Introduction
Synchronous bilateral Paget’s disease of the nipple
is extremely rare. Paget’s disease of the breast represents
approximately 1–3% of all breast malignancies (1,2) and is
characterized pathologically by the presence of round
intraepidermal cells of the nipple. Paget’s disease can present in
conjunction with an underlying invasive cancer, in conjunction with
underlying ductal carcinoma in situ (DCIS), or alone without
any underlying invasive breast carcinoma or DCIS (3). The majority of cases of Paget’s
disease have an underlying breast malignancy (1,2);
however, 66–86% of patients without a clinical mass on physical
examination or mammogram have DCIS alone. Early reports described
the occurrence of Paget’s disease alone without an underlying
cancer as rare, representing at most 8% of patients with Paget’s
disease (3). The prognosis of
patients who present with Paget’s disease of the breast is
primarily determined by the extent of the associated carcinoma
(1,2), and adjuvant treatment is administered
depending on nodal and receptor status.
In this study, we present a case of synchronous
bilateral Paget’s disease of the breast without underlying
carcinoma, which is an extremely rare phenomenon.
Case report
A 45-year-old Chinese woman admitted to our
department with a 6-month history of eczema and itching in both
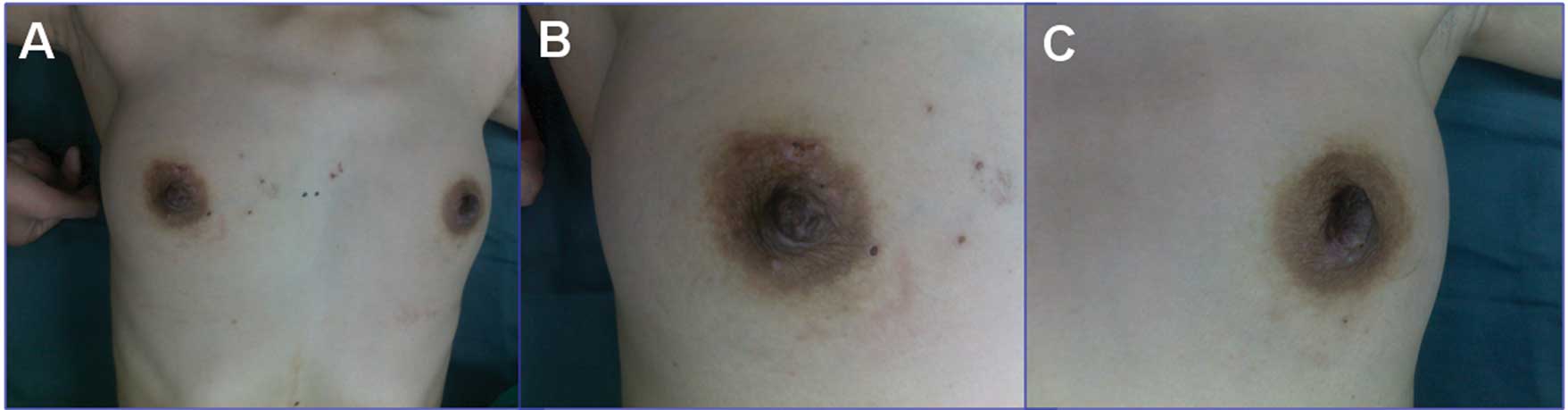
nipples. The patient presented with no palpable mass at either
breast, no obvious nipple inversion, ulceration or active nipple
discharge and no sign of any palpable swelling in the lymph nodes
of the axilla or supraclavicular region (Fig. 1). The patient had a medical history
of gastric cancer. She had neither family history nor risk factors
of breast cancer. Mammography showed no microcalcifications or mass
on the breast. Chest computed tomography and breast ultrasonography
revealed no abnormalities, and bone scintigraphy showed no site of
distant metastasis.
The clinical hypothesis of Paget’s disease resulted
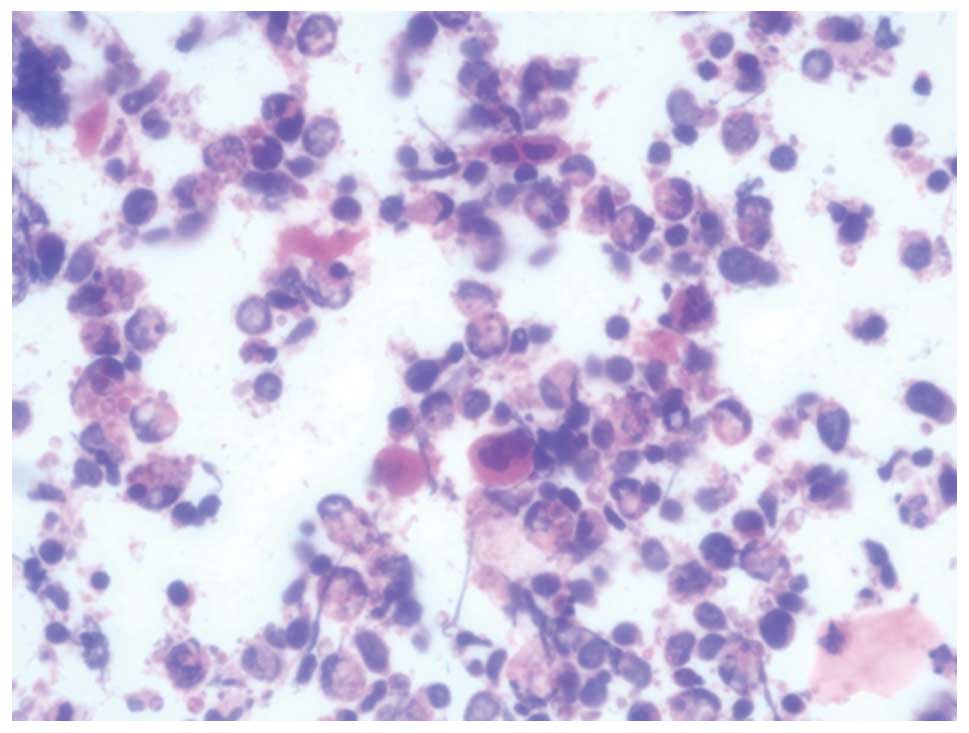
in the patient undergoing a nipple scrape cytology, which showed
isolated malignant cells of Paget’s type. The tumor cells had clear
cytoplasm and irregular nuclei with prominent nucleoli, which are
typical features of Paget’s disease (Fig. 2). These results confirmed a
diagnosis of Paget’s disease of the nipple, and the patient was
scheduled for mastectomy and sentinel node biopsy. No complications
occurred during or after surgery and she recovered well
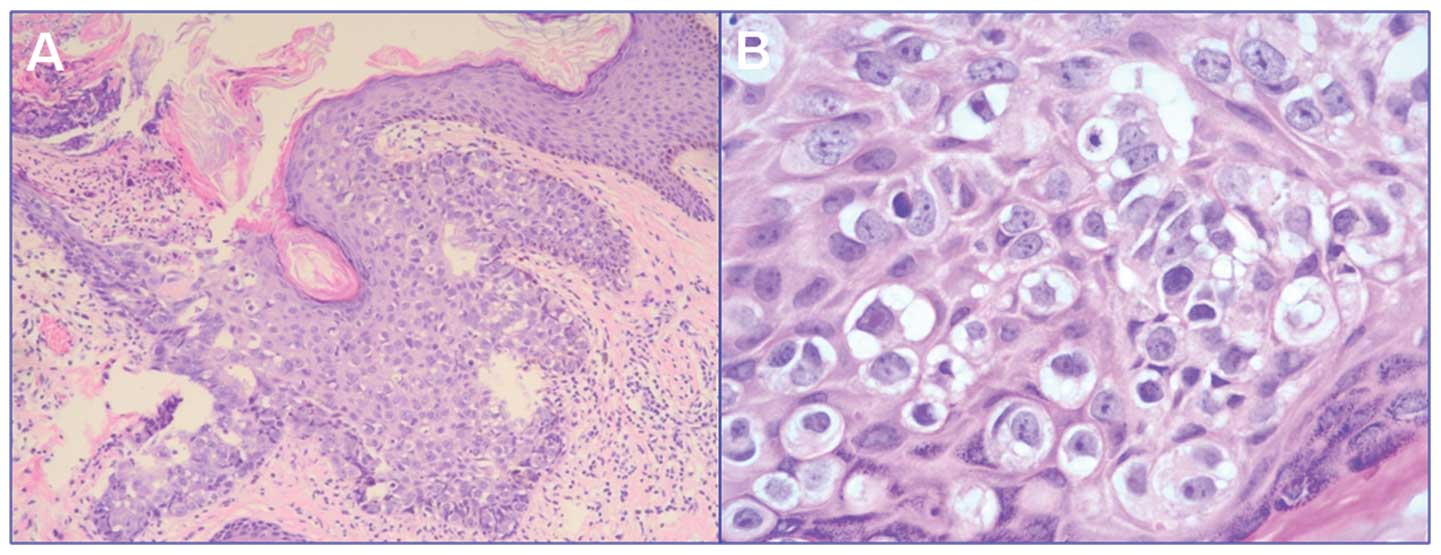
post-operatively. Histopathological examination of the specimen
identified it as Paget’s disease of the breast (Fig. 3) with no evidence of underlying
invasive ductal carcinoma, DCIS of the breast tissue or lymph node
invasion. Immunohistochemical staining showed a negative expression
of oestrogen and progesterone receptors (data not shown). The
patient has been disease-free for 7 months following surgery.
Discussion
Paget’s disease of the nipple, also known as Paget’s
disease of the breast, was initially described by Sir James Paget
in 1874 (1,2). Paget’s disease was defined as the
relationship between chronic eczema of the nipple and underlying
impalpable breast carcinoma. The disease is frequently mistaken for
a benign dermatological condition, such as dermatitis of the
nipple. This results in delayed diagnosis. Thus, a skin biopsy or
scrape cytology of the eczematous area is highly recommended to
exclude or confirm a diagnosis of Paget’s disease in such
cases.
Although Paget’s disease of the breast and
synchronous bilateral breast cancer are uncommon (4), synchronous bilateral Paget’s disease
is extremely rare (5,6). Only a few cases of synchronous
bilateral breast cancer with Paget’s disease have been described
worldwide (5,6). To the best of our knowledge, there are
fewer than 10 cases of bilateral Paget’s disease of the breast
described in the literature. We did not encounter any cases of
synchronous bilateral Paget’s disease of the breast without any
underlying invasive breast carcinoma or DCIS.
Although the presence of the intraepidermal Paget’s
cell is the main pathological characteristic of this disease, the
origin of the Paget’s cell has yet to be conclusively determined
(7). Two main hypotheses have been
proposed to explain its pathogenesis (7). The epidermotropic hypothesis states
that Paget’s cells originate from ductal epithelium, where they
migrate towards the epidermis. This hypothesis is verified by the
association between Paget’s disease and an underlying breast
carcinoma in the majority of patients. Conversely, the
intraepidermal transformation hypothesis states that malignant
keratinocytes originate from the areolar epidermis (7). Currently, the epidermotropic
hypothesis is more widely accepted. However, findings of our case
support the intraepidermal transformation hypothesis, since there
is no underlying carcinoma.
The above-mentioned theories are plausible; however,
each theory of pathogenesis entails a markedly different approach
to treatment (7). Mastectomy or
subcutaneous mastectomy with nipple excision has conventionally
been recommended based on Paget’s original report and several
historical studies (1,8). However, advances have been made in the
treatment of the Paget’s disease of the breast. In their study,
Pierce et al (9) examined 30
patients with Paget’s disease of the breast who did not present
with a palpable breast mass or mammographic density involving
complete resection of the nipple-areola complex followed by
definitive radiotherapy. Their results indicated that
breast-conserving therapy is a viable alternative to mastectomy in
the treatment of Paget’s disease. A high rate of false-negative
findings on mammography and a high incidence of multicentric or
multifocal in situ and invasive carcinomas were identified
in mastectomy specimens. In short, Paget’s disease is frequently
associated with underlying peripheral or multicentric breast
cancer. Therefore, mastectomy with sentinel node biopsy (SNB) is
probably the best treatment option for the majority of patients
(10).
The prognosis of patients with synchronous bilateral
breast cancer does not differ from that of patients with unilateral
breast cancer. Our patient had bilateral early lesions, both of
which were Paget’s disease. She has been healthy without any
evidence of recurrence for seven months following surgery. Thus, it
is reasonable that all nipple changes be explained at the first
opportunity to diagnose the disease when no palpable mass is
present (11). Mammography is
crucial for patients with clinical evidence of Paget’s disease of
the nipple in order to plan an optimal therapeutic strategy once
the underlying malignancy has been detected. Improvements in
mammography and, in particular, magnetic resonance imaging (MRI)
techniques have resulted in a higher rate of detection of
early-stage breast cancers (12).
It is possible that synchronous bilateral breast cancer, with or
without Paget’s disease, may be identified by chance. When we
encounter a patient with Paget’s disease, the possibility of
non-palpable early lesions underlying the breast should be
considered (13).
Acknowledgements
This study was supported by Zhejiang Provincial
Medical and Healthy Science and Technology Projects (Grant no.
2011KYB137), Science Research Fund of Taizhou (Grant no. A102KY09),
Science Research Fund of Shaoxing (Grant no. 2011D10013), and
Science Research Fund of Zhuji (Grant no. 2011CC7874).
Reference
|
1
|
Ashikari R, Park K, Huvos AG and Urban JA:
Paget’s disease of the breast. Cancer. 26:680–685. 1970.
|
|
2
|
Marshall JK, Griffith KA, Haffty BG, Solin
LJ, Vicini FA, McCormick B, Wazer DE, Recht A and Pierce LJ:
Conservative management of Paget disease of the breast with
radiotherapy: 10- and 15-year results. Cancer. 97:2142–2149.
2003.PubMed/NCBI
|
|
3
|
Chen CY, Sun LM and Anderson BO: Paget
disease of the breast: changing patterns of incidence, clinical
presentation, and treatment in the U.S. Cancer. 107:1448–1458.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakayama H, Masuda H, Ugajin W, Nakamura
Y, Akiyama K, Suzuki K and Amano S: Quadruple cancer including
bilateral breasts, Vater’s papilla, and urinary bladder: report of
a case. Surg Today. 29:276–279. 1999.PubMed/NCBI
|
|
5
|
Anderson WR: Bilateral Paget’s disease of
the nipple: case report. Am J Obstet Gynecol. 134:877–878.
1979.
|
|
6
|
Fernandes FJ, Costa MM and Bernardo M:
Rarities in breast pathology. Bilateral Paget’s disease of the
breast – a case report. Eur J Surg Oncol. 16:172–174.
1990.PubMed/NCBI
|
|
7
|
Sakorafas GH, Blanchard K, Sarr MG and
Farley DR: Paget’s disease of the breast. Cancer Treat Rev.
27:9–18. 2001.
|
|
8
|
Dixon AR, Galea MH, Ellis IO, Elston CW
and Blamey RW: Paget’s disease of the nipple. Br J Surg.
78:722–723. 1991.
|
|
9
|
Pierce LJ, Haffty BG, Solin LJ, McCormick
B, Vicini FA, Wazer DE, Recht A, Strawderman M and Lichter AS: The
conservative management of Paget’s disease of the breast with
radiotherapy. Cancer. 80:1065–1072. 1997.
|
|
10
|
Siponen E, Hukkinen K, Heikkilä P, Joensuu
H and Leidenius M: Surgical treatment in Paget’s disease of the
breast. Am J Surg. 200:241–246. 2010.
|
|
11
|
Piekarski J, Jeziorski A, Baklinska M,
Szymczak W, Zadrozny M and Berner J: Patients with Paget disease of
nipple and with palpable mass in breast have unfavorable prognosis.
J Exp Clin Cancer Res. 23:33–37. 2004.PubMed/NCBI
|
|
12
|
Frei KA, Bonel HM, Pelte MF, Hylton NM and
Kinkel K: Paget disease of the breast: findings at magnetic
resonance imaging and histopathologic correlation. Invest Radiol.
40:363–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kijima Y, Owaki T, Yoshinaka H and Aikou
T: Synchronous bilateral breast cancer with Paget’s disease and
invasive ductal carcinoma: report of a case. Surg Today.
33:606–608. 2003.
|