Introduction
Although overall mortality from breast cancer is on
the decline, a particularly challenging situation involves patients
with locally advanced breast cancer (LABC). Clinically, this
subgroup is divided into two distinct categories: first, patients
with large clinical stage IIA, IIB and T3N1M0 tumors who are
operable but require a mastectomy; and second, those who are
unlikely to have a complete surgical resection due to significant
tumor burden (i.e., stage TanyN2M0). Overall, patients in the
second category have a poorer prognosis due to the advanced stage
and aggressive nature of their disease and the high likelihood of
leaving residual disease at surgery (1,2).
Due to these challenges, the treatment of locally
advanced disease requires the use of neoadjuvant chemotherapy to
reduce the tumor burden sufficiently to facilitate surgical
resection. The National Surgical Adjuvant Breast and Bowel Project
(NSABP) B18 and B27 trials demonstrated an improved relapse
survival rate in patients who achieved a pathological complete
response (pCR) compared to patients who did not achieve pCR, with
the combination of doxorubicin and cyclophosphamide followed by
docetaxel (AC-D) achieving a pCR in 26% of patients (3,4). At
present, there is no method to distinguish patients who is likely
to respond to neoadjuvant chemotherapy; therefore, it is current
practice to initiate treatment and assess the patient clinically
for a response. Patients who fail to demonstrate an adequate
response are administered salvage treatment, either with
alternative chemotherapy agents or radiotherapy. However, this
salvage approach is unlikely to benefit patients. The NSABP B27,
Aberdeen and GEPARTRIO studies showed that patients who were
non-responders to initial anthracycline therapy were unlikely to
achieve a pCR through the addition of other cytotoxic agents
(4–6). This lack of benefit is of concern as a
number of neoadjuvant regimens result in significant toxicity. For
example, use of AC-D in NSABP B27 yielded a 23.7% grade 4–5
toxicity rate (7). In addition,
there are concerns of potential cardiac toxicity due to the
administration of anthracyclines (8).
Response to chemotherapy is likely dependent on the
molecular profile of a patient’s tumor. Identification of
biomarkers that predict response to neoadjuvant chemotherapy may
prevent unnecessary exposure to cytotoxic agents and lead to the
initiation of either experimental regimens or radiotherapy more
rapidly, potentially leading to better outcomes. One possible
molecular marker is breast cancer 1 (BRCA1) expression.
BRCA1 is a well-known tumor suppressor gene with carriers at
high risk of developing breast and ovarian cancer. Preclinical
evidence suggests that BRCA1 expression is a potential prognostic
or predictive marker for chemotherapeutic response since functional
BRCA1 is able to increase the efficacy of DNA repair (9). Studies have shown that in tumors with
functional BRCA1 protein, increased BRCA1 mRNA levels are
associated with reduced chemotherapy efficacy and poorer outcomes
(10). Reduced intracellular BRCA1
protein levels appear to be a common finding in human breast
cancer, as indicated in 30 of 108 tumor samples in a Japanese
breast cancer cohort (11). Based
on these findings, the expression of BRCA1 may affect response and
outcomes to neoadjuvant AC-D chemotherapy.
Another candidate molecular marker is PIK3CA
mutation status. PIK3CA encodes a catalytic subunit of the
class I phosphoinositide-3-kinases (PI3K). PIK3CA is mutated
in approximately 25% of breast cancers with activating mutations in
exons 9 and 20 of PIK3CA generating constitutively active
forms of the PI3-kinase (12).
Hyperactivation is involved in chemoresistance through the
stimulation of cell survival pathways through Akt and mTOR
(13). Although results of recent
studies have shown that numerous tumors with PIK3CA
mutations are less aggressive, tending to present at an earlier
stage of disease (14), Papaxoinis
et al have reported that PIK3CA mutations were a
negative prognostic factor for survival in patients presenting with
high-risk breast cancer requiring chemotherapy, possibly due to
chemoresistance (15). Due to its
potential role in chemoresistance, activating PIK3CA
mutations may affect response to neoadjuvant chemotherapy and the
likelihood of achieving a pCR.
Currently, little data is available as to whether
BRCA1 protein levels or PIK3CA mutations are predictive for
pCR when AC-D was utilized in the neoadjuvant setting. Therefore,
we conducted a retrospective exploratory analysis to address
whether: i) lower levels of BRCA1 protein in breast cancer cells
were associated with improved AC-D response; and ii) activating
PIK3CA mutations were associated with AC-D resistance.
Materials and methods
Patient samples
This is a retrospective analysis which compared
chemotherapy response in patients to the protein level of BRCA1 and
PIK3CA mutation status. The study design was reviewed by the
Ottawa Hospital Research Institute Research Ethics Board and was
given approval prior to proceeding. Three criteria were established
for inclusion in this study: i) A confirmed diagnosis of LABC or
IBC via core biopsy prior to initiation of any therapy; ii) a clear
clinical history regarding the chemotherapy regimens undertaken by
the patient, and iii) overall response. Potential participants were
identified using the Ottawa Hospital Cancer Centre Breast Cancer
database between 2006 and 2008. Informed patient consent for use of
their tissue in this study was obtained from potential
participants. Individuals who agreed to participate in the study
were enrolled while those who refused participation were not
contacted further by the study team.
Individuals who met the inclusion criteria and
consented to participate had their clinical data derived from
hospital records available at the Ottawa Hospital Cancer Centre.
Clinical data of interest included patient demographics, hormone
receptor status, HER-2 status, and clinical stage. Response to AC-D
chemotherapy was recorded as per the RECIST criteria, a
standardized, peer-reviewed reporting system designed to assess
chemotherapy response (16). Tumor
samples were then requisitioned from pathology, either from within
the Ottawa Hospital system or externally if the biopsy was
performed elsewhere.
BRCA1 protein analysis
Formalin-fixed, paraffin-embedded patient tumor
samples were collected and immunohistochemical (IHC) analysis and
scoring were conducted as described in a previous study (17). Formalin-fixed, paraffin-embedded
blocks representing areas of tumor were retrieved from the archives
of the Department of Pathology and Laboratory Medicine. Step
sections were obtained from these blocks for H&E and IHC
analysis for BRCA1 protein. IHC was performed in batches to
maintain uniformity in the intensity and distribution of staining.
The scoring method was adapted from the Allred and Quick scoring
methods used for the scoring of estrogen and progesterone receptor
immunoreactivity in breast carcinomas (18). The scores for distribution were
represented as the percentage of tumor cell nuclei that stained
positive from 0 to 3 (0, negative; 1, <30%; 2, 30–70% and 3,
>70%). Staining intensity was also scored from 0–3 (0, negative;
1, mild; 2, moderate and 3, strong). Thus, we were able to pool
cases with low intensity (0 or 1) or distribution (0 or 1) as
negative (comprehensive scores 0 or 1). Cases with higher (2 or 3)
scores in either distribution or intensity received higher
comprehensive scores. Pathologists were blinded to each other’s
scores and to the clinical outcome data.
PIK3CA mutation analysis
Sections (5 μM) from paraffin-embedded specimens
were subjected to either macrodissection or laser capture
microdissection to ensure that all DNA samples were derived from
>80% cancer cells. DNA was isolated using QuickExtract DNA
extraction kit (Epicentre Biotechnologies, Madison, WI, USA).
Regions of PIK3CA exons 9 and 20 were amplified using nested
PCRs (primers available from corresponding author on request). PCR
products from the second round of the nested PCR were screened for
the presence of mutations using high resolution melting in a
Corbett Rotorgene 6000. For PCR products with melting curve
deflections, the presence and specific nature of mutations was
confirmed by dideoxy-sequencing. PIK3CA was considered
mutant only if the well-characterized mutations E542K, E545K and
H1047R were detected.
Statistical analysis
Primary statistical questions were posed for the
exploratory analysis. These included whether the i) reduced levels
of BRCA1 protein within the tumor specimen would correlate with an
increased likelihood of achieving a pCR when AC-D chemotherapy was
utilized; ii) a significant difference would be found in the
likelihood of achieving a pCR based on the PIK3CA mutation
status when AC-D chemotherapy was utilized; iii) BRCA1 protein
levels and PIK3CA mutations were associated with achieving a
partial response or better when AC-D chemotherapy was utilized; and
iv) the protein level of BRCA1 or PIK3CA mutation status
would affect the likelihood of being diagnosed with inflammatory
breast cancer.
For BRCA1 protein levels, initial scoring occurred
in a range from 0–9, with 0 representing absent protein and 9
representing high levels of protein. In order to create a
dichotomous variable of relatively ‘high’ and ‘low’ BRCA1
expression, a minimal p-value test was conducted. Based on this
analysis, tumor samples scored as ≤ 4 were considered to have low
levels of BRCA1 protein, while samples scored > 4 were
considered to have high levels of protein. BRCA1 protein levels
were then tested for correlation with pCR and a partial response or
better using a Chi-square test and logistic regression analysis.
For PIK3CA mutations, correlation with pCR and a partial
response or better was performed using a Fisher’s exact test and a
logistic regression analysis. For the evaluation of whether BRCA1
protein levels and PIK3CA mutations affect the likelihood of
developing inflammatory disease, exact logistic regression was
used.
Results
Patient characteristics
For the years 2006–2008, the Ottawa Hospital Cancer
Centre breast cancer database identified 136 eligible participants
who met the inclusion criteria. A total of 65 individuals consented
to participate; however, only 59 samples could be analyzed.
Participant baseline characteristics are shown in Table I. The participants were administered
AC-D chemotherapy followed by 1 year of trastuzumab initiated with
docetaxel if HER2 was positive, with the exception of one
participant who received 6 cycles of carboplatin/taxotere due to a
pre-existing cardiac issue. Partial response or better to therapy
was observed in 68.3% of participants, with 23.7% achieving a
pCR.
 | Table IBaseline characteristics of
participants (n=59). |
Table I
Baseline characteristics of
participants (n=59).
| Age
(mean/median) | 51.6 years/53
years |
| Hormone receptor
status |
| ER-positive | 72.8% |
| PR-positive | 61.0% |
| HER2 overexpression
status | 28.8% |
| Tumor grade |
| Grade 1 | 6.8% |
| Grade 2 | 47.5% |
| Grade 3 | 45.7% |
| Median | 2 |
| Participants with
inflammatory disease | 20.3% |
| Histological
subtype |
| Ductal | 91.5% |
| Lobular | 8.5% |
| Response to
neoadjuvant AC-D |
| Stable disease | 15.3% |
| Partial
response | 55.9% |
| Pathological
complete response | 23.7% |
| Progressive
disease | 5.1% |
BRCA1 protein staining from tumor
samples
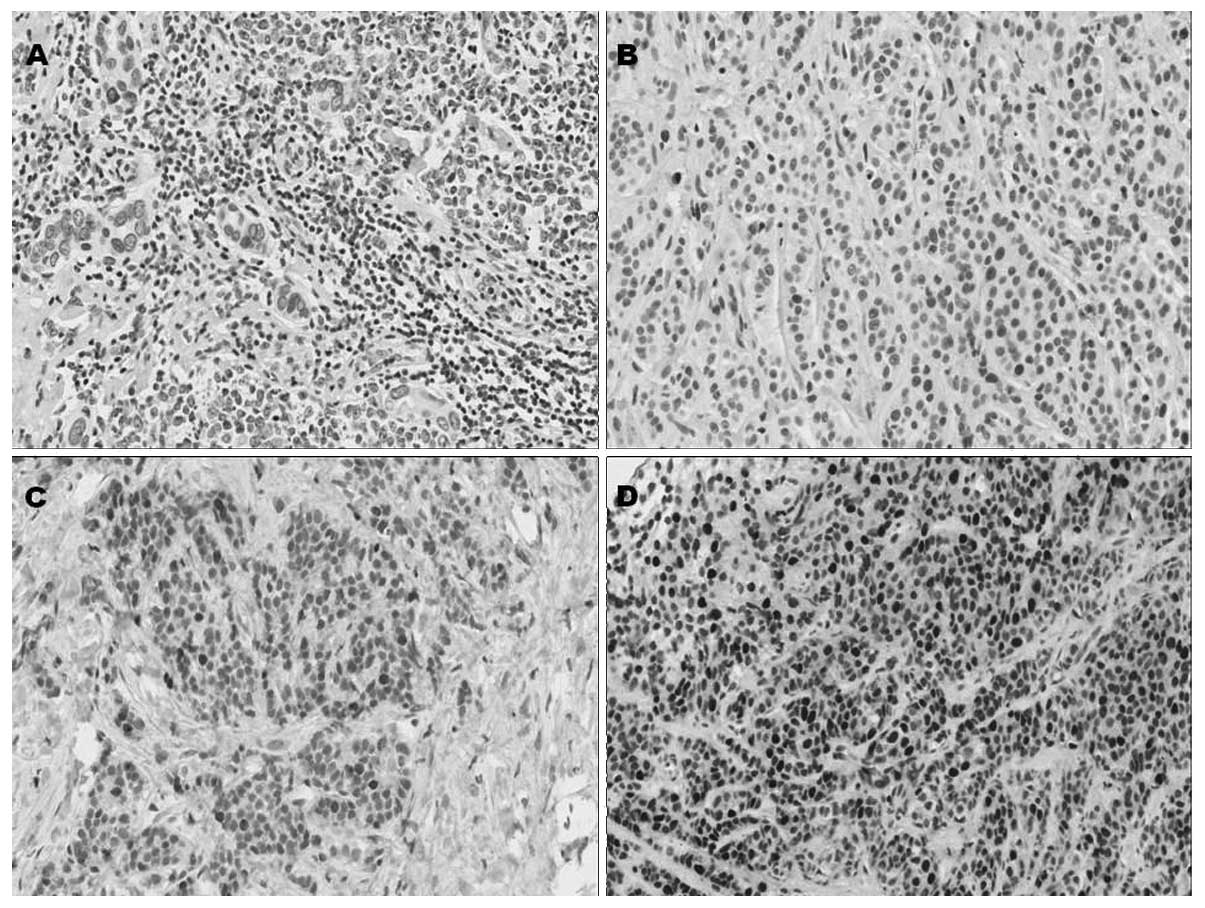
In tumor cells, immunostaining for BRCA1 was mostly
nuclear, with occasional cytoplasmic hue in certain samples. Normal
appearing breast epithelial cells adjacent to the tumor did not
show any immunostaining, although occasional nuclei in
myoepithelial cells showed strong immunoreactivity (Fig. 1). Based on the dichotomous variable
of relatively ‘high’ and ‘low’ levels of BRCA1 expression
established by the minimal p-value test (≤ 4 vs. > 4), 33
samples were scored as having ‘low’ levels of BRCA1 expression,
while 21 samples were scored as having ‘high’ levels of BRCA1
expression.
Correlation of PIK3CA mutations and BRCA1
protein expression to chemotherapy response
With regards to PIK3CA mutations, 27% of
patients had a mutation in either exon 9 or 20, with exon 20
mutations being the most common (18%). This is similar to the
mutation frequencies that have been reported for breast cancer as a
whole (19,20). The presence of a mutation did not
appear to affect the likelihood of achieving a pCR to neoadjuvant
chemotherapy compared to tumor samples possessing a wild-type
PIK3CA mutation (OR=0.977; p=1.00). A similar observation
was found when PIK3CA mutations were evaluated against
achieving a partial response or better (OR=1.03; p=0.971) (Table II).
 | Table IICorrelations of BRCA1 protein level
and PIK3CA mutations to pCR. |
Table II
Correlations of BRCA1 protein level
and PIK3CA mutations to pCR.
| Statistical
comparison | Result |
|---|
| PI3KCA
mutation status and pCR | No association
OR=0.977 if wild type (p=1.00) |
| PI3KCA
mutation status and partial response or better | No association
OR=1.03 if wild type (p=0.971) |
| BRCA1 protein level
and pCR | No association
OR=1.74 if low (p=0.44) |
| BRCA1 protein level
and partial response or better | No association
OR=1.18 if low (p=0.82) |
A relatively low level of BRCA1 protein expression
was more likely associated with achieving a pCR compared to a high
level of BRCA1 expression, although this result was not
statistically significant (OR 1.74; p=0.44). In addition, low
levels of BRCA1 protein expression do not appear to be associated
with the likelihood of achieving a partial response or better to
neoadjuvant chemotherapy (OR=1.18; p=0.82) (Table II).
Participants with PIK3CA wild-type proteins
were more likely to be diagnosed with inflammatory disease compared
to those with mutations in PIK3CA, but this finding did not
reach statistical significance (OR=5.34 for wild type; p=0.109).
Participants with low levels of BRCA1 protein were at an increased
risk of being diagnosed with inflammatory disease, but this finding
did not reach statistical significance (OR=2.86; p=0.407).
Discussion
Inoperable locally advanced disease is a challenging
subtype of potentially curable breast cancer. As cure is dependent
on achieving a complete surgical excision of the primary tumor,
patients with inoperable disease require neoadjuvant chemotherapy
in the hope of reducing the disease burden significantly enough to
allow for surgery to occur. In addition, the NSABP B18 and B27
trials have demonstrated an improved relapse survival rate in
patients who achieve a pCR when neoadjuvant chemotherapy is
utilized (3,4). By contrast, other women present with
operable disease but opt to undergo neoadjuvant chemotherapy to
allow breast conserving surgery to be performed. In all of these
cases, predictive biomarkers for response to neoadjuvant
chemotherapy may allow treating physicians to predict who is likely
to benefit from this approach and possibly select other treatment
modalities or clinical trial protocols in the hope of benefitting
their patients.
In this exploratory study, we evaluated
PIK3CA mutations and intracellular BRCA1 protein levels to
see whether they are associated with response to neoadjuvant
chemotherapy. Preclinical evidence suggests that the two factors
may affect response to chemotherapy. PIK3CA mutations in
breast cancer lead to increased PI3K pathway signalling, which
increased the activity of the Akt and mTOR proteins, leading to
increased cell survival and resistance to cancer therapy (14). Increased BRCA1 protein levels are
able to increase the efficacy of DNA repair in the presence of
chemotherapy (9,21). Thus, we hypothesized that these two
factors may be associated with chemotherapy resistance and be
worthy of exploration in the neoadjuvant setting.
In our study, no statistical association was
identified between PIK3CA mutations or BRCA1 protein levels
and response to neoadjuvant chemotherapy in patients with LABC.
This was an exploratory study with a limited number of available
samples for analysis. However, results of our study suggest trends
for each of these factors to provide a foundation for a larger,
prospective study. In terms of PIK3CA mutations, such a
trend is clearly not present for either the achievement of pCR
(OR=0.977, p=1.00) or response to chemotherapy (OR=1.03, p=0.971).
This result is noteworthy, given the strong preclinical rationale
for this pathway in terms of chemotherapy resistance. Notably,
PIK3CA mutations appeared to associate with the type of
disease at presentation. Patients with activating mutations were
much less likely to present with inflammatory disease, an
aggressive subtype of LABC with a poor prognosis. Although to the
best of our knowledge this is the first study to examine
PIK3CA mutations specifically in LABC, the data obtained are
consistent with previous studies in which PIK3CA mutations
are associated with hormone receptor positivity and less aggressive
disease (14).
Low BRCA1 protein levels appear to be more promising
in predicting for pCR, but the current results are not definitive
(OR=1.71, p=0.44). Given that BRCA1 is a predictive and prognostic
factor for improved chemotherapy response and outcome in sporadic
ovarian cancer (20), more studies
with a larger sample size are required.
There are certain limitations to our study. First,
our sample size was small, reducing the overall power. It is
possible that clearer associations could have been detected with a
larger data set. Second, although we adopted a generalized
approach, it is possible that had we focused on only particular
pathological features, such as ER-positive or HER2-positive, a more
distinct association might have been detected. However, the
population group included in this study was insufficient for these
specific subgroup analyses.
In conclusion, results of our exploratory study did
not show that PIK3CA mutations or BRCA1 protein levels are
associated with response to neoadjuvant chemotherapy in patients
with LABC, although the small sample size limits the conclusions
that may be drawn. Thus, the role of BRCA1 protein levels in
chemotherapy response should be investigated, given that there was
a trend towards greater response in tumors expressing low levels of
the protein and evidence of correlation with chemotherapeutic
response in ovarian cancer. Such studies should be prospective and
a larger number of participants should be included.
Acknowledgements
This study was possible due to generous funding
provided by the Ottawa Regional Cancer Foundation.
References
|
1
|
Gralow JR, Burstein HJ, Wood W, et al:
Preoperative therapy in invasive breast cancer: pathologic
assessment and systemic therapy issues in operable disease. J Clin
Oncol. 26:814–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleer CG, van Golen KL and Merajver SD:
Molecular biology of breast cancer metastasis. Inflammatory breast
cancer: clinical syndrome and molecular determinants. Breast Cancer
Res. 2:423–429. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher B, Bryant J, Wolmark N, et al: The
effect of preoperative chemotherapy on the outcome of women with
operable breast cancer. J Clin Oncol. 16:2672–2685. 1998.PubMed/NCBI
|
|
4
|
Bear HD, Anderson S, Smith RE, et al:
Sequential preoperative or postoperative docetaxel added to
preoperative doxorubicin plus cyclophosphamide for operable breast
cancer: national surgical adjuvant breast and bowel project
protocol B-27. J Clin Oncol. 24:2019–2027. 2006. View Article : Google Scholar
|
|
5
|
Smith IC, Heys SD, Hutcheon AW, et al:
Neoadjuvant chemotherapy in breast cancer: significantly enhanced
response with docetaxel. J Clin Oncol. 20:1456–1466. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Von Minckwitz G, Blohmer JU, Raab G, et
al: In vivo chemosensitivity-adapted preoperative
chemotherapy in patients with early-stage breast cancer: the
GEPARTRIO pilot study. Ann Oncol. 16:56–63. 2005.
|
|
7
|
Bear HD, Anderson S, Brown A, et al: The
effect on tumor response of adding sequential preoperative
docetaxel to preoperative doxorubicin and cyclophosphamide:
preliminary results from national surgical adjuvant breast and
bowel project protocol B-27. J Clin Oncol. 21:4165–4174. 2003.
View Article : Google Scholar
|
|
8
|
Smith LA, Cornelius VR, Plummer CJ, et al:
Cardiotoxicity of anthracycline agents for the treatment of cancer:
systematic review and meta-analysis of randomised controlled
trials. BMC Cancer. 10:337–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weberpals JI, Clark-Knowles KV and
Vanderhyden BC: Sporadic epithelial ovarian cancer: clinical
relevance of BRCA1 inhibition in the DNA damage and repair pathway.
J Clin Oncol. 26:3259–3267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gennari A, Sormani M, Varesco L, et al:
Prognostic significance of BRCA1, PARP1 and PARP2 in sporadic
breast cancer. J Clin Oncol. 27s:e221142009.
|
|
11
|
Yoshikawa K, Honda K, Inamoto T, et al:
Reduction of BRCA1 protein expression in Japanese sporadic breast
carcinomas and its frequent loss in BRCA1-associated cases. Clin
Cancer Res. 5:1249–1261. 1999.PubMed/NCBI
|
|
12
|
Zhao L and Vogt PK: Class I PI3K in
oncogenic cellular transformation. Oncogene. 27:5486–5496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalinsky K, Jacks LM, Heguy A, et al:
PIK3CA mutation associates with improved outcome in breast cancer.
Clin Cancer Res. 15:5049–5059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papaxoinis G, Pectasides D, Wirtz RM, et
al: Prognostic significance of PI3K mRNA expression in patients
with operable, high risk breast cancer. J Clin Oncol.
27s:5772009.
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumors: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
17
|
Weberpals JI, Tu D, Squire JA, et al:
Breast cancer 1 (BRCA1) protein expression as a prognostic marker
in sporadic epithelial ovarian carcinoma: an NCIC CTG OV. 16
correlative study. Ann Oncol. 22:2403–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
19
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcription and translation. Nat Rev
Cancer. 5:921–929. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karakas B, Bachman KE and Park BH:
Mutation of the PIK3CA oncogene in human cancers. Br J Cancer.
94:455–459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carser JE, Quinn JE, Michie CO, et al:
BRCA1 is both a prognostic and predictive biomarker of response to
chemotherapy in sporadic epithelial ovarian cancer. Gynecol Oncol.
123:492–498. 2011. View Article : Google Scholar : PubMed/NCBI
|