Introduction
Pancreatic cancer is one of the leading causes of
cancer-related mortality worldwide (1). The 5-year survival rate of patients
with pancreatic cancer is only approximately 4 to 5%; therefore,
the mortality rate is almost on par with the incidence rate
(2). Resectability is considered as
the most significant prognostic factor (3,4).
However, even after curative resection, the 5-year survival rate is
only 10 to 25%, seemingly due to the high rates of local
recurrence, liver metastasis, lymph node recurrence and peritoneal
dissemination (5). Gemcitabine, a
pyrimidine analog, was launched in 1996, and has been used as the
first-line agent for the treatment of pancreatic cancer (6). Recently, the efficacies of adjuvant
and neoadjuvant chemotherapy (NAC) with gemcitabine for pancreatic
cancer were reported (7,8).
Chemotherapy and radiotherapy is known to induce
apoptotic cell death in malignant tumors (9). In addition, it has recently been
reported that anti-cancer treatments may also induce
epithelial-to-mesenchymal transition (EMT) in cancer cells, which
may play an important role in the aggressive behavior of tumors
(10–12). Previous studies in solid tumor cell
lines have linked the development of chemoresistance to the
occurrence of EMT (10,13). EMT is a key event in the tumor
invasion process, whereby epithelial cell layers lose polarity and
cell-cell contacts and undergo a marked remodeling of the
cytoskeleton (14). Among the
hallmarks of EMT are the loss of E-cadherin (14), expression of N-cadherin, the
so-called cadherin switch (15),
expression of vimentin, a mesenchymal marker, nuclear translocation
of β-catenin, and increased production of transcription factors,
including Snail, Twist and Slug (16). Although the precise mechanisms
underlying these alterations are unknown, they appear to be linked
to alterations in β-catenin signaling, and E-cadherin functions,
and activation of the transcription factors Twist, Slug, and/or
Snail (16,17). Shah et al demonstrated an
increased number of cancer stem cells, which show aggressive
behavior and EMT in a gemcitabine-resistant pancreatic cancer cell
population (13).
Accumulating evidence suggests that pancreatic
stromal cells promote the progression and EMT of pancreatic cancer
by increasing cancer cell proliferation and invasion as well as by
protecting the cells from radiation- and gemcitabine-induced
apoptosis (18). There are a number
of reports on the induction of chemoresistance linked to EMT and
cancer stem cells in vitro. In stromal-rich tumors, such as
scirrhous gastric cancer and pancreatic cancer, the correlation
between the tumor and stromal cells is particularly important.
However, there is at present no evidence on the expression of EMT
and stem cell markers in pancreatic cancer cells following
chemotherapy in vivo.
In this study, we comparatively evaluated the
expression of the apoptosis marker M30 (caspase-cleaved cytokeratin
18 fragments), EMT marker Snail and stem cell marker CD44 in
resected pancreatic cancer specimens from patients administered,
and those not administered, preoperative NAC with gemcitabine.
Material and methods
Patients
Between January 2005 and December 2010, 34
pancreatic ductal adenocarcinoma patients (21 males and 13 females)
underwent surgery at the Department of Gastroenterologic Surgery,
Kanazawa University Hospital (Kanazawa, Japan). Among them, 13
patients received preoperative chemotherapy with gemcitabine (NAC
group). During the same period, 21 patients did not receive
preoperative chemotherapy but underwent surgery (control group).
Written informed consent was obtained from all 34 patients prior to
their enrollment in the study, and the treatment was undertaken
with the approval of the local medical ethics committee.
Pathological specimens
Formalin-fixed and paraffin-embedded specimens were
retrieved from the surgical pathology files of the Pathology
Department of Kanazawa University Hospital. The grading system of
Evans et al (19) was used
to determine the pathological effects of preoperative chemotherapy.
The number of cytological changes and the amount of destruction of
the tumor were graded on a scale of I–IV, as follows: grade I,
characteristic cytological changes of malignancy are present, but
little (<10%) or no tumor cell destruction is evident; grade
IIa, destruction of 10–50% of the tumor cells; grade IIb,
destruction of 51–90% of tumor cells; grade III, few (<10%)
viable-appearing tumor cells are present; grade IIIM, sizable pools
of mucin are present; grade IV, no viable tumor cells are present;
grade IVM, acellular pools of mucin are present.
Immunohistochemical examination
For immunohistochemical staining, the Dako Envision
system, which uses dextran polymers conjugated with horseradish
peroxidase (Dako, Carpinteria, CA, USA), was employed to avoid any
endogenous biotin contamination. Tissues were fixed with 10%
formaldehyde in phosphate-buffered saline, embedded in paraffin,
and cut into 5-μm tissue sections. The sections were deparaffinized
in xylene and rehydrated in a graded ethanol series. Endogenous
peroxidase was blocked by immersing the sections in 3%
H2O2 in 100% methanol for 20 min at room
temperature. Antigen retrieval was achieved by microwaving sections
at 95°C for 10 min in 0.001 M citrate buffer (pH 6.7). After
blocking the endogenous peroxidase, the sections were incubated
with Protein Block Serum-Free (Dako) at room temperature for 10 min
to block non-specific staining. The sections were then incubated
for 2 h at room temperature with 1:50 diluted mouse monoclonal
antibodies against M30 (Peviva, Bromma, Sweden), CD44 (Thermo
Fisher Scientific Anatomical Pathology, Fremont, CA, USA) and Snail
(Santa Cruz Biotechnology, Santa Cruz, CA, USA). Peroxidase
activity was detected with the enzyme substrate 3-amino-9-ethyl
carbazole. For the negative controls, the sections were incubated
with Tris-buffered saline without the primary antibodies. Samples
in which at least 10% of tumor cells were slightly counterstained
with Meyer hematoxylin were defined as showing positive staining.
The frequency of cells positive for each antibody was reported semi
quantitatively as follows: (−), no reaction; (+), mild, with
<30% of cells positive; (++), moderate, with 30–70% of cells
positive; and (+++), marked, with >70% of cells positive. A
positive expression was defined as staining of >30% of the
cancer cells (++ or +++).
Statistical analysis
Categorical variables were compared using the
Chi-square test. For statistical analysis, P values were calculated
using a two-tailed test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Between January 2005 and December 2010, 34
pancreatic ductal adenocarcinoma patients (21 males and 13 females)
underwent surgery. Among them, 13 patients, 7 males and 6 females,
with an average age of 62.6 years (range, 51–77) underwent
preoperative chemotherapy with gemcitabine (NAC group). During the
same period, 21 pancreatic cancer patients who did not receive
preoperative chemotherapy also underwent surgical resection
(control group). These 21 patients comprised 14 male and 7 female
patients, with an average age of 66.0 years (range, 52–80). The
patient and tumor characteristics are shown in Table I. There were no significant
differences in these characteristics between the two treatment
groups.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | NAC | Control | P-value |
|---|
| Patients (n) | 13 | 21 | |
| Gender (n) |
| Male | 7 | 14 | |
| Female | 6 | 7 | N.S. |
| Age (years) |
| Median | 62.6 | 66.0 | |
| Range | 51–77 | 52–80 | N.S. |
| Location (n) |
| Head of
pancreas | 9 | 11 | |
| Pancreas body and
tail | 3 | 10 | N.S. |
Histopathological characteristics and
pathological response
The histopathological characteristics of the tumors
in each group are shown in Table
II. Nno significant differences were found with respect to the
tumor size, invasion of the serosa, retroperitoneum, vessel or
nerve, or lymph node metastasis between the NAC group and the
control group. In the NAC group, the tumor specimens showed
evidence of tumor cell injury, although, none of the patients
exhibited a complete pathological response. The treatment effect
was judged by Evans grading to be grade IIa in 11 patients and
grade IIb in 2 patients.
 | Table IIHistopathological characteristics. |
Table II
Histopathological characteristics.
| Histopathological
characteristics | NAC | Control | P-value |
|---|
| Tumor size (mm) |
| Average | 30.1 | 30.6 | |
| Range | 16–55 | 17–53 | N.S. |
| Serosal invasion | 53.8% | 40.0% | N.S. |
| Retroperitoneal
invasion | 84.6% | 61.9% | N.S. |
| Vascular
invasion | 84.6% | 90.5% | N.S. |
| Lymph node
metastasis | 76.9% | 57.1% | N.S. |
| Perineural
invasion | 100.0% | 90.5% | N.S. |
Immunohistochemical examination
Approximately 34 surgically resected specimens of
pancreatic ductal adenocarcinoma were immunohistochemically
examined for M30, CD44 and Snail expression (Table III).
 | Table IIIExpression of M30, CD44 and
Snail. |
Table III
Expression of M30, CD44 and
Snail.
| Markers | NAC | Control | P-value |
|---|
| M30 |
| − or + | 5 | 19 | |
| ++ or +++ | 8 | 2 | <0.05 |
| CD44 |
| − or + | 6 | 18 | |
| ++ or +++ | 7 | 3 | 0.02 |
| Snail |
| − or + | 6 | 12 | |
| ++ or +++ | 7 | 9 | N.S. |
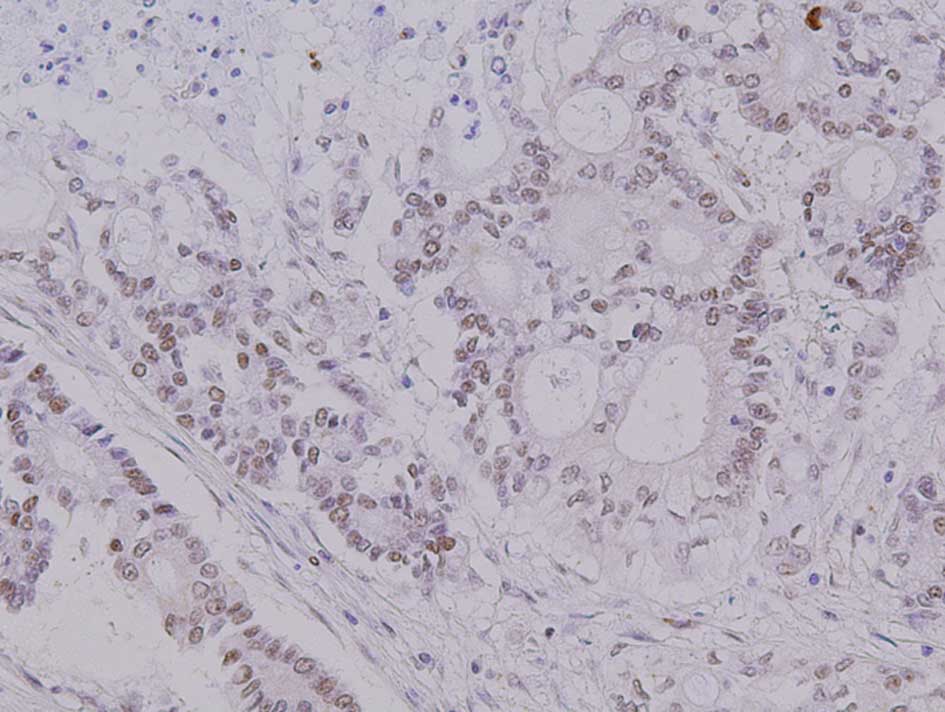
The apoptosis marker M30 was mainly expressed in the
nuclei of the cancer cells (Fig.
1). A positive M30 expression was found in 8 of the 13 cases
(61.5%) of the NAC group and in 2 of the 21 cases (9.5%) of the
control group; the difference between the two groups was
statistically significant (P=0.002).
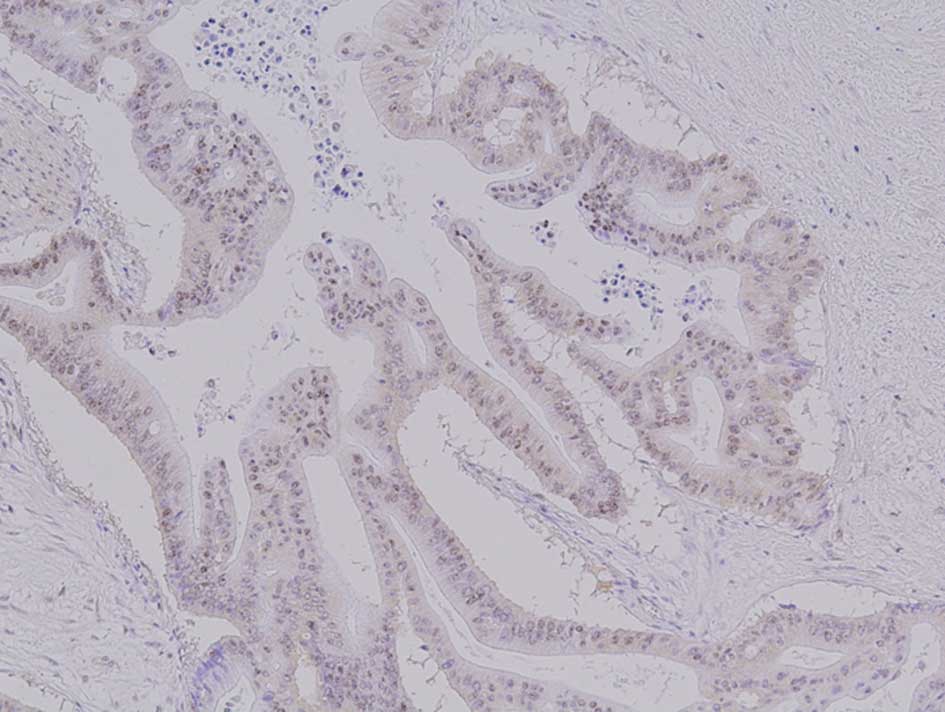
The pancreatic cancer stem cell marker CD44 was
mainly expressed in the cell surface and cytoplasm of a proportion
of cancer cells (Fig. 2). A
positive CD44 expression was found in 7 of the 13 cases (53.8%) in
the NAC group and in 3 of the 21 cases (14.3%) in the control
group; the difference between the two groups was significant
(P=0.02).
The EMT marker Snail was mainly expressed in the
nuclei and cytoplasm of the cancer cells (Fig. 3). A positive Snail expression was
observed in 7 of the 13 cases (53.8%) in the NAC group, and in 9 of
the 21 cases (42.9%) in the control group; the difference between
two groups was not statistically significant. We also examined the
expression of other EMT markers such as E-cadherin, N-cadherin and
vimentin. However, no significant differences were found in the
frequency of expression of these markers between the NAC group and
the control group (data not shown).
Discussion
Despite the rapid advances in diagnostic and
surgical techniques, the prognosis of pancreatic cancer remains
dismal. Pancreatic ductal adenocarcinoma is characterized by its
high malignant potential, showing rapid progression, early
metastasis, and limited response to chemotherapy and radiotherapy
(18). The efficacies of adjuvant
and neoadjuvant chemotherapy with gemcitabine for pancreatic cancer
(7,8) and the induction of apoptotic cell
death in vitro by chemotherapy and radiotherapy in malignant
tumors (9) were reported.
Caspase-mediated cleavage of the cytokeratin 18 (CK18) contributes
to the degradation of the intracellular cytoskeleton if epithelial
cells undergo apoptosis. Recently, plasma caspase-cleaved CK18
fragments (M30) have been reported as specific apoptosis markers
and have important clinical biomarker utility (20). In this study, all tumor specimens
showed evidence of tumor cell injury following preoperative
gemcitabine chemotherapy, and significantly higher expression
levels of the apoptosis marker M30 were detected in the NAC group
as compared with those in the control group. This finding suggests
that gemcitabine induces apoptosis in pancreatic cancer in
vivo.
By contrast, gemcitabine-resistant pancreatic tumor
cells are more invasive, and show increased migratory potential and
increased expression levels of the stem cell marker CD44 (21). It has been reported that
chemoradiation-resistant pancreatic cancer cells are similar to
cancer stem cells and undergo EMT, suggesting a possible link
between chemoradiation resistance-induced EMT and generation of
cancer stem cells (13). Moreover,
cancer cells showing expression of the stem cell marker CD44 have
been reported to be responsible for gemcitabine resistance of
pancreatic cancer (22). In this
study, the NAC group showed a significantly higher expression
frequency of CD44 than the control group. In vivo pancreatic
cancers resistant to gemcitabine are rich in cancer stem cells. In
cases in which chemotherapy was effective (grade IIb), the majority
of the residual tumor cells were found to be positive for CD44
expression.
The interaction between pancreatic cancer cells and
stromal cells, such as pancreatic stellate cells and
myofibroblasts, is receiving increasing attention. Accumulating
evidence suggests that pancreatic stellate cells promote the EMT of
cancer cells and the progression of pancreatic cancer by increasing
cancer cell proliferation/invasion and by protecting them from
radiation- and gemcitabine-induced apoptosis (18,23).
Such evidence may explain the limited response of pancreatic cancer
in vivo to chemotherapy and radiotherapy. In the present
study, the EMT marker Snail was detected at the same level in the
NAC and control groups. It has been reported that Snail expression
in pancreatic cancer is observed in approximately 36% of the cells,
and that over-expression of Snail increases chemoresistance
(24). These results suggest that
EMT is induced in pancreatic cancer cells, irrespective of whether
the patient receives chemotherapy or not, and the interaction
between cancer cells and stromal cells assumes a crucial role in
pancreatic cancer.
In the NAC group, the tumor specimens showed
evidence of tumor cell injury; however, there were no cases showing
a complete pathological response. Furthermore, preoperative
chemotherapy with gemcitabine did not result in any significant
reduction of serosal/retroperitoneal/perineural/vascular invasion
or lymph node metastases. These results indicate that preoperative
chemotherapy for pancreatic cancer with gemcitabine is somewhat
effective, but that it does not reduce the tumor burden or the
required extent of resection. Therefore, chemoradiotherapy is
frequently required for the treatment of unresectable pancreatic
cancer (25). Findings of a
randomized clinical trial demonstrated that preoperative
chemo-radiotherapy is effective for the local control of pancreatic
cancer, but that additional radiotherapy tended to increase distant
metastasis (26). Moreover, it is
reported that several anti-cancer treatments are capable of
inducing EMT in cancer tissues (10–12).
In conclusion, preoperative chemotherapy with
gemcitabine is effective for pancreatic cancer in vivo.
However, this therapy does not result in any decrease of the tumor
burden in tumors rich in cancer stem cells or in the required
extent of surgical resection. Therefore, counter measures for
cancer stem cells and EMT induced by stromal cells are important in
pancreatic cancer treatment.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 58:71–96. 2008.
|
|
3
|
Conlon KC, Klimstra DS and Brennan MF:
Long-term survival after curative resection for pancreatic ductal
adenocarcinoma. Clinico-pathologic analysis of 5-year survivors.
Ann Surg. 223:273–279. 1996.PubMed/NCBI
|
|
4
|
Matsuno S, Egawa S, Fukuyama S, et al:
Pancreatic cancer registry in Japan: 20 years of experience.
Pancreas. 28:219–230. 2004.PubMed/NCBI
|
|
5
|
Kayahara M, Nagakawa T, Ueno K, et al: An
evaluation of radical resection for pancreatic cancer based on the
mode of recurrence as determined by autopsy and diagnostic imaging.
Cancer. 72:2118–2123. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burris HA, Moore MJ, Andersen J, et al:
Improvements in survival and clinical benefit with gemcitabine as
first-line therapy for patients with advanced pancreatic cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
7
|
Ottle H, Post S, Neuhaus P, et al:
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative-intent resection of pancreatic cancer: a
randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar
|
|
8
|
Golcher H, Brunner T, Grabenbauer G, et
al: Preoperative chemo-radiation in adenocarcinoma of the pancreas.
A single centre experience advocating a new treatment strategy.
EJSO. 34:756–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goses MJ, Dressen RC, Rutten HJ, et al:
Prepoerative radio-chemotherapy is successful also in patients with
locally advanced rectal cancer who have intrinsically high
apoptotic tumors. Ann Oncol. 19:2026–2032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-mesenchymal transition in
colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsukamoto H, Shibata K, Kajiyama H, et al:
Irradiation-induced epithelial-mesencymal transition (EMT) related
to invasive potential in endometrial carcinoma cells. Gynecol
Oncol. 107:500–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tajima H, Ohta T, Shoji Y, et al:
Expression of epithelial-mesenchymal transition markers in locally
recurrent hepatocellular carcinoma after radiofrequency ablation.
Exp Therap Med. 1:347–350. 2010. View Article : Google Scholar
|
|
13
|
Shah AN, Summy JM, Zhang J, Park SI,
Darikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hay ED and Zuk A: Transformations between
epithelium and mesenchyme: normal, pathological and experimentally
induced. Am J Kidney Dis. 26:678–690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiery JP: Epithelial-mesenchymal
transitions in tumor progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kikuta K, Masamune A, Watanabe T, et al:
Pancreatic stellate cells promote epithelial-mesenchymal transition
in pancreatic cancer cells. Biochem Biophys Res Commun.
403:380–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Evans DB, Rich TA, Byrd DR, et al:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dive C, Smith RA, Garner E, et al:
Considerations for the use of plasma cytokeratin 18 as a biomarker
in pancreatic cancer. Br J Cancer. 102:577–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du Z, Qin R, Wei C, et al: Pancreatic
cancer cells resistant to chemoradiotherapy rich in
‘stem-cell-like’ tumor cells. Dig Dis Sci. 10:741–750.
2010.PubMed/NCBI
|
|
22
|
Hong SP, Wen J, Bang S, Park S and Song
SY: CD44-positive cells are responsible for gemcitabine resistance
in pancreatic cancer cells. Int J Cancer. 125:2323–2331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Müerköster SS, Werbing V, Koch D, et al:
Role of myofibroblasts in innate chemoresistance of pancreatic
carcinoma – epigenetic downregulation of caspase. Int J Cancer.
123:1751–1760. 2008.PubMed/NCBI
|
|
24
|
Yin T, Wang C, Liu T, Zhao G, Zha Y and
Yang M: Expression of Snail in pancreatic cancer promotes
metastasis and chemoresistsance. J Surg Res. 141:196–203. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cauffert B, Mornex F, Bonnetain F, et al:
Phase III trial comparing intensive induction chemoradiotherapy (60
Gy, infusional 5-FU and intermittent cicplatin) followed by
maintenance gemcitabine with gemcitabine alone for locally advanced
unresectable pancreatic cancer. Definitive results of the 2000-01
FFCD/SFRO study. Ann Oncol. 19:1592–1599. 2008.
|
|
26
|
Van Laethem JL, Hammel P, Mornex F, et al:
Adjuvant gemcitabine alone versus gemcitabine-based
chemoradiotherapy after curative resection for pancreatic cancer: a
randomized EORTC-40013-22012 /FFCD-9203/GERCOR phase II study. J
Clin Oncol. 28:4450–4456. 2010.
|