattern of aberrant
methylation in females with or without dysplasia may help identify
subgroups at an increased risk of histological progression or
cancer development.
Acknowledgements
We acknowledge the financial assistance provided by
the Council of Scientific and Industrial Research (CSIR), India, to
AKJ.
References
|
1
|
Globocan. 2008, http://wwwdep.iarc.fr/globocan/database.htm.
|
|
2
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Virmani AK, Muller C, Rathi A,
Zoechbauer-Mueller S, Mathis M and Gazdar AF: Aberrant methylation
during cervical carcinogenesis. Clinical Cancer Res. 7:584–589.
2001.PubMed/NCBI
|
|
4
|
Hirama T and Koeffer HP: Role of the
cyclin dependent kinase inhibitors in the development of cancer.
Blood. 86:841–854. 1995.PubMed/NCBI
|
|
5
|
Hannon GJ and Beach D: p15INK4B is a
potential effector of TGF-beta-induced cell cycle arrest. Nature.
371:257–261. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liggett WH Jr and Sidransky D: Role of the
p16 tumor suppressor gene in cancer. J Clin Oncol. 16:1197–1206.
1998.PubMed/NCBI
|
|
7
|
Merlo A, Herman JG, Mao L, Lee DJ,
Gabrielson E, Burger PC, Baylin SB and Sidransky D: 5′ CpG island
methylation is associated with transcriptional silencing of the
tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med.
1:686–692. 1995.
|
|
8
|
Zhang SJ, Endo S, Ichikawa T, Washiyama K
and Kumanishi T: Frequent deletion and 5 CpG island methylation of
the p16 gene in primary malignant lymphoma of the brain. Cancer
Res. 58:1231–1237. 1998.PubMed/NCBI
|
|
9
|
Esteller M, Corn PG, Baylin SB and Herman
JG: A gene hypermethylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
10
|
Rocco JW and Sidransky D:
p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res.
264:42–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng Q, Balasubramanian A, Hawes SE, Toure
P, Sow PS, Dem A, Dembele B, Critchlow CW, Xi L, Lu H, McIntosh MW,
Young AM and Kiviat NB: Detection of hypermethylated genes in women
with and without cervical neoplasia. J Natl Cancer Inst.
97:273–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D’Costa J, Saranath D, Dedhia P, Sanghvi V
and Mehta RA: Detection of HPV-16 genome in human oral cancers and
potentially malignant lesions from India. Oral Oncol. 34:413–420.
1998.PubMed/NCBI
|
|
13
|
Park JS, Dong SM, Kim HS, Lee JY, Jong Um
S, Park IS, Kim SJ and Namkoong SE: Detection of p16 gene
alteration in cervical cancer using tissue microdissection and LOH
study. Cancer Lett. 136:101–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeh KT, Chang GA, Lin TH, Wang YF, Tien N,
Chang JY, Chen CJ and Shih MC: Epigenetics changes of tumor
suppressor genes, p15, p16, VHIL and p53 in oral
cancer. Oncol Rep. 10:659–663. 2003.PubMed/NCBI
|
|
15
|
Esteller M, Tortola S, Toyota M, Capella
G, Peinado MA, Baylin SB and Herman JG: Hypermethylation-associated
inactivation of p14ARF is independent of
p16INK4a methylation and p53
mutational status. Cancer Res. 60:129–133. 2000.
|
|
16
|
Herman JG, Jen J, Merlo A and Baylin SB:
Hypermethylation associated inactivation indicates a tumor
suppressor role for p15INK4b. Cancer Res. 56:722–727.
1996.PubMed/NCBI
|
|
17
|
Perrone F, Tabano S, Colombo F, Dagrada G,
Birindelli S, Gronchi A, Colecchia M, Pierotti MA and Pilotti S:
p15INK4b, p14ARF
and p16INK4a inactivation in sporadic and
neurofibromatosis type 1-related malignant peripheral nerve sheath
tumors. Clinical Cancer Research. 9:4132–4138. 2003.
|
|
18
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tay SK and Tay KJ: Passive cigarette
smoking is a risk factor for cervical neoplasia. Gynecol Oncol.
93:116–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Louie KS, de Sanjose S, Diaz M,
Castellsagué X, Herrero R, Meijer CJ, Shah K, Franceschi S, Muñoz N
and Bosch FX; International Agency for Research on Cancer
Multicenter Cervical Cancer Study Group. Early age at first sexual
intercourse and early pregnancy are risk factors for cervical
cancer in developing countries. Br J Cancer. 100:1191–1197. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jha AK, Nikbakht M, Parashar G,
Shrivastava A, Capalash N and Kaur J: Reversal of hypermethylation
and reactivation of the RARβ2 gene by natural compounds in cervical
cancer lines. Folia Biol (Praha). 56:195–200. 2010.PubMed/NCBI
|
|
22
|
Jha AK, Kumar S, Nikbakht M, Sharma V and
Kaur J: Epigenetics and its role in ageing and cancer. J Med Med
Sci. 2:696–713. 2011.
|
|
23
|
Narayan G, Arias-Pulido H, Koul S, Vargas
H, Zhang FF, Villella J, Schneider A, Terry MB, Mansukhani M and
Murty VV: Frequent promoter methylation of CDH1, DAPK, RARβ and
HIC1 genes in carcinoma of cervix uteri: its relationship to
clinical outcome. Mol Cancer. 2:242003.PubMed/NCBI
|
|
24
|
Ishida E, Nakamura M, Ikuta M, Shimada K,
Matsuyoshi S, Kirita T and Konishi N: Promotor hypermethylation of
p14ARF is a key alteration for progression of oral
squamous cell carcinoma. Oral Oncol. 41:614–622. 2005.
|
|
25
|
Kurakawa E, Shimamoto T and Utsumi K:
Hypermethylation of p16INK4a and
p15INK4b gene in non-small cell lung cancer. Int J Oncol.
19:277–281. 2001.
|
|
26
|
Jeong DH, Youm MY, Kim NY, Lee KB, Sung
MS, Yoon HK and Kim KT: Promoter methylation of p16, DAPK, CDH1 and
TIMP-3 genes in cervical cancer: correlation with clinicopathologic
characteristics. Int J Gynecol Cancer. 16:1234–1240. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tripathi A, Banerjee S, Roy A,
Roychowdhury S and Panda CK: Alterations of the p16 gene in uterine
cervical carcinoma from Indian patients. Int J Gynecol Cancer.
13:472–479. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Attaleb M, El hamadani W, Khyatti M,
Benbacer L, Benchekroun N, Benider A, Amrani M and El Mzibri M:
Status of p16INK4a and E-cadherin gene promoter
methylation in Moroccan patients with cervical carcinoma. Oncol
Res. 184:185–192. 2009.
|
|
29
|
Lea JS, Coleman R, Kurien A, Schorge JO,
Miller SD, Minna JD and Muller YC: Aberrant p16 methylation is a
biomarker for tobacco exposure in cervical squamous cell
carcinogenesis. Am J Obstet Gynecol. 190:674–679. 2004. View Article : Google Scholar : PubMed/NCBI
|