Introduction
Lung cancer is a major cause of mortality from
malignant disease, due to its high incidence, malignant behavior
and the lack of major advancements in treatment strategy (1). A great deal of progress has been made
in the target therapy for non-small cell lung cancer (NSCLC),
largely owing to the development of small molecular inhibitors,
including epidermal growth factor receptor (EGFR) (2–6) and
ALK (7). By contrast, patients with
the principal subtype of NSCLC, squamous cell lung cancer, rarely
respond to these agents. Mutations in the discoidin domain receptor
(DDR) kinase gene have been identified in squamous cell lung cancer
from large-scale Sanger sequencing (8). DDRs have been shown to exhibit altered
expression patterns in multiple types of human cancer, including
lung cancers (9,10). The mechanism by which the receptors
may contribute to oncogenesis is not well known; however, given the
important role of the receptors in transmitting signals from the
extracellular matrix (ECM), it is possible that the receptors act
as regulators of cell proliferation, adhesion, migration and tumor
metastasis (9). The downregulation
of DDR2 mRNA expression has been reported in NSCLCs
(10). Davies et al screened
for mutations in patients with lung cancer by comprehensively
sequencing 518 kinases in the human genome and described a novel
DDR2 gene mutation (R105S) in the discoidin domain (11). However, the mutation status of the
DDR2 gene in the Japanese population has not been well
reported.
In this study, the mutation status of DDR2 at
the discoidin and kinase domains in lung squamous histology tumors
was investigated. This study also investigated DDR2 mRNA
levels via real-time PCR using LightCycler. The findings were
compared with the clinicopathological features of lung cancer.
Patients and methods
Patients
The study group included patients with lung cancer
who had undergone surgery at the Department of Surgery II, Nagoya
City University Hospital (Nagoya, Japan). Patient consent was
obtained from the patients or a family member. The study wa
approved by the ethics committee of the hospital. Tumor samples
were immediately frozen and stored at −80°C until they were
assayed. Hammerman et al demonstrated that the DDR2
mutations were present within the squamous histology of lung cancer
(8), therefore this study focused
on squamous cell carcinomas. The clinical and pathological
characteristics of the 166 patients with lung cancer for
DDR2 gene analyses were as follows: 143 (86.1%) were male
and 23 were female; 143 (86.1%) were diagnosed with squamous cell
carcinomas and 22 were adenosquamous cell carcinomas; 153 (92.2%)
were smokers and 13 were non-smokers. The clinical and pathological
characteristics of the 92 patients with lung cancer for DDR2
gene mRNA expression analyses were as follows: 80 (87%) were male
and 12 were female; 87 (94.6%) were diagnosed with squamous cell
carcinomas and four were adenosquamous cell carcinomas; five (5.4%)
were light-smokers (Brinkman index <100) and 52 (56.5%) were of
pathological stage I.
PCR assays for DDR2 mRNA expression
Total RNA was extracted from lung cancer tissues
using an Isogen kit (Nippon Gene, Tokyo, Japan) according to the
manufacturer’s instructions. The RNA concentration was determined
using a spectrophotometer and adjusted to a concentration of 200
ng/ml. Approximately 10 cases were excluded for each assay as there
were not enough tumor cells to extract sufficient tumor RNA. RNA (1
μg) was reverse transcribed using Superscript II enzyme (Gibco-BRL,
Gaithersburg, MD, USA) with 0.5 μg oligo (dT)12–16
(Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). The
reaction mixture was incubated at 42°C for 50 min and then at 72°C
for 15 min. PCR analyses were performed using 1 μl of each DNA
sample. The PCR was performed using LA-Taq kit (Takara Bio Inc.,
Shiga, Japan) in a 25-μl reaction vessel. The primer sequences for
the discoidin domain of the DDR2 gene were as follows:
forward: 5′-CAGCTTCCAGTCAGTGGTCA-3′ and reverse:
5′-GCCAGCCCACATAGTCATAG-3′ (643 bp, exons 3–7). The cycling
conditions were as follows: initial denaturation at 94°C for 5 min,
followed by 40 cycles at 94°C for 45 sec, 60°C for 45 sec and 72°C
for 45 sec. The primer sequences for the DDR2 gene kinase
domain were as follows: forward: 5′-TTTGGGGAGGTTCATCTCTG-3′ and
reverse: 5′-GTCAGGACAAATGGCTGGTT-3′ (747 bp, exons 9–12). The
cycling conditions were as follows: initial denaturation at 94°C
for 5 min, followed by 40 cycles at 94°C for 45 sec, 60°C for 45
sec and 72°C for 45 sec. The products were purified by a Qiagen PCR
purification kit (Qiagen, Valencia, CA, USA). The samples were
sequenced using the ABI prism 3100 analyzer (Applied Biosystems
Japan Ltd., Tokyo, Japan) and analyzed by BLAST and chromatograms
via manual review.
To ensure the fidelity of mRNA extraction and
reverse transcription, the samples were subjected to PCR
amplification with oligonucleotide primers specific for the
constitutively expressed gene β-actin and normalized using a
β-actin detection kit (Nihon Gene Research Laboratories, Miyagi,
Japan). The primer sequences for the DDR2 gene were as
follows: forward: 5′-CCACTATGCAGAGGCTGACA-3′ and reverse:
5′-CAGAGATGAACCTCCCCAAA-3′ to amplify a 183-bp fragment. The
cycling conditions were as follows: initial denaturation at 95°C
for 10 min, followed by 50 cycles at 95°C for 5 sec, 60°C for 5 sec
and 72°C for 8 sec. PCR reactions were performed using a
LightCycler-FastStart DNA Master SYBR-Green I kit (Roche Molecular
Biochemicals, Mannheim, Germany) and quantified.
Statistical analysis
Statistical analysis was performed using the
Mann-Whitney U test for unpaired samples and Wilcoxon’s signed-rank
test for paired samples. Linear correlations between the variables
were determined by means of a simple linear regression. Correlation
coefficients were determined by rank correlation using the
Spearman’s test and ξ2 test. The overall survival rate
of patients with lung cancer was examined using the Kaplan-Meier
method and differences were examined using the log-rank test.
Analyses were performed using the Stat-View software package
(Abacus Concepts Inc., Berkeley, CA, USA). P<0.05 was considered
to indicate a statistically significant result.
Results
DDR2 gene mutation status in Japanese
patients with lung cancer
The current study sequenced the kinase domain of
DDR2 gene for 173 squamous histology NSCLC samples. Of 173
patients, from direct sequencing using cDNA samples, no mutations
were identified. This study also sequenced the discoidin domain of
the DDR2 gene for 166 squamous histology NSCLC samples,
where 148 samples overlapped. Of 166 patients, from direct
sequencing using cDNA samples, no mutations were identified.
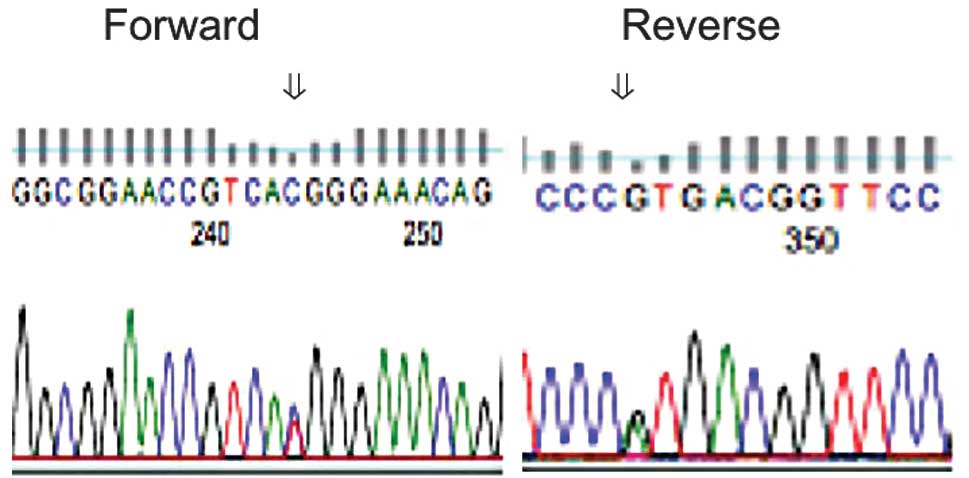
However, 14 DDR2 polymorphism cases (8.4%) were identified
(Fig. 1). The nucleotide T at 408
was changed to C. The amino acid was not converted from histidine
(CAT>CAC, His>His). This DDR2 polymorphism status was
not correlated with gender (male 11/143 vs. female; 3/23,
p=0.3914), age (age ≤65, 7/71 vs. >65, 7/104; p=0.5724) or
smoking status (smoker 14/153 vs. non-smoker 0/13; p=0.2544). The
polymorphism cases tended to be higher in squamous cell carcinomas
(p=0.0796) when compared with the adenosquamous carcinomas. The
polymorphism cases were predominantly observed in advanced stages
(stage II–IV, 11/74 vs. stage I, 3/92; p=0.0075).
DDR2 gene expression status in Japanese
patients with lung cancer
In the 92 tissues from histologically confirmed lung
squamous cell carcinoma, the mean value for DDR2 mRNA level
as standardized by the mRNA level of β-actin (10.915±1.546, mean ±
standard deviation) was significantly lower than the tissues from
non-malignant lung tissue (22.790±3.382, p=0.0013). The T/N ratios
of the DDR2 mRNA levels for each sample were: stage I,
0.978±0.187; stage II, 1.027±0.369; stage III, 1.532±0.611; and
stage IV, 0.17 (Table I). The
DDR2 mRNA T/N ratios in squamous cell carcinoma
(1.113±1.765) and adenosquamous cell carcinomas (0.676±0.423) were
not significantly different (p=0.5839). No significant difference
in DDR2 mRNA levels T/N ratio was observed between gender
and age. The patient groups were further stratified according to
clinicopathological factors. The T/N ratio was not significantly
different between the light-smokers (Brinkman index <100;
0.386±0.368) and smokers (>100; 1.129±1.765; p=0.1479). The T/N
ratio of DDR2 mRNA levels in each sample were: T1,
0.884±0.240; T2, 0.990±0.232; T3, 1.295±0.406; and T4, 1.975±1.048.
Although there was no significant difference, DDR2 mRNA
levels were higher in advanced T stages (Table I).
 | Table IClinicopathological data of 92
patients with lung cancer. |
Table I
Clinicopathological data of 92
patients with lung cancer.
| DDR2 |
|---|
|
|
|---|
| Factors | No. of patients n
(%) | T-N ratio of
DDR2/β-actin mRNA levels | P-value |
|---|
| Mean age (years) | 66.7±9.0 | 92 | |
| Stage |
| I | 52 (56.5) | 0.978±0.187 | NS |
| II | 21 (22.8) | 1.027±0.369 | |
| III | 18 (19.6) | 1.532±0.611 | |
| IV | 1 (1.1) | 0.17 | |
| Tumor status |
| T1 | 30 (32.6) | 0.884±0.240 | NS |
| T2 | 34 (37.0) | 0.990±0.232 | |
| T3 | 8 (8.7) | 1.295±0.406 | |
| T4 | 10 (10.8) | 1.975±1.048 | |
| Lymph node
metastasis |
| N0 | 65 (70.7) | 1.025±0.162 | NS |
| N1 | 15 (16.3) | 1.682±0.835 | |
| N2 | 12 (13.0) | 0.753±0.272 | |
| Pathological
subtype |
| Adenosquamous | 5 (5.4) | 0.676±0.423 | 0.5839 |
| SCC | 87 (94.6) | 1.113±1.765 | |
| DDR2
polymorphism |
| T408C | 7 (8.2) | 3.301±3.972 | 0.0465 |
| T408T | 78 (91.8) | 0.929±1.717 | |
| Smoking |
| BI<100 | 5 (5.4) | 0.386±0.368 | 0.1479 |
| BI>100 | 78 (94.6) | 1.129±1.760 | |
| Age (years) |
| ≤65 | 35 (38.0) | 1.318±1.716 | 0.3204 |
| >65 | 57 (62.0) | 0.948±1.724 | |
| Gender |
| Male | 80 (87.0) | 1.115±1.823 | 0.3272 |
| Female | 12 (13.0) | 0.916±0.765 | |
Correlation between DDR2 and DDR2
polymorphisms
Of the 85 patients that overlapped, 7 polymorphism
cases were found at the discoidin domain (C408T, H136H; Fig. 1). The DDR2 polymorphism in
lung cancer had a significantly higher DDR2 mRNA level (T/N
ratio, 3.301±3.972) than the wild-type DDR2 for lung cancer
(T/N ratio, 0.929±1.717; p=0.0465). The overall survival rate of
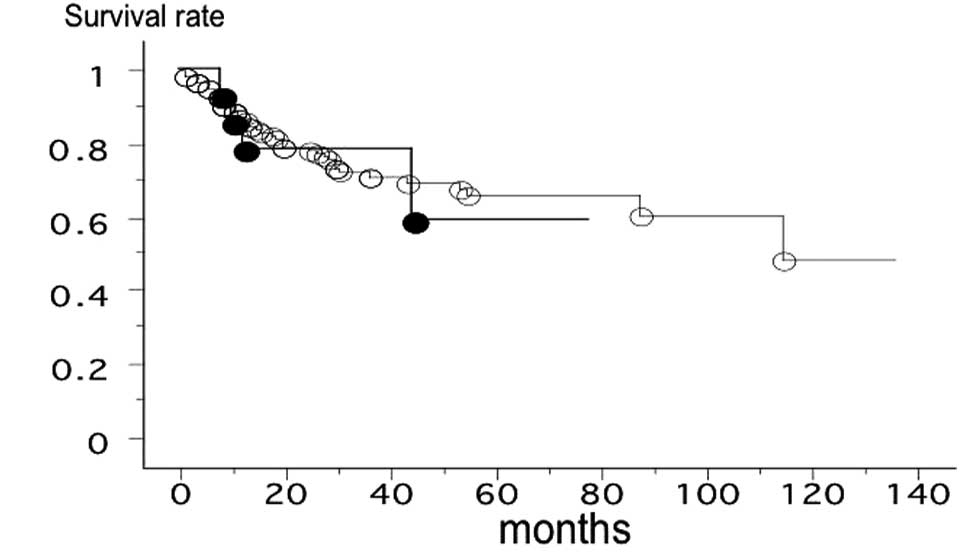
162 patients with lung cancer from Nagoya City University, with
follow-up until August 31, 2011, was studied with reference to the
DDR2 gene status. The survival rate of patients with the
DDR2 gene polymorphism (n=14, 5 mortalities) and the
patients with the wild-type DDR2 (n=148, 41 mortalities) was
not significantly different (log-rank test, p=0.7944; Fig. 2).
Discussion
The present study has shown that DDR2 mRNA
expression is significantly deregulated in NSCLC when compared with
normal lung tissue. However, DDR2 mRNA levels were higher in
the DDR2 polymorphism cases. The polymorphism cases (8.4%)
were predominantly observed within the advanced lung cancers. The
collagen-binding RTK DDRs have previously been linked to various
human diseases, including cancers (12–15).
Although the sample size was not large, no DDR2 mutations
were observed in this cohort. However, the DDR2 expression
pattern in lung cancer suggests that DDR2 contributes to the
pathogenesis of lung cancer.
Previous studies have reported somatic mutations in
the DDR2 gene at the discoidin or kinase domain (8,11),
however, the present study did not confirm the existence of the
DDR2 mutation. There are several explanations for this
discrepancy. Lung cancer encompasses a broad range of clinical
subtypes, in which the two cohorts differed. In addition, an ethnic
difference between the studies on mutant DDR2 may exist, as
in EGFR gene mutations (2–6). The
present study cannot conclude that differences in the DDR2
sequence were due to methodology or PCR and sequencing methods.
However, this study detected 8.4% of polymorphism cases at the
discoidin domain in this cohort.
The mechanism by which DDRs may contribute to
oncogenesis is not well known, however, given their role in
transmitting signals from the ECM, it is likely that DDRs act as
regulators of cell proliferation, adhesion, migration and
subsequent tumor metastasis. Prolonged stimulation of DDR2 is
associated with the upregulation of MMP-1 expression (16). DDR2 is also important in mediating
fibroblast migration and proliferation via a MMP-2-dependent
mechanism (17,18). Activated DDR2 has been noted to
induce the expression of MMP-1, MMP-2 and MMP-13 (17,19).
Similar to EGFR, it is conceivable that an altered expression of
DDRs triggers abnormal activity, ultimately leading to enhanced
proliferation and oncogenesis. In this study, the synonymous
nucleotide change in DDR2 was present in approximately 10%
of the present clinical cohort. This polymorphism was correlated
with an increased DDR2 expression and advanced pathological
stages of lung cancers.
It has been reported that the development of
experimental liver metastasis using melanoma cells, which were
stably transfected with a small interfering RNA for DDR2,
was reduced compared with mock transfected clones (20). Findings of a previous study revealed
that imatinib, nilotinib and dasatinib are potent inhibitors of the
kinase activity of DDRs (21). The
kinase profiles of the three compounds were addressed in two
chemical proteomic studies which observed that the compounds also
bind to the DDRs (21,22). Of these compounds, dasatinib was the
most potent inhibitor of DDRs (17). Recently, it was observed that only
dasatinib produced a response in mutant-DDR2 lung cancer
cell lines (8). These findings may
contribute to a greater understanding of the therapeutic potential
of these inhibitor compounds with DDR2.
In conclusion, the DDR2 mutation in lung
cancers of Japanese patients was observed to be extremely rare.
However, the DDR2 polymorphism and/or its expression may be
involved in the progression of lung cancer.
Acknowledgements
The authors thank Mrs. Miki Mochizuki for her
technical assistance. This study was supported by Grants-in-Aid for
Scientific Research, Japan Society for the Promotion of Science
(JSPS; Nos. 23659674, 24592097, 21591820) and a grant for the
Cancer Research Program for Developing the Supporting System for
Upgrading Education and Research (2009) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan.
References
|
1
|
Ginsberg RJ, Kris K and Armstrong G:
Cancer of the lung. Principles and Practice of Oncology. 4th
edition. Lippincott; Philadelphia: pp. 673–682. 1993
|
|
2
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasaki H, Endo K, Konishi A, et al: EGFR
mutation status in Japanese lung cancer patients: genotyping
analysis using LightCycler. Clin Cancer Res. 11:2924–2929. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sasaki H, Shimizu S, Endo K, et al: EGFR
and erbB2 mutation status in Japanese lung cancer patients. Int J
Cancer. 118:180–184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukuoka M, Wy YL, Thongprasert S, et al:
Biomarker analyses and first overall survival results from a phase
III, randomized, open-label, first-line study of gefitinib versus
carboplatin/paclitaxel in clinically selected patients with
advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol.
29:2866–2874. 2011. View Article : Google Scholar
|
|
7
|
Soda M, Choi YL, Enomoto M, et al:
Identification of the transforming EML4-ALK fusion gene in
non-small-cell lung cancer. Nature. 448:561–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hammerman PS, Sos ML, Ramos AH, et al:
Mutations in the DDR2 kinase gene identify a novel therapeutic
target in squamous cell lung cancer. Cancer Discovery. 1:78–89.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogel WF, Abdulhussein R and Ford CE:
Sensing extracellular matrix: an update on discoidin domain
receptor function. Cell Signal. 18:1108–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ford CE, Lau SK, Zhu CQ, et al: Expression
and mutation analysis of the discoidin domain receptors 1 and 2 in
non-small cell lung carcinoma. Br J Cancer. 96:808–814. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davies H, Hunter C, Smith R, et al:
Somatic mutations of the protein kinase gene family in human lung
cancer. Cancer Res. 65:7591–7595. 2005.PubMed/NCBI
|
|
12
|
Johnson JD, Edman JC and Rutter WJ: A
receptor tyrosine kinase found in breast carcinoma cell has an
extracellular discoidin I-like domain. Proc Natl Acad Sci USA.
90:5677–5681. 1993. View Article : Google Scholar
|
|
13
|
Alves F, Vogel W, Mossie K, et al:
Distinct structural characteristics of discoidin I subfamily
receptor tyrosine kinases and complementary expression in human
cancer. Oncogene. 10:609–618. 1995.
|
|
14
|
Barker KT, Martindale JE, Mitchell PJ, et
al: Expression patterns of the novel receptor-like tyrosine kinase,
DDR, in human breast tumors. Oncogene. 10:569–575. 1995.PubMed/NCBI
|
|
15
|
Nemoto T, Ohashi K, Akashi T, et al:
Overexpression of protein tyrosine kinases in human esophageal
cancer. Pathobiology. 65:195–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vogel W, Gish GD, Alves F, et al: The
discoidin domain receptor tyrosine kinases are activated by
collagen. Mol Cell. 1:13–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olaso E, Labrador JP, Wang L, et al:
Discoidin domain receptor 2 regulates fibroblast proliferation and
migration through the extracellular matrix in association with
transcriptional activation of matrix metalloproteinase-2. J Biol
Chem. 277:3606–3613. 2002. View Article : Google Scholar
|
|
18
|
Olaso E, Ikeda K, Eng FJ, et al: DDR2
receptor promotes MMP-2-mediated proliferation and invasion by
hepatic stellate cells. J Clin Invest. 108:1369–1378. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu L, Peng H, Wu D, et al: Activation of
the discoidin domain receptor 2 induces expression of matrix
metalloproteinase 13 associated with osteoarthritis in mice. J Biol
Chem. 280:548–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badiola I, Villacé P, Basaldua I, et al:
Downregulation of discoidin domain receptor 2 in A375 human
melanoma cells reduces its experimental liver metastasis ability.
Oncol Rep. 26:971–978. 2011.PubMed/NCBI
|
|
21
|
Day E, Waters B, Spiegel K, et al:
Inhibition of collagen-induced discoidin domain receptor 1 and 2
activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol.
599:44–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bantscheff M, Eberhard D, Abraham Y, et
al: Quantitative chemical proteomics reveals mechanisms of action
of clinical ABL kinase inhibitors. Nat Biotech. 25:1035–1044. 2007.
View Article : Google Scholar : PubMed/NCBI
|