Introduction
The identification of tumor markers leads to a
significant improvement in cancer therapy and aids investigators in
understanding cancer biology (1).
Tumor markers are useful in cancer detection and classification
(2). Moreover, in treating
patients, serum levels of such tumor markers are helpful in the
determination of cancer prognosis and likelihood of recurrence
(3). Among several well-known tumor
markers, cancer-associated antigen (CA)125 has garnered increasing
attention in lung cancer research. CA125 is a glycoprotein found on
the cell membrane which has been used as a standard tumor marker
for the diagnosis and follow-up of ovarian cancers (4,5).
Moreover, a significant increase of CA125 has been reported in
other cancers including breast and lung cancers has been noted
(6,7).
Among the various anti-cancer agents used for lung
cancer treatment, cisplatin [cis-diamminedichloroplatinum (II)] is
commonly prescribed (8). Cisplatin
mediates cancer cell apoptosis through reactive oxygen species
(ROS)-dependent and DNA adduct pathways (9,10).
Cisplatin-induced apoptosis is mainly mediated through the caspase
signaling pathway (11). Although
available lung cancer cell lines have been widely used for the
investigation of cancer cell biology and chemotherapeutic
susceptibility, none of the cell lines were from Thai patients.
Since ethnic diversity is known to be a factor affecting tumor
marker expression and chemotherapeutic response (12,13)
and knowledge regarding such cancer signatures of Thai-originated
cancer cells remains elusive, the present study aimed to generate
knowledge about the tumor marker expression and chemotherapeutic
response of lung cancer cells obtained from a Thai patient.
Consequently, we generated primary cancer cells collected from the
pleural effusion fluid of a Thai patient and characterized the lung
cancer signatures of the cells compared to the established lung
cancer H460 cells. The obtained cells were evaluated for CA125
expression and response to cisplatin, one of the most widely used
chemotherapeutic agents. The results and methodology described in
this study may aid the development of lung cancer diagnostic and
therapeutic approaches and, in particular, advance understanding of
ethnic differences.
Materials and methods
Clinical specimens and reagents
Pleural effusions were collected from a 76-year-old
male Thai patient with suspected lung adenocarcinoma. Informed
consent was obtained from the patient and the study was approved by
the ethics committee of the Faculty of Medicine and the ethics
committee of the faculty of Pharmaceutical Sciences, Chulalongkorn
University. Human proximal tubular epithelial renal cells (HK-2)
and human lung cancer epithelial (H460) cells were obtained from
the American Type Culture Collection (Manassas, VA, USA). H460
cells were cultured in RPMI-1640 while HK-2 cells were cultured in
DMEM, supplemented with 10% fetal bovine serum (FBS), 2 mM
L-glutamine and 100 U/ml penicillin/streptomycin in a 5%
CO2 environment at 37°C. Cisplatin, propidium iodide
(PI) and Hoechst 33342 were obtained from Sigma Chemical, Inc. (St.
Louis, MO, USA). Resazurin was purchased from Invitrogen (Carlsbad,
CA, USA). Specific antibodies for CA125, CYFRA 21-1, and SCCA were
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Resazurin and Alexa Fluor 488 goat anti-rabbit IgG (H+L) and Alexa
Fluor 590 goat anti-mouse IgG (H+L) conjugated secondary antibody
were purchased from Invitrogen.
Specimen preparation
Pleural effusion was centrifuged at 1600 × g for 10
min at room temperature. The pellet was resuspended with 4 ml
sterile balanced salt solution and then centrifuged on a Ficoll
gradient (Ficoll-Paque™, GE Healthcare, Piscataway, NJ, USA) at 400
× g for 40 min at 20°C to separate the tumor cells from
erythrocytes. The layer of mononuclear cells were collected and
washed twice with 3 volumes of RPMI-1640 by centrifugation at 400 ×
g for 10 min at 20°C. The pellet was then resuspended and the cells
were cultured in ACL-4 medium supplemented with 5% FBS at 37°C and
5% CO2.
Growth properties and cell
morphology
Cells were initially seeded at a density of
1×104 cells in a 24-well plate and the population
doubling time was determined. At various time points, cells were
trypsinized by 0.25% trypsin-EDTA treatment and the number of cells
was calculated by the trypan blue exclusion method. Population
doubling times were determined from an exponential regression of
viable cell counts over nine days.
Immunofluorescence
Cells (5×104/well) were seeded in 6-well
plates for 24 h to allow the cells to completely adhere to the
surface. The cells were then fixed in 3.7% formaldehyde for 10 min
at room temperature, permeabilized and blocked in a solution
containing 0.5% saponin, 1% FBS and 1.5% goat serum for 30 min.
Following primary antibody incubation with CA125, CYFRA 21-1 or
SCCA antibody at 1:100 dilution for 1 h, cells were washed and
incubated together with Alexa Fluor 488 goat anti-rabbit IgG
(H+L)-conjugated secondary antibody (Invitrogen) or Alexa Fluor 590
goat anti-mouse IgG (H+L)-conjugated secondary antibody
(Invitrogen) for 30 min. Nuclei were stained with Hoechst dye
(Invitrogen). Images were visualized by fluorescence microscopy
(Olympus IX51 with DP70).
Cytotoxicity and apoptosis assay
Cytotoxicity was determined by a Presto Blue
fluorescence assay. Following specific treatments, cells in a
96-well plate were incubated with 1:50 resazurin for 1 h at 37°C.
Fluorescence intensity of resazurin product (resorufin) was
measured at 530 nm (excitation wavelength) and 590 nm (emission
wavelength) using a microplate reader. Cell viability was
calculated as a percentage relative to non-treated cells. Analyses
were performed independently in triplicate. Apoptosis was
determined by a Hoechst 33342 DNA fragmentation assay. Briefly,
cells were incubated with 10 μg/ml of Hoechst 33342 for 30 min and
analyzed for apoptosis by scoring the percentage of cells having
intensely condensed chromatin and/or fragmented nuclei on
fluorescence microscopy (Olympus IX51 with DP70). The apoptotic
index was calculated as the percentage of cells with apoptotic
nuclei over the total number of cells.
Statistical analysis
The data are presented as the means ± SD from three
or more independent experiments. Statistical analysis was performed
using the Student’s t-test at a significance level of
P<0.05.
Results
Growth properties and morphology of lung
carcinoma cells
Pleural effusion fluids of an untreated Thai patient
were first centrifuged and the suspended cells were collected. The
cells obtained were cultured under adherent conditions in lung
cancer cell-selective ACL-4 medium as described by the National
Cancer Institute (NCI) for the selective growth of non-small cell
lung cancer, while rarely sparing the growth of normal cells, such
as fibroblasts and macrophages. In the present study, those cells
continuously propagating for at least six months were designated as
P1 cells. The clinical and pathological details of the patient from
whom P1 cells were obtained are described in Materials and methods.
Six months later, cells were routinely cultured in ACL-4 medium
supplemented with 10% FBS. P1 cells exhibited epitheloid morphology
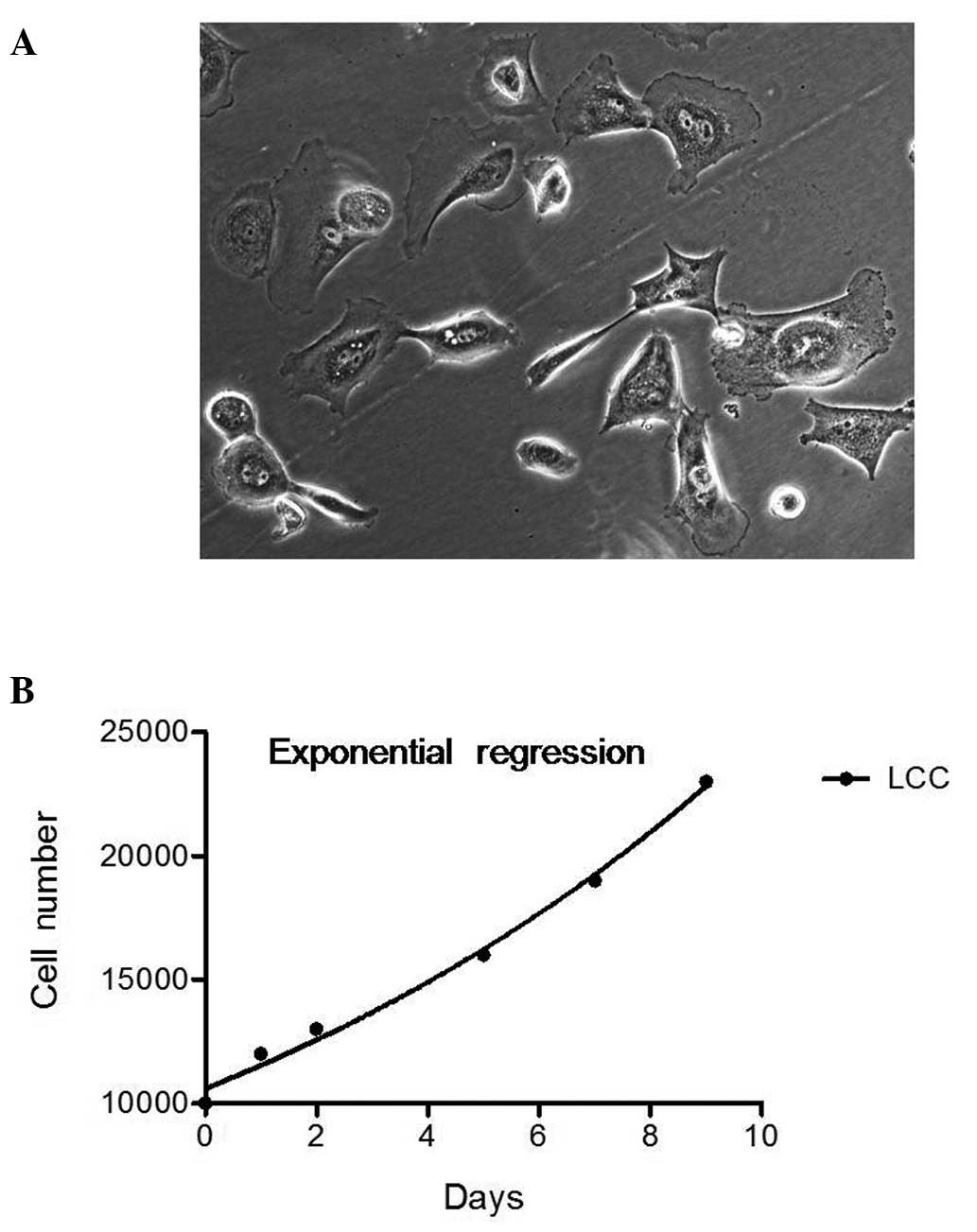
with a bipolar, spindle-shaped appearance (Fig. 1A). Cells grew individually with
little cell-cell contact. This morphology of P1 cells was markedly
similar to the previously established non-small cell lung cancer
cell lines from solid tumor specimens (14). Population doubling time of P1 cells
was calculated based on the exponential regression of cell growth
(Fig. 1B). The best-fit population
doubling value was 8.127 days. Although the cells were
slow-growing, they could be maintained in ACL-4 medium and continue
to be cultured for a long period of time.
Expression of CA125 in P1 cells
Recent evidence suggests that CA125 may be an
important tumor marker for lung cancer. Data obtained from the
present study revealed that the expression profile of primary lung
cancer cells originating from a Thai patient confirms this
hypothesis and may facilitate the development of a lung cancer
marker; we therefore evaluated the expression of CA125 in P1 cells.
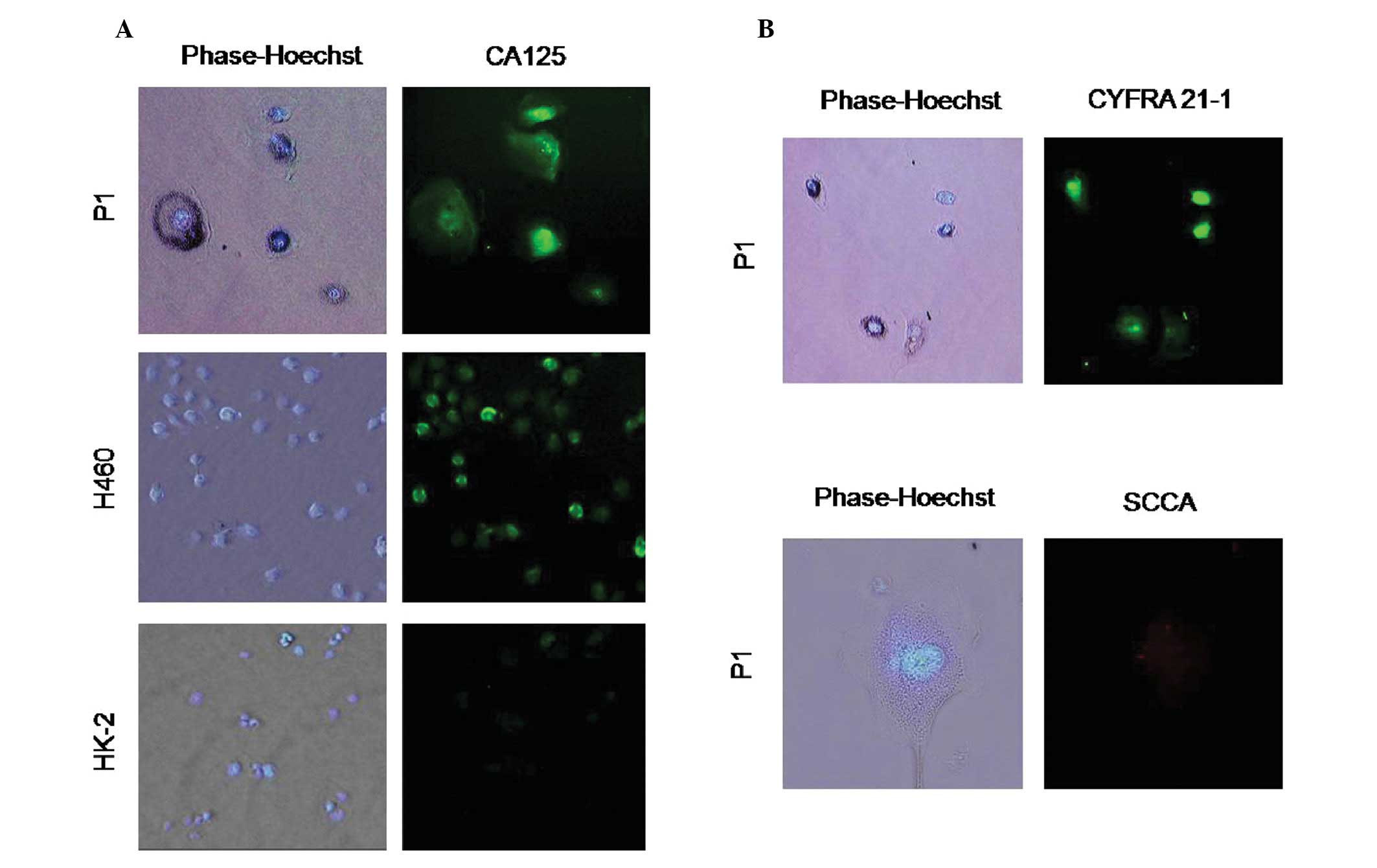
Immunohistochemical investigation using anti-CA125 antibody
indicated that CA125 was highly expressed in P1 cells as in the
positive control lung carcinoma H460 cells. Notably, CA125 signals
originated from the nuclear region of the cells as well as the cell
surface (Fig. 2A). Moreover, we
evaluated the specificity of CA125 expression on tumor cells by
staining the non-cancerous proximal tubule epithelial HK-2 cells.
Results indicated that even though HK-2 cells were stained by the
CA125 antibody, the signal was only slightly increased (Fig. 2A). To provide supporting evidence
for the histological sub-type of P1 cells, we performed an
additional immunofluorescence study using CYFRA 21-1 (a marker for
non-small cell carcinoma) and anti-SCCA (a marker for squamous cell
carcinoma) antibodies. P1 cells were found to express CYFRA 21-1
but not SCCA protein (Fig. 2B). The
results indicated that P1 may be either adenocarcinoma or large
cell carcinoma, but not squamous carcinoma.
Cisplatin response of P1 cells
Disruption of apoptosis contributes to malignant
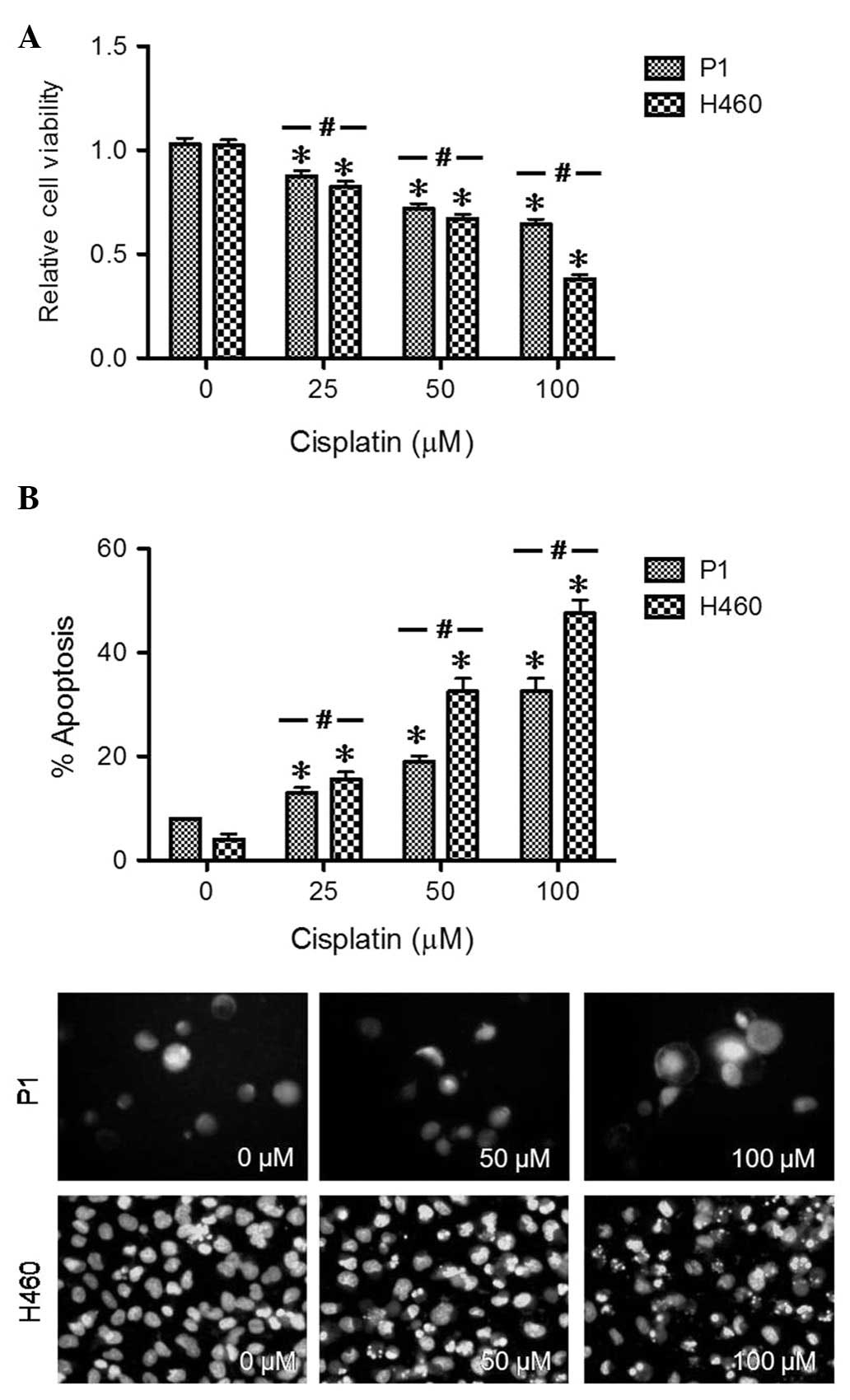
cell growth and chemotherapeutic resistance. To determine the
apoptosis phenotype of P1 cells, the cells were treated with
various concentrations of cisplatin (0–200 μM) and analyzed for
cell toxicity by resazurin assay and apoptosis by Hoechst 33342
assay. H460 cells were used as a standard phenotype for non-small
cell lung cancer. Cells having intensely condensed and/or
fragmented nuclei were considered apoptotic. Treatment with
cisplatin induced a dose-dependent decrease in cell viability and a
concomitant increase of apoptotic cells in P1 and H460 cells
(Fig. 3). P1 cells acquired higher
apoptosis resistance to cisplatin as compared to H460 cells
(Fig. 3B). We summarized the
IC50 values of P1, H460, and the additional A549 lung
cancer cell line in Table I. Since
apoptotic resistance is a key characteristic of all cancer cells,
this finding suggested the lung cancer-like signature of P1
cells.
 | Table IComparison of cisplatin susceptibility
of cells. |
Table I
Comparison of cisplatin susceptibility
of cells.
| Cells | IC50
(μM) |
|---|
| H460 | 80±8.61 |
| P1 | 151±10.51 |
| A549 | 62±5.32 |
Discussion
Improved understanding of cancer cell biology is
likely to benefit the overall improvement of cancer therapy. In
Thailand, the number of diagnosed lung cancer patients is on the
increase and has become a primary cause of cancer-related mortality
(15). Satisfactory clinical
outcomes for lung cancer treatment are currently limited by two
main obstacles which include late detection and the presence of
chemotherapeutic resistance (16).
Overcoming these barriers is vital, with improvement of basic
knowledge of lung cancer biology, tumor markers and cellular
response to chemotherapeutic agents being crucial. The present
study generated P1 cells from the pleural effusion of a Thai male
who was diagnosed as a lung cancer patient. The cells demonstrated
typical cancerous characteristics including CYFRA 21-1
expression.
CA125 expression in lung cancer cells has previously
been reported and the accumulated data suggested that this tumor
marker may be of significance for lung cancer therapy (6). Furthermore, CA125 expression in lung
cancer patients may be a good predictive tool for patient outcome
(17). CA125 is an important tumor
marker recognized by a monoclonal antibody OC125. CA125 is elevated
in the serum of ovarian cancer patients and has been identified as
a useful tool for the screening of ovarian cancer (4,5,18). In
lung cancer, investigators have found a significant expression of
CA125 in a number of lung cancer cell lines (7). In accordance with these findings, we
found a strong expression of such tumor markers in lung carcinoma
H460 (obtained from ATCC) and Thai-originated lung cancer P1
cells.
Accumulating evidence indicates that ethnic
difference is a notable factor in the determination of the
chemotherapeutic response. In the case of anti-cancer agents,
regimens including doses and drug manipulating protocols are often
used for different ethnic populations. In response to
chemotherapeutic agents, a number of cell mechanisms are activated,
supporting the idea that genetic variation by ethnicity may affect
the drug responsiveness. Cisplatin is widely used for the treatment
of numerous solid tumors including lung cancers (19). The mechanisms of action of the drug
on tumor cells is through ROS production and DNA adduct formation
(9,10). To induce cell death, several
signaling pathways are activated in the regulation of survival and
apoptosis and the variation in genetics may alter the cell
response. Variation in response to cisplatin among cells was
revealed in the present study. Two lung cancer cell models obtained
from ATCC, H460 and A549, cells were used as standard cells in the
evaluation of cisplatin susceptibility. Results of viability in
response to cisplatin revealed that P1 exhibited slight resistance
to cisplatin-mediated death compared to H460 (Fig. 3) and A549 cells (data not shown).
For the apoptotic evaluation, the number of apoptotic cells in
response to cisplatin in the P1 population significantly decreased
as compared to those of H460 cells (Fig. 3B), suggesting that P1 cells
exhibited relative cisplatin resistance. Consistent results
obtained from further investigation revealed that the cisplatin
IC50 of P1, H460, and A549 cells was 151±10.51, 80±8.61,
and 62±5.32 μM, respectively.
Based on these data, it is possible that the
variation in ethnicity plays a significant role in drug
susceptibility. However, investigations as to this role are
required. The present study provides a basis for the better
understanding of ethnic difference in cancer cell biology.
Acknowledgements
This study was supported by the higher education
research promotion and national research university project of
Thailand, office of the higher education commission and
postdoctoral fellowship (Ratchadaphiseksompot Endowment Fund,
Chulalongkorn University). The authors would like to thank Mr.
Krich Rajprasit, a proofreader.
References
|
1
|
Pamies RJ and Crawford DR: Tumor markers -
an update. Med Clin N Am. 80:185–199. 1996. View Article : Google Scholar
|
|
2
|
Trape J, Molina R and Sant F: Clinical
evaluation of the simultaneous determination of tumor markers in
fluid and serum and their ratio in the differential of serous
effusions. Tumor Biol. 25:276–281. 2004. View Article : Google Scholar
|
|
3
|
Hayes DF, Bast RC, Desch CE, et al: Tumor
marker utility grading system: a framework to evaluate clinical
utility of tumor markers. J Natl Cancer I. 88:1456–1466. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zurawski VR Jr, Knapp RC, Einhorn N, et
al: An initial analysis of preoperative serum CA 125 levels in
patients with early stage ovarian carcinoma. Gynecol Oncol.
30:7–14. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bast RC Jr, Klul TL and St John E: A
radioimmunoassay using a monoclonal antibody to monitor the course
of epithelial ovarian cancer. New Engl J Med. 309:883–887. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimura Y, Fujii T, Hamamoto K, et al:
Serum CA125 level is a good prognostic indicator in lung cancer.
Brit J Cancer. 62:676–678. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Homma S, Satoh H, Kagohashi K, et al:
Production of CA125 by human lung cancer cell lines. Clin Exp Med.
4:139–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arriagada R, Bergman B, Dunant A, et al:
Cisplatin-based adjuvant chemotherapy in patients with completely
resected non-small-cell lung cancer. New Engl J Med. 350:351–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Chanvorachote P, Toledo D, et al:
Peroxide is a key mediator of Bcl-2 down-regulation and apoptosis
induction by cisplatin in human lung cancer cells. Mol Pharmacol.
73:119–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu YJ, Muldoon LL and Neuwelt EA: The
chemoprotective agent N-acetylcyseine blocks cisplatin-induced
apoptosis through caspase signaling pathway. J Pharmacol Exp Ther.
312:424–463. 2005.PubMed/NCBI
|
|
12
|
Gilliland FD: Ethnic differences in cancer
incidence: a marker for inherited susceptibility? Environ Health
Persp. 105:897–900. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O’Donnell PH and Dolan ME: Cancer
pharmacoethnicity: ethnic differences in susceptibility to the
effects of chemotherapy. Clin Cancer Res. 15:4806–4814.
2009.PubMed/NCBI
|
|
14
|
Musuda N, Fukuoka M, Takada M, Kudoh S and
Kusunoki Y: Establishment and characterization of 20 human
non-small cell lung cancer cell lines in a serum-free defined
medium (ACL-4). Chest. 100:429–438. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vatanasapt V, Sriamporn S and Vatanasapt
P: Cancer control in Thailand. Jpn J Clin Oncol. 32:S82–S91. 2002.
View Article : Google Scholar
|
|
16
|
Read C, Janes S, George J and Spiro S:
Early lung cancer: screening and detection. Prim Care Respir J.
15:332–336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buccheri G and Ferrigno D: Lung tumour
markers in oncology practice: a study of TPA and CA125. Brit J
Cancer. 87:1112–1118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacobs IJ, Skates SJ, MacDonald N, et al:
Screening for ovarian cancer: a pilot randomized controlled trial.
Lancet. 353:1207–1210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|