Introduction
Metastasis is a multi-step biological process and
becomes the primary cause of cancer-related mortality in most solid
tumors. Therefore, preventing the metastatic spread of cancer cells
is a principal task in cancer therapy. During the metastatic
process, cells were required to detach from their primary site, and
then to migrate to the lymphatic and circulatory systems (1). In metastasis, the majority of cells
undergo anoikis, which is induced by the detachment of
anchorage-dependent cells from the surrounding extracellular matrix
(2). Acquisition of
anoikis-resistance is therefore a prerequisite for the metastasis
of cancer cells. Recently, several reports have confirmed that the
breakdown of anoikis contributes prominently to the malignancy of
breast (3), colon (4), lung (5) and head and neck carcinomas (6). However, few studies have focused on
the role of anoikis-resistance in hepatocellular carcinoma (HCC)
metastasis.
In recent years, accumulating studies indicate that
anoikis is mainly mediated by the death receptor pathway activated
by caspase (7,8). This process occurs in hepatocytes
(9). Activation of growth factor
signaling pathways, which commonly occurs in carcinoma cells,
enables cells to acquire anoikis resistance (10,11).
Numerous kinase/phosphatase signaling molecules are known to be
involved in anoikis as central regulators, such as Ras (12), Akt and raf (13). Other reports have also confirmed
that cell cytoskeletal rearrangement is critical for the cells to
survive anoikis resistance (14,15).
Moreover, this process is especially associated with cadherin
family members, which are the key elements in the complex network
of survival signaling (16).
CD147, a member of the immunoglobulin superfamily,
was initially characterized as an inducer of matrix
metalloproteinase (MMP) synthesis (17). CD147 is known to play a vital role
in tumor progression. High expression levels of CD147 in epithelial
carcinoma tissue are significantly associated with poor prognosis
(18,19). Recently, CD147 has been found to
promote epithelial-mesenchymal transition (EMT) through
transforming growth factor (TGF)-β signaling (20) and serve as an anoikis suppressor by
the inhibition of Bim, a pro-apoptotic protein interacting with
Bcl-2 to mediate cell death in breast cancer cells (21). In addition, a radio-immunoconjugate
131I-labeled antibody HAb18 F(ab’)2 against CD147,
registered as Licartin, was used to treat primary HCC and prevent
tumor recurrence of post-orthotopic liver transplantation in
advanced HCC patients (22).
However, the role of CD147-induced anoikis resistance in HCC
metastasis remains poorly elucidated.
The aim of the present study was to investigate the
upregulation of CD147-conferred HCC cell resistance to anoikis
partially through activation of the phosphoinositide-3 kinase
(PI3K)/Akt pathway, thereby providing a novel mechanism supporting
HCC metastasis.
Materials and methods
Cell culture and anoikis assay
The SMMC-7721 human HCC cell line was purchased from
the Shanghai Institute for Biological Sciences (Shanghai, China)
and routinely cultured in RPMI-1640 medium (Hyclone Laboratories,
Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco,
Rockville, MD, USA). HEK293ar cells were obtained as previously
reported (23). The anoikis assay
was essentially performed as described by Frisch (24). Briefly, flasks were pre-coated with
2% sterilized agar. Cells were trypsinized and plated onto
pre-coated flasks. Suspension medium consisted of RPMI-1640
supplemented with 1% methocel and 10% FBS. According to the process
of anoikis adaptation for HEK293ar cells (23), a subpopulation of SMMC-7721 cells
resistant to anoikis was obtained. Cultures were maintained
routinely at 37°C under a mixture of 95% air and 5%
CO2.
Immunofluorescence
SMMC-7721 cells were suspended in methocel medium
for the indicated times. Cells were harvested and dried on
coverslips. Double-staining of Hoechst 33342 and propidium iodide
(PI) was developed to evaluate the phenotype and viability of
treated cells. To examine the expression levels of CD147 in treated
cells, the cells were firstly fixed and blocked, and then incubated
with anti-CD147 monoclonal antibody (1:200) (25). After washing, the cell samples were
incubated with FITC-conjugated goat anti-mouse IgG antibody
(1:1,000; Pierce, Rockford, IL, USA) and the nuclei were stained
with DAPI (1:100; Biotium, Hayward, CA, USA). The stained cells
were examined with a laser scanning microscope (Olympus, Tokyo,
Japan).
Antibodies and immunoblot analysis
For the immunoblot analysis, cells were lysed in
RIPA buffer (Beyotime Inc., NanTong, China). Protein concentration
was determined with the Bradford reagent (Beyotime Inc.). Equal
amounts of total proteins were separated and transferred to
polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA).
The membranes were subsequently immunoblotted with the appropriate
primary antibody. The following primary antibodies were used in
this study: anti-CD147 antibody (25), anti-Akt and anti-phosphorylated Akt
(ser 473) antibody from Cell Signaling Technology (Beverly, MA,
USA). A secondary horseradish peroxidase-conjugated goat anti-mouse
antibody (Pierce) was finally used for signal detection with an ECL
kit (Pierce) according to the manufacturer’s instructions.
Trypan blue exclusion assay
The anoikis-induced cell death was evaluated by
trypan blue exclusion assay as previously described (26). In brief, the parental SMMC-7721 and
SMMC-7721 cells resistant to anoikis were suspended in methocel
medium for the indicated times. Cells were harvested, washed three
times with PBS and resuspended in 100 μl PBS. After mixing with 100
μl of 0.8% trypan blue, the cells were counted using a
hemocytometer.
In vitro invasion assay
The migration ability was measured by in
vitro invasion assay as previously reported (27). The parental SMMC-7721 and SMMC-7721
cells resistant to anoikis were suspended in methocel medium for 48
h, resuspended in serum-free DMEM medium containing 0.5% FBS and
then added to the upper compartment of a chamber at a total number
of 2×105 cells per chamber. After incubation for 24 h,
the number of cells migrating through the filter was counted by
crystal violet staining and plotted as the mean number of migrating
cells per optic field in three independent experiments.
siRNA transfection
siRNA sequences corresponding to the cDNA sequences
of CD147 (Genebank accession NM_001728) were designed
(5’-GGUUCUUCGUGAGUUCCUCdTdT-3’ and 5’-GAGGAACUCACGAAGAACCdTdT-3’).
Briefly, the subpopulation of SMMC-7721 cells resistant to anoikis
was transfected with CD147 siRNA (100 nM) using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA). Scrambled negative control (SNC)
siRNA was used as mock control. Transiently-transfected cells were
recovered for 24 h and re-plated on agar-coated 6-well plates.
Apoptosis assay
SMMC-7721 cells resistant to anoikis transfected
with CD147 siRNA or control siRNA were suspended in fresh methocel
medium in an agar-coated plate for 24 h. Cells were harvested and
treated with 10 mmol/l EDTA to disrupt the cell-cell contacts. The
cells were then analyzed by double-staining with Annexin V-FITC and
PI using an Apoptosis Detection kit (Calbiochem, San Diego, CA,
USA) followed by detection using a FACSCalibur flow cytometer.
Early apoptotic cells were labeled as
Annexin+/PI- and necrotic cells were labeled
as PI+. The percentages of cells at early apoptosis or
necrotic stage were analyzed at least three times.
Statistical analysis
Statistical analyses were performed using the SPSS
16.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Statistical significance of the differences was determined using
Student’s t-test. All P-values were based on two-sided tests.
P<0.05 was considered statistically significant.
Results
Anoikis of suspended SMMC-7721 cells and
formation of multi-cellular spheroids
To characterize the morphological change of
SMMC-7721 cells after suspension in methocel medium, typical images
representing the morphology of cells after treatment for 0, 24 and
48 h are shown in Fig. 1A.
Cell-cell contacts were spontaneously and gradually formed and
multi-cellular spheroids were generated in a time-dependent manner.
The volume of the aggregated spheroids was significantly increased
at the time point of 48 h as indicated in Fig. 1B. The viability of the SMMC-7721
cells was further analyzed by double-staining of Hoechst 33342 and
PI under a laser microscope. At 0 h, the staining assay indicated
that the treated cells were alive (exclusion of PI). After cells
were cultured for 24 h, anoikis with positive staining of PI was
induced in some of the cells and apoptotic bodies were also found
along the cytoplasmic membrane. Multi-cellular spheroids were found
at the time-point of 48 h, when the majority of cells underwent
anoikis with a strong staining of PI and a weak staining of Hoechst
33342 (Fig. 3C).
CD147 was upregulated on the cell-cell
contacts in suspended multi-cellular spheroids
To explore the role of CD147 in anoikis resistance,
we examined the expression levels of CD147 in HCC cells. Western
blotting showed that SMMC-7721 cells in suspended culture exhibited
a notable time-dependent accumulation of the CD147 protein
(Fig. 2A). Immunofluorescence
staining confirmed the elevated levels of CD147 in SMMC-7721 cells
after suspension for 48 h (Fig.
2B). Additionally, in a large multi-cellular spheroid, the
strong CD147 staining areas were restricted to the cell-cell
contacts for both SMMC-7721 and HEK293ar cells as indicated by the
yellow arrows in Fig. 2B.
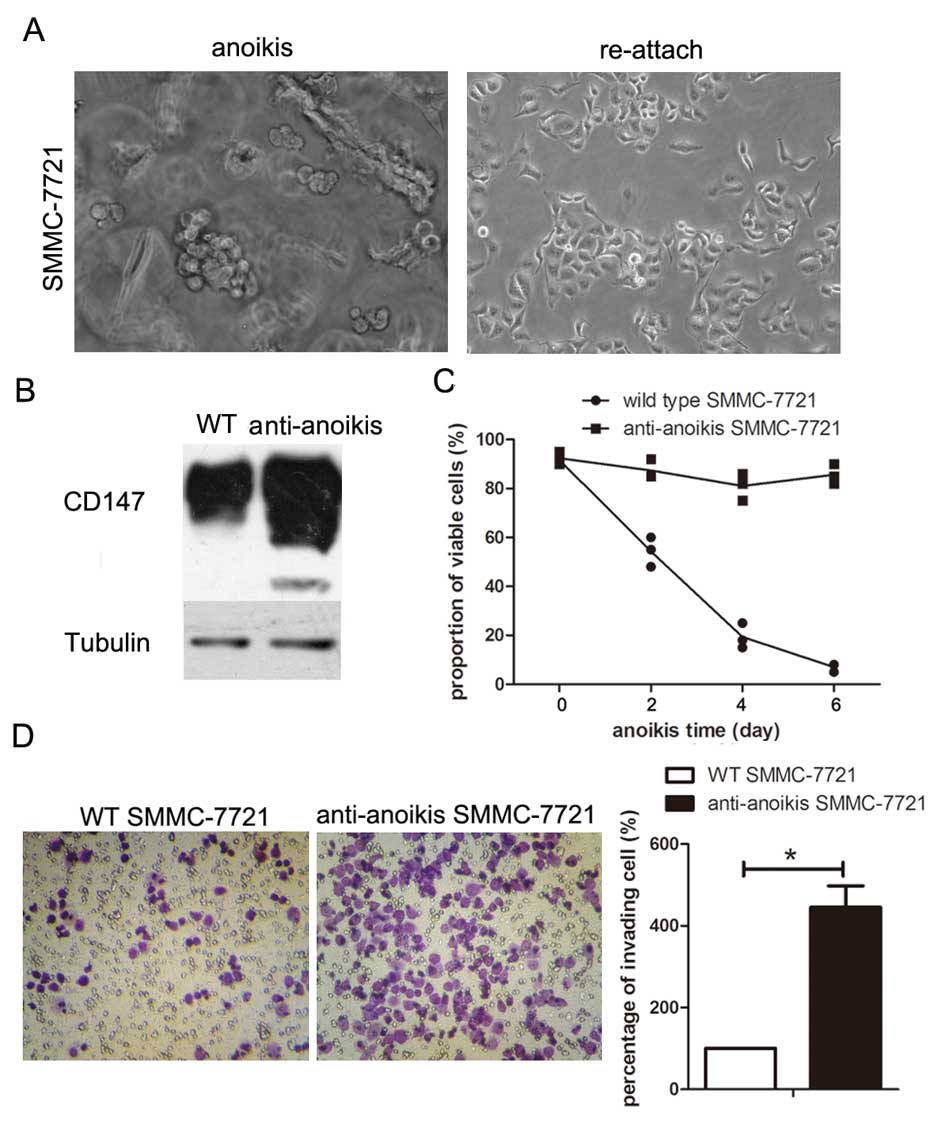
CD147 was significantly upregulated in
anoikis-resistant SMMC-7721 cells with an increase in both
viability and invasion ability
Based on a previous study (23), a subpopulation of anoikis-resistant
SMMC-7721 cells was obtained to explore the correlation between
CD147 expression levels and anoikis resistance. Morphological
changes occurred in cells during the adaptation period for
SMMC-7721 cells in suspension culture (Fig. 3A). Western blotting indicated that
CD147 was significantly upregulated in the anoikis-resistant
SMMC-7721 cells, compared to the parental SMMC-7721 cells (Fig. 3B). The trypan blue exclusion assay
demonstrated that a high percentage of the anoikis-resistant
SMMC-7721 cells remained alive following suspension in culture
medium for 6 days, while almost all the parental SMMC-7721 cells
underwent death (Fig. 3C). In
vitro invasion assay showed that the invasion ability was
significantly increased in SMMC-7721 cells resistant to anoikis,
compared to the parental SMMC-7721 cells (Fig. 3D).
Anoikis was induced by the knockdown of
CD147 expression in the subpopulation of SMMC-7721 cells resistant
to anoikis through inactivation of the PI3K/Akt pathway
To prove the direct involvement of CD147 during the
adaptation process of anoikis resistance, we knocked down the
expression levels of CD147 in the subpopulation of
anoikis-resistant SMMC-7721 cells through transfection with
specific small interfering RNA (siRNA). Immunofluorescence assay
results indicated that the staining intensity of CD147 in the area
of cell-cell contacts was significantly reduced in
anoikis-resistant SMMC-7721 cells tansfected with CD147 siRNA,
compared to those transfected with SNC siRNA (Fig. 4A). Similarly, the cell nuclei tended
to be cracked in cells transfected with CD147 siRNA following
suspension in methocel medium for 24 h as indicated by the white
arrows (Fig. 4A). Flow cytometry
showed that the inhibition of CD147 expression significantly
induced anoikis (both early apoptosis and necrosis) in
anoikis-resistant SMMC-7721 cells (Fig.
4B). To elucidate the possible signaling pathway involved in
the CD147-mediated anoikis resistance, western blot analysis was
performed to examine the expression levels of CD147, phosphorylated
Akt and Akt in anoikis-resistant SMMC-7721 cells transfected with
CD147 siRNA and SNC siRNA, respectively. We found that the
knockdown of CD147 resulted in a significant decrease of
phosphorylated Akt levels, whereas the expression of Akt was not
affected (Fig. 4C). These data
suggest that activation of the PI3K-Akt signaling pathway may be
involved in CD147-mediated anoikis resistance.
Discussion
This study provides evidence that an elevated
endogenous expression of CD147 contributes to anoikis resistance in
SMMC-7721 cells partially through activation of the PI3K/Akt
survival pathway, which has been considered an important process
for the metastasis of HCC cells.
We first established a model for cell suspension
culture to characterize the morphology of SMMC-7721 cells following
suspension in methocel medium. A number of studies have validated
that anoikis can be inhibited despite loss of cell-matrix adhesion
when cell-cell adhesion is preserved among various epithelial
carcinoma cell lines (16,28,29).
Our research model also revealed a multi-cellular spheroid
formation in a time-dependent manner. Furthermore, we observed a
significant increase of CD147 expression in SMMC-7721 cells under
suspension culture, especially restricted to the region along the
cytoplasmic membrane of cell-cell contacts, which indicates a
potential role of CD147 in mediating the formation of
multi-cellular spheroids. Earlier evidence has shown that CD147
promotes breast cancer cell survival by regulating intercellular
contacts and inhibiting anoikis through BIM (21). Besides, our previous study has
verified a vital role of CD147 involved in cell-cell contacts in an
E-cadherin-dependent manner in HEK293 cells (23). These data suggest that CD147 may
also be involved in anoikis resistance in HCC when cells are
exposed to a condition of extracellular matrix-detached
culture.
The direct role of CD147 in the adaptation of
anoikis resistance was investigated. The SMMC-7721 cell line has
been extensively studied in the anoikis resistance of HCC (30,31).
Therefore, we established an anoikis-resistant cell model
(SMMC-7721 cells resistant to anoikis) to investigate the mechanism
of anoikis suppression. The adaptation process in this cell model
is closer to the natural in vivo status of the physiological
conditions compared to earlier research models that directly
modified the expression levels of target gene by manual
intervention (21). The
significantly high expression levels of CD147 led to
anoikis-resistant SMMC-7721 cells exhibiting a notably higher
viability and invasion ability compared to their parental SMMC-7721
cells. To provide direct evidence that CD147 is involved in the
process of anoikis resistance in the subpopulation of SMMC-7721
cells resistant to anoikis, we inhibited CD147 through transfection
of specific targeting siRNA in anoikis-resistant SMMC-7721 cells.
The proportion of the apoptotic and necrotic cells was greatly
increased compared to the mock control after the cells were
suspended in methocel medium for 24 h. Accordingly, the morphology
of nuclei tended to split after the inhibition of CD147. These
results strongly suggest that the upregulation of CD147 is
important in rendering cell anoikis resistance, which is a premise
for the metastasis of primary cells.
The present study also provided a potential
molecular mechanism supporting the hypothesis that the upregulation
of CD147 induces anoikis resistance in SMMC-7721 cells. A number of
published observations have demonstrated the principal role of
PI3K/Akt pathway activation in anoikis resistance (32–34).
Activation of PI3K/Akt is essential for the integrity of adherent
junctions and is associated with the rearrangement of the
cytoskeleton (35). Our previous
studies have demonstrated a vital role of CD147 in the activation
of the PI3K/Akt pathway to protect cell survival under the
different stress exposition (26,36).
Therefore, we explored the correlation between the upregulation of
CD147 expression and activation of the PI3K/Akt pathway in our
anoikis-resistant cell model. Our results have shown that the
knockdown of CD147 significantly decreased the levels of pAkt,
suggesting that activation of the PI3K/Akt pathway is involved in
anoikis resistance by the upregulation of CD147. However, our
preliminary data only provide a potential molecular mechanism of
CD147-induced anoikis resistance in HCC cells. The possible
involvement of other signaling molecules cannot be excluded.
Moreover, a thorough evaluation of other related signaling pathways
would shed light on the mechanisms of CD147-mediated anoikis
resistant regulation.
In conclusion, the upregulation of CD147
significantly contributes to anoikis resistance in SMMC-7721 cells
following suspension in methocel medium. Spontaneous upregulation
of the expression levels of CD147 in SMMC-7721 cells resistant to
anoikis may promote the cell-cell contacts and then activate the
PI3K/Akt pathway. Thus, our findings provide a new mechanism
underlying the metastasis of HCC cells, which emphasizes an
important role of CD147 in anoikis resistance regulation (Fig. 5).
Acknowledgements
The authors are thankful to Mr. Kai Qu for his
technical assistance.
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
PI3K
|
phosphoinositide-3 kinase
|
|
PI
|
propidium iodide
|
|
siRNA
|
small interfering RNA
|
|
SNC
|
scrambled negative control
|
References
|
1
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simpson CD, Mawji IA, Anyiwe K, et al:
Inhibition of the sodium potassium adenosine triphosphatase pump
sensitizes cancer cells to anoikis and prevents distant tumor
formation. Cancer Res. 69:2739–2747. 2009. View Article : Google Scholar
|
|
4
|
Taddei ML, Parri M, Angelucci A, et al:
EphA2 induces metastatic growth regulating amoeboid motility and
clonogenic potential in prostate carcinoma cells. Mol Cancer Res.
9:149–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pongrakhananon V, Nimmannit U, Luanpitpong
S, Rojanasakul Y and Chanvorachote P: Curcumin sensitizes non-small
cell lung cancer cell anoikis through reactive oxygen
species-mediated Bcl-2 downregulation. Apoptosis. 15:574–585. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kumar P, Yadav A, Patel SN, et al:
Tetrathiomolybdate inhibits head and neck cancer metastasis by
decreasing tumor cell motility, invasiveness and by promoting tumor
cell anoikis. Mol Cancer. 9:2062010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoudjit F and Vuori K: Matrix attachment
regulates Fas-induced apoptosis in endothelial cells: a role for
c-flip and implications for anoikis. J Cell Biol. 152:633–643.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mawji IA, Simpson CD, Hurren R, et al:
Critical role for Fas-associated death domain-like
interleukin-1-converting enzyme-like inhibitory protein in anoikis
resistance and distant tumor formation. J Natl Cancer Inst.
99:811–822. 2007. View Article : Google Scholar
|
|
9
|
Smets FN, Chen Y, Wang LJ and Soriano HE:
Loss of cell anchorage triggers apoptosis (anoikis) in primary
mouse hepatocytes. Mol Genet Metab. 75:344–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Attwell S, Roskelley C and Dedhar S: The
integrin-linked kinase (ILK) suppresses anoikis. Oncogene.
19:3811–3815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Terada LS and Nwariaku FE: Escaping
anoikis through ROS: ANGPTL4 controls integrin signaling through
Nox1. Cancer Cell. 19:297–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosen K, Rak J, Leung T, Dean NM, Kerbel
RS and Filmus J: Activated Ras prevents downregulation of Bcl-X(L)
triggered by detachment from the extracellular matrix. A mechanism
of Ras-induced resistance to anoikis in intestinal epithelial
cells. J Cell Biol. 149:447–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boisvert-Adamo K and Aplin AE: B-RAF and
PI-3 kinase signaling protect melanoma cells from anoikis.
Oncogene. 25:4848–4856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frisch SM, Vuori K, Ruoslahti E and
Chan-Hui PY: Control of adhesion-dependent cell survival by focal
adhesion kinase. J Cell Biol. 134:793–799. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Zhang Y, Ithychanda SS, et al:
Migfilin interacts with Src and contributes to cell-matrix
adhesion-mediated survival signaling. J Biol Chem. 284:34308–34320.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang HG, Jenabi JM, Zhang J, et al:
E-cadherin cell-cell adhesion in ewing tumor cells mediates
suppression of anoikis through activation of the ErbB4 tyrosine
kinase. Cancer Res. 67:3094–3105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caudroy S, Polette M, Tournier JM, et al:
Expression of the extracellular matrix metalloproteinase inducer
(EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary
and breast lesions. J Histochem Cytochem. 47:1575–1580. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinheiro C, Longatto-Filho A, Simoes K, et
al: The prognostic value of CD147/EMMPRIN is associated with
monocarboxylate transporter 1 co-expression in gastric cancer. Eur
J Cancer. 45:2418–2424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo H, Li R, Zucker S and Toole BP:
EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis,
also binds interstitial collagenase to the tumor cell surface.
Cancer Res. 60:888–891. 2000.PubMed/NCBI
|
|
20
|
Wu J, Ru NY, Zhang Y, et al: HAb18G/CD147
promotes epithelial-mesenchymal transition through TGF-beta
signaling and is transcriptionally regulated by Slug. Oncogene.
30:4410–4427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JM, O’Neill P, Jin W, et al:
Extracellular matrix metalloproteinase inducer (CD147) confers
resistance of breast cancer cells to Anoikis through inhibition of
Bim. J Biol Chem. 281:9719–9727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J, Shen ZY, Chen XG, et al: A
randomized controlled trial of Licartin for preventing hepatoma
recurrence after liver transplantation. Hepatology. 45:269–276.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma XK, Wang L, Li Y, et al: HAb18G/CD147
cell-cell contacts confer resistance of a HEK293 subpopulation to
anoikis in an E-cadherin-dependent manner. BMC Cell Biol.
11:272010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frisch SM: Evidence for a function of
death-receptor-related, death-domain-containing proteins in
anoikis. Curr Biol. 9:1047–1049. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen ZN: Significance and application of
anti-malignant hepatoma MAb HAb18 in radioimmunal diagnosis of
human hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 14:9–12.
1992.(In Chinese).
|
|
26
|
Gou X, Ru Q, Zhang H, et al: HAb18G/CD147
inhibits starvation-induced autophagy in human hepatoma cell
SMMC7721 with an involvement of Beclin 1 down-regulation. Cancer
Sci. 100:837–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu J, Xu HY, Zhang Q, et al: HAb18G/CD147
functions in invasion and metastasis of hepatocellular carcinoma.
Mol Cancer Res. 5:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Derksen PW, Braumuller TM, van der Burg E,
et al: Mammary-specific inactivation of E-cadherin and p53 impairs
functional gland development and leads to pleomorphic invasive
lobular carcinoma in mice. Dis Model Mech. 4:347–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh AB, Sugimoto K and Harris RC:
Juxtacrine activation of epidermal growth factor (EGF) receptor by
membrane-anchored heparin-binding EGF-like growth factor protects
epithelial cells from anoikis while maintaining an epithelial
phenotype. J Biol Chem. 282:32890–32901. 2007. View Article : Google Scholar
|
|
30
|
Wu Y, Zuo J, Ji G, et al: Proapoptotic
function of integrin beta(3) in human hepatocellular carcinoma
cells. Clin Cancer Res. 15:60–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng Y, Wang LY, Cai T, et al:
All-trans-retinoic acid increased the expression of integrin
alpha5beta1 and induced ‘anoikis’ in SMMC-7721 hepatocarcinoma
cell. J Exp Clin Cancer Res. 20:429–438. 2001.PubMed/NCBI
|
|
32
|
McFall A, Ulku A, Lambert QT, Kusa A,
Rogers-Graham K and Der CJ: Oncogenic Ras blocks anoikis by
activation of a novel effector pathway independent of
phosphatidylinositol 3-kinase. Mol Cell Biol. 21:5488–5499. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Isakoff SJ, Engelman JA, Irie HY, et al:
Breast cancer-associated PIK3CA mutations are oncogenic in mammary
epithelial cells. Cancer Res. 65:10992–11000. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang K, Sun J, Cheng J, Djeu JY, Wei S
and Sebti S: Akt mediates Ras downregulation of RhoB, a suppressor
of transformation, invasion, and metastasis. Mol Cell Biol.
24:5565–5576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frisch SM and Ruoslahti E: Integrins and
anoikis. Curr Opin Cell Biol. 9:701–706. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Zhang H, Gou X, Horikawa Y, Xing J
and Chen Z: Upregulation of HAb18G/CD147 in activated human
umbilical vein endothelial cells enhances the angiogenesis. Cancer
Lett. 278:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|