Introduction
Haemangiopericytoma (HPC) is a mesenchymal neoplasm
accounting for a minority of all vascular tumours. The term was
first coined in 1942 by Stout and Murray for tumours thought to
originate from pericytes, which are modified dendritic-like smooth
muscle cell encircling blood vessels (1). According to the World Health
Organisation (WHO) classification of tumours of soft tissues and
bone, the term ‘haemangiopericytoma’ is used to refer to a variety
of tumours, which have in common the presence of a thin-walled
branching ‘staghorn’ vascular pattern and resemble cellular areas
of solitary fibrous tumours (SFTs) (2). As a result, there are difficulties in
predicting clinical behaviour for a given neoplasm, and thus in
establishing specific treatment modalities, since this tumour
category remains to be clarified. Numerous entities have
progressively escaped from the ill-defined haemangiopericytoma
tumour category over the past 10 years, and those which remain tend
to now be recognized as cell or malignant forms of SFT (3). Up to 15% of soft tissue neoplasms show
HPC-like features, at least focally. HPC is currently no longer
considered a specific entity, but rather as a growth pattern,
common to a number of often unrelated neoplasms.
HPC mostly arises in the lower extremities and the
retroperitoneum, while the head and neck area is the third most
common site (2). The majority of
HPCs are histologically benign. However, a small percentage of HPCs
possess atypical features, such as a high mitotic rate, high
cellularity and foci of necrosis. Following curative surgical
treatment, recurrent or metastatic tumours, or both, develop in
certain patients with HPC (2). The
lungs are the most common metastatic site, followed by the bones
and liver. However, predicting the clinical behaviour of this
tumour following the initial resection and determining the
appropriate treatment for recurrent or metastatic tumours can be
difficult.
We report a case of metastatic HPC in the thyroid
gland and discuss the histological and immunohistochemical features
and the clinical presentation.
Patients and methods
Patient
A 67-year-old male was referred to the Department of
Surgery for intrathoracic multinodular toxic goiter. Seven years
earlier the patient had undergone surgical resection at another
hospital for an abdominal wall tumour that had been diagnosed as a
classical HPC. One year prior to the referral, the patient
developed lung metastases that were treated with sunitinib and
radiotherapy. Preoperative CT scanning showed nodular areas in the
right basal lung; the nodular areas ranged in size between 13 and
20 mm.
At the time of thyroid surgery, the patient had
hyperthyroidism (laboratory findings: TSH 3.72; fT3 1.62; fT4 0.84)
and the thyroid volume was 150 ml. Radiological evaluation revealed
conspicuous compression of the trachea. The thyroid gland had a
prevalent left intrathoracic growth, which caused right tracheal
deviation, and a total thyroidectomy was performed.
The patient is currently receiving chemotherapeutic
treatment with sunitinib.
Institutional approval from the ethics committee was
obtained for the study. Informed consent was obtained from the
patient with regard to the use of the tumor samples.
Specimens
Specimens were surgically obtained and fixed in 10%
neutral-buffered formaldehyde and embedded in paraffin. Routine
haematoxylin and eosin staining was performed on the microtomic
section for histopathological examination.
Immunohistochemistry
A paraffin block for immunohistochemical study was
selected. Immunohistochemistry was carried out using the
avidin-biotin-peroxidase complex method. Antibodies were purchased
from Ventana Medical Systems (Tucson, AZ, USA). The antibodies
employed included CD34, CD99, bcl-2, vimentin, cytokeratin, actin,
desmin, thyroglobulin and TTF-1. All the antibodies were
pre-diluted.
Results
Specimens were obtained from a 67-year-old male who
underwent thyroidectomy following examination and radical
evaluation. The gross appearance of the pathological specimen was a
multinodular goiter with right and left solid nodules, measuring a
maximum of 5.3 cm. Furthermore, the patient had a solid nodule in
the right thyroid lodge measuring 1.1 cm. On the cut surface, the
nodules appeared as greyish masses with haemorrhagic changes.
Microscopic examination revealed multiple hypercellular nodules
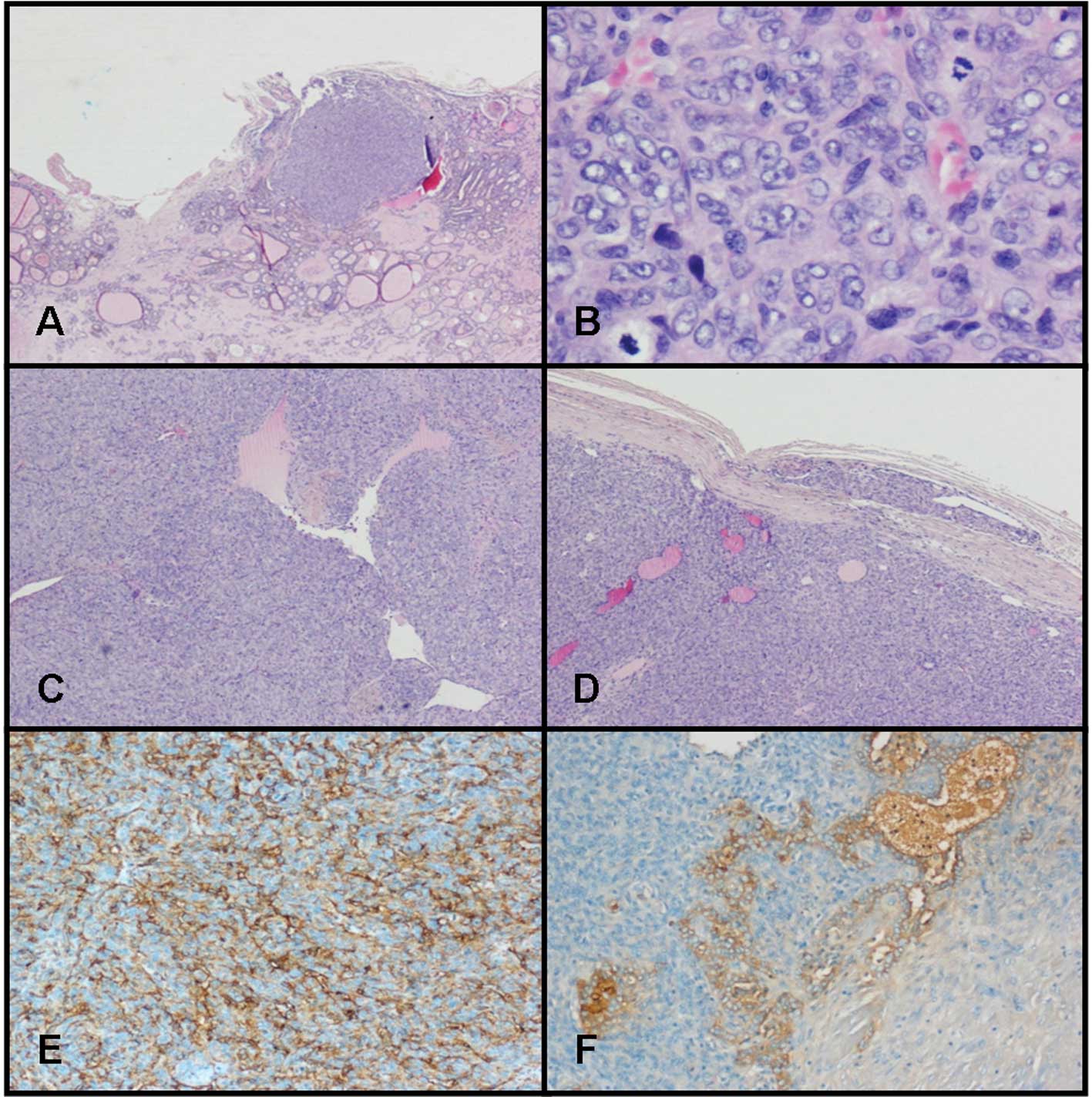
with an infiltrative growth pattern (Fig. 1A). These nodules consisted of
tightly packed fusiform or spindle-shaped cells with nuclear
polymorphism. An increased mitotic rate was observed (>4 MF/10
HPF) (Fig. 1B). The cells were
arranged around a prominent vasculature, which formed a ramifying
network, occasionally with a ‘staghorn’ configuration (Fig. 1C). The vessels were lined with flat
endothelial cells. Small foci of necrosis and emboli were present
(Fig. 1D).
The tumour cells exhibited a marked expression of
CD34 (Fig. 1E), CD99, Bcl-2,
vimentin and Factor VIII, and a negative expression of
thyroglobulin (Fig. 1F). Cells were
negative for cytokeratins, thyroglobulin, TTF-1, actin and desmin.
The proliferation marker Ki-67 was positive in up to 60% of the
tumour cells. The collateral thyroid parenchyma was
multinodular.
Discussion
HPC is a rare mesenchymal tumour. According to
cumulative data, it is mainly a tumour of adult patients (4), and according to Espat et al
(5), the median patient age is 45
years. In their series, tumours of the extremities, pelvis, and
head and neck occurred with similar frequency. Extremity tumours
were mostly in the axilla and thigh, and the head and neck group
accounts for tumours of the meninges and of the cheek (5).
Pain is a late symptom, associated with an enlarging
mass; however, specific symptoms are associated with the location
of the tumour. Hypoglycemia has been reported in approximately 5%
of HPC, most often located in the pelvis and retroperitoneum. In
1990, Benn et al showed that hypoglycemia is mediated by the
production of insulin-like growth factor by the tumour (6).
HPC grows in deep soft tissues as a circumscribed
brown lesion surrounded by a pseudo-capsule, or as an exophytic
lesion from the serosal surfaces. HPCs are extremely variable in
appearance, depending on the relative proportion of cells and
fibrous stroma. Classical HPC consists of tightly packed round to
fusiform cells, arranged around an elaborate vasculature. The
vascular network is ramified and shows variation in calibre, with a
‘staghorn’ configuration. Frequently the vessels are surrounded by
a thick coat of collagen, which extends into the interstitium.
Enzinger and Weiss suggest that when the prevalent component of the
tumours are spindle cells arranged in short, ill-defined fascicles,
with a striking hyalinisation, the lesions should be recognized as
classical solitary fibrous tumours (2). The criteria for malignancy proposed by
Enzinger and Smith in classical HPC identify overtly malignant or
high-grade lesions, but fail to address low-grade lesions (7). In their study, large-sized tumours
(>5 cm), increased the mitotic rate (≥4 MF/10 HPF), high
cellularity, presence of immature and pleomorphic tumour cells, and
foci of haemorrhage and necrosis predicted a highly malignant
course. Enzinger and Weiss employed the term ‘low-malignant
potential’ for lesions with lower levels of mitotic activity (1–3
MF/10 HPF), particularly if they have any degree of atypia and
cellularity (2). However, it should
be emphasized that a number of cases exhibit high-grade features.
Numerous HPCs express CD34; however, desmin and cytokeratin are
usually absent (3).
The inability to render an accurate assessment of
the biological potential of the tumour is a significant problem for
pathologists. At present, no prognostic criteria exist for which
there is universal agreement. However, the prognosis of HPC is
generally favourable, although recurrent or metastatic tumours, or
both, develop in some patients following curative surgical
treatment (2). In 2002, Espat et
al reported a 93 and 80% 2- and 5-year survival rate,
respectively, for classical HPC (5). It was noted that all patients
undergoing complete surgical excision were alive at 5 years after
surgery. Significantly higher disease-free survival and lower local
recurrence rates were associated with extremity lesions versus
meningeal and retroperitoneal lesions. In 1998, Spitz et al
(8)reported a local failure rate of
32% in patients treated with curative intent. However, these
authors suggested that local recurrence is not an indicator of poor
prognosis in these patients (8).
Frequently, patients develop metastases together with the primary
tumour. Classical HPC has a low disease-associated mortality,
whereas malignant tumours have a more variable outcome.
Approximately one-half of the patients were cured of their tumours
following excision, whereas the remainder developed recurrence,
metastases or both (2). Metastases
were noted in approximately 30% of patients, with a 5-year
actuarial survival of 71% (8). The
most common metastatic sites were the lungs, bones and liver.
HPC is a rare tumour of the thyroid and can invade
neighbouring tissues. Primary diagnosis with fine-needle aspiration
biopsy (FNAB) is extremely difficult; however, it has a
characteristic and easily diagnosed histopathological
appearance.
In this study, we report a case of classical
abdominal HPC that presented 7 years after the first surgical
resection with thyroid metastases of malignant HPC. A review of the
literature (Table I) regarding HPC
of the thyroid gland showed that it is extremely rare (9–10),
with only 9 cases being reported thus far (11–19).
 | Table ISummary of the cases with thyroidal
haemangiopericytoma. |
Table I
Summary of the cases with thyroidal
haemangiopericytoma.
| Author | Year | Age | Gender | Side | Size (cm) | Pleomorphism | Mitosis (MF/10
HPF) | Necrosis |
|---|
| Prokš (11) | 1961 | 37 | F | R | 6 | + | Great number | + |
| Tano Assini et
al (12) | 1968 | 31 | F | L | 4 | NR | NR | NR |
| Kallenberg and
Anagnostaki (13) | 1979 | 59 | M | L | NA | NA | NA | NA |
| Tytor and Olofsson
(14) | 1986 | 79 | F | R | NR | NR | NR | NR |
| Justrabo et al
(15) | 1989 | 77 | F | L | 11.5 | − | − | − |
| Geisinger et
al (16) | 1990 | 66 | F | NR | 8 | NR | 6 | NR |
| Dictor et al
(17) | 1992 | 5 | M | L | 8 | + | 0–1 | − |
| Cameselle-Teijeiro
et al (18) | 2003 | 36 | M | L | 6 | NR | − | − |
| Hansen et al
(19) | 2004 | 15 | F | L | 5 | + | 4–5 | − |
| Present case | 2011 | 67 | M | LR | 7 | − | >4 | + |
According to the cumulative data, including the
present case, the mean age of the patients is 47 years (range,
5–79). There is a predominant incidence in females, with a
female:male ratio of 3:2 (F=6; M=4). The mean size of the tumours
is 7 cm (range, 4–11.5). Six tumours (77%) were located in the left
lobe, one case was bilateral and in one case the location of the
lesion was not reported. The reported cases were primitive thyroid
gland HPCs with at least one histological feature (pleomorphism,
mitosis rate or necrosis) suggestive for potentially malignant
behaviour, with the exception of the case studies by
Cameselle-Teijeiro et al (7), which was a lipomatous HPC, and that of
Justrabo et al (15), which
was a benign HPC. No evidence of local recurrence or distant
metastases was reported.
Our case showed an uncommon location of a metastatic
classical HPC, which recurred following surgical treatment and
chemo-radiotherapy. In the metastatic site, the tumour acquired the
histological features of a malignant HPC. We can speculate as to
how a classical HPC with clinically aggressive behaviour may be
associated with malignant histological changes. Furthermore, we
support the study by Espat et al, who, after observing a
mortality rate of greater than 50% in patients affected by
conventional HPC (5), emphasized
the importance of applying strict diagnostic criteria in making the
most appropriate diagnosis.
Acknowledgements
This study has been supported in parts by grants
from the Ministero dell’Istruzione, dell’Università e della
Ricerca, Associazione Italiana per la Ricerca sul Cancro, Istituto
Toscano Tumori and Ministero della Salute.
References
|
1
|
Stout AP and Murray MR:
Hemangiopericytoma: a vascular tumor featuring Zimmermann’s
pericytes. Ann Surg. 116:26–33. 1942.PubMed/NCBI
|
|
2
|
Enzinger FM and Weiss SW: Soft Tissue
Tumors. 5th edition. Mosby; New York, NY: 2008
|
|
3
|
Gengler C and Guillou L: Solitary fibrous
tumour and haemangiopericytoma: evolution of a concept.
Histopathology. 48:63–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enzinger FM and Weiss SW: Soft Tissue
Tumors. 3rd edition. Mosby; New York, NY: 1995
|
|
5
|
Espat NJ, Lewis JJ, Leung D, Woodruff JM,
Antonescu CR, Shia J and Brennan MF: Conventional
hemangiopericytoma. Cancer. 95:1746–1751. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benn JJ, Firth RG and Sönksen PH:
Metabolic effect of an insulin-like factor causing hypoglycemia in
a patient with haemangiopericytoma. Clin Endocrinol. 32:769–780.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Enzinger FM and Smith BH:
Hemangiopericytoma. An analysis of 106 cases Hum Pathol. 7:61–82.
1976.
|
|
8
|
Spitz FR, Bouvet M, Pisters PW, Pollock RE
and Feig BW: Hemangiopericytoma: a 20-year single-institution
experience. Ann Surg Oncol. 5:350–355. 1998.PubMed/NCBI
|
|
9
|
Brandwein MS, Kapadia SB and Gnepp DR:
Nonsquamous pathology of the larynx, hypopharynx, and trachea.
Diagnostic Surgical Pathology of the Head and Neck. Gnepp DR:
Saunders; Philadelphia, PA: pp. 304–305. 2001
|
|
10
|
Weis SW and Goldblum JR: Soft Tissue
Tumors. Mosby; St Louis: 2001
|
|
11
|
Prokš C: Generalized hemangiopericytoma of
the thyroid gland (report of a case). Neoplasma. 8:219–224.
1961.PubMed/NCBI
|
|
12
|
Tano Assini MT, Oliva Otero G and Gomez
SC: Visceral hemangiopericytoma. Presentation of 2 cases. Prensa
Med Argent. 55:996–1000. 1968.PubMed/NCBI
|
|
13
|
Kallenberg F and Anagnostaki L:
Hemangiopericytoma of the thyroid gland. Ugeskr Laeger.
141:3530–3531. 1979.PubMed/NCBI
|
|
14
|
Tytor M and Olofsson J: Thyroid tumors
invading the larynx and trachea. J Otolaryngol. 15:74–79.
1986.PubMed/NCBI
|
|
15
|
Justrabo E, Michiels JF, Maire J, Jacquot
JP and Levillain P: Hemangiopericytoma of the thyroid gland. Ann
Endocrinol (Paris). 50:26–30. 1989.
|
|
16
|
Geisinger KR, Silverman JF, Cappellari JO
and Dabbs DJ: Fine-needle aspiration cytology of malignant
hemangiopericytomas with ultrastructural and flow cytometric
analyses. Arch Pathol Lab Med. 114:705–710. 1990.PubMed/NCBI
|
|
17
|
Dictor M, Elner A, Andersson T and Fernö
M: Myofibromatosis- like hemangiopericytoma metastasizing as
differentiated vascular smooth-muscle and myosarcoma. Myopericytes
as a subset of ‘myofibroblasts’. Am J Surg Pathol. 16:1239–1247.
1992.PubMed/NCBI
|
|
18
|
Cameselle-Teijeiro J, Manuel Lopes J,
Villanueva JP, Gil-Gil P and Sobrinho-Simões M: Lipomatous
haemangiopericytoma (adipocytic variant of solitary fibrous tumour)
of the thyroid. Histopathology. 43:406–408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hansen T, Gaumann A, Ghalibafian M,
Höferlin A, Heintz A and Kirkpatrick CJ: Haemangiopericytoma of the
thyroid gland in combination with Hashimoto’s disease. Virchows
Arch. 445:315–319. 2004.
|