Introduction
Based on the 2006 Surveillance Epidemiology and End
Results (SEER) database, the incidence of melanoma is 19.6 per
100,000 population per year in the US, with mortality rates at 2.7
per 100,000 (1). Rates in Israel
are not significantly different with 1,200 new cases being reported
per year per 7,000,000 population. The favorable incidence to
mortality ratio reflects the fact that most melanoma patients are
diagnosed at an early, surgically curable stage. Approximately
8,400 patients per year in the US either presented with or
developed incurable disease, and succumbed to melanoma (1).
Although surgery provides the best opportunity for
cure, it is frequently futile or impossible in patients with
advanced stage III or IV metastatic disease. Thus, a
multidisciplinary approach is an option that should be applied to
this subgroup of patients, in an attempt at improving their
outcome.
In their 1998 phase II chemobiotherapy (CBT) study,
Buzaid et al achieved 6.5% pathological complete response
(pCR) and 43.5% partial response (PR) in 65 potentially resectable
stage III melanoma patients (2).
Stein et al used CBT to achieve 17% CR in 29 stage IV
patients with 8 months median time to progression (3). Gibbs et al reported clinical
complete response (cCR) in 2.8% of 48 stage III patients, pCR in
11.1% and 36% PR, using combined preoperative and postoperative
CBT, with 64.6% of patients being disease-free following a median
follow-up of 31 months (4).
Researchers from Yale University identified 16 patients who
responded to combination chemotherapy and had their residual tumor
resected. Eleven of these 16 were alive with no evidence of disease
(NED) following a median of 35 months (5).
Our own experience with CBT echoes the results of
these publications with a 44% (28 of 57 patients) response rate,
25% (14 patients) CR and 19% (11 patients) PR (6).
Based on these observations, and specifically, the
high response rate, the CBT regimen was considered and applied as
the treatment of choice to precede or be combined with surgery for
selected patients who were deemed potentially resectable or became
potentially resectable during treatment.
Patients and methods
Patient selection
The Institutional Melanoma Tumor Boards selected 37
cutaneous melanoma patients diagnosed between January 1983 and
October 2006 in a non-randomized manner. These patients presented
with or developed very advanced stage III or IV disease, and were
deemed eligible for induction CBT followed by potentially curative
surgery (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Total number of
patients | 37 |
| Male/female | 22/15 |
| Age at diagnosis
(years) |
| Median/range | 44/20–71 |
| Melanoma site |
| Trunk | 14 |
| Extremity | 13 |
| Head and neck | 3 |
| Unknown primary | 8 |
| Breslow thickness
(mm) |
| Median/range | 3.7/0.45–26 |
| Stage |
| Advanced III/IV | 17/20 |
| Earlier CBT
regimena |
| Number of
patients | 13 |
| Median number of
courses (range) | 4 (2–6) |
| Later CBT
regimena |
| Number of
patients | 24 |
| Median number of
courses (range) | 4 (2–6) |
Very advanced stage III was defined as irresectable
or marginally resectable regional disease, based on surgical
oncologist’s clinical examination, computed tomography (CT) or
positron emission tomography-computed tomography (PET-CT) plus
magnetic resonance imaging (MRI) of brain and multidisciplinary
team deliberation. Stage IV for inclusion in this study required
biopsy-proven distant metastases combined with progression on
imaging.
The study was approved by the ethics committee of
the hospital. Signed informed consent was obtained from the patient
prior to commencing chemotherapy treatment.
Methods
Patients had to be on no active treatment for at
least two weeks prior to the initiation of CBT. All 37 patients had
an ECOG performance status of 0–2; white blood cell count >3000;
platelet count >100,000; normal renal function tests; bilirubin
<2 mg%; and normal thyroid function tests. Symptomatic patients
or those aged >50 underwent a Thallium 201 Effort Stress Cardiac
Perfusion Test and/or lung function test (>75% of predicted).
Systemic evaluation included head, chest and abdominal CT or PET-CT
with MRI of brain. Patients on corticosteroids, with other
malignancies or brain metastases or known mucosal primary
malignancies were excluded.
Two chemotherapy regimens were used (Table II), both using interferon-α (Intron
A®; Schering, Kenilworth, NJ, USA), dacarbazine and
cisplatin. BCNU and granulocyte-monocyte colony stimulating factor
(GMCSF), which were part of the first regimen were replaced by
decrescendo interleukin 2 (IL-2) (Proleukin®; Hoffman La
Roche, Nutley, NJ, USA) due to the questionable contribution of
BCNU and its significant side-effects, the unavailability of GMCSF
in Israel, and the activity of IL-2 in metastatic melanoma.
 | Table IIChemobiotherapy (CBT) regimens. |
Table II
Chemobiotherapy (CBT) regimens.
| A, Earlier regimen
(N=13), Q-21 day (16). |
|---|
|
|---|
| Regimen | Dose | Initiation point |
|---|
| INF α (s.c.) | 3 mU/days 1,3,5 | Week I |
| DTIC (i.v.) | 220
mg/m2/days 1–3 | Week II |
| CDDP (i.v.) | 25
mg/m2/days 1–3 | Week II |
| BCNU (i.v.) | 150
mg/m2/day 1 | Week II |
| | (every 2nd
course) |
| GMCSF (s.c.) | 20
μg/m2/days 1–7 | Week III |
|
| B, Later regimen
(N=24), Q-21 day. |
|
| Regimen | Dose | |
|
| INF α (s.c.) | 5 mU/days 1–21 | |
| DTIC (i.v.) | 220
mg/m2/days 1–3 | |
| CDDP (i.v.) | 25
mg/m2/days 1–3 | |
| IL-2 (i.v.) | 18
mU/m2/day 1 | |
| 9
mU/m2/day 2 | |
| 4.5
mU/m2/days 3–4 | |
Response evaluation was performed at the end of
every two courses combining clinical examination, CT or PET-CT
comparative study and multidisciplinary team evaluation. Surgical
intervention was considered at each interval. CBT was stopped if
progressive disease was noted.
Results
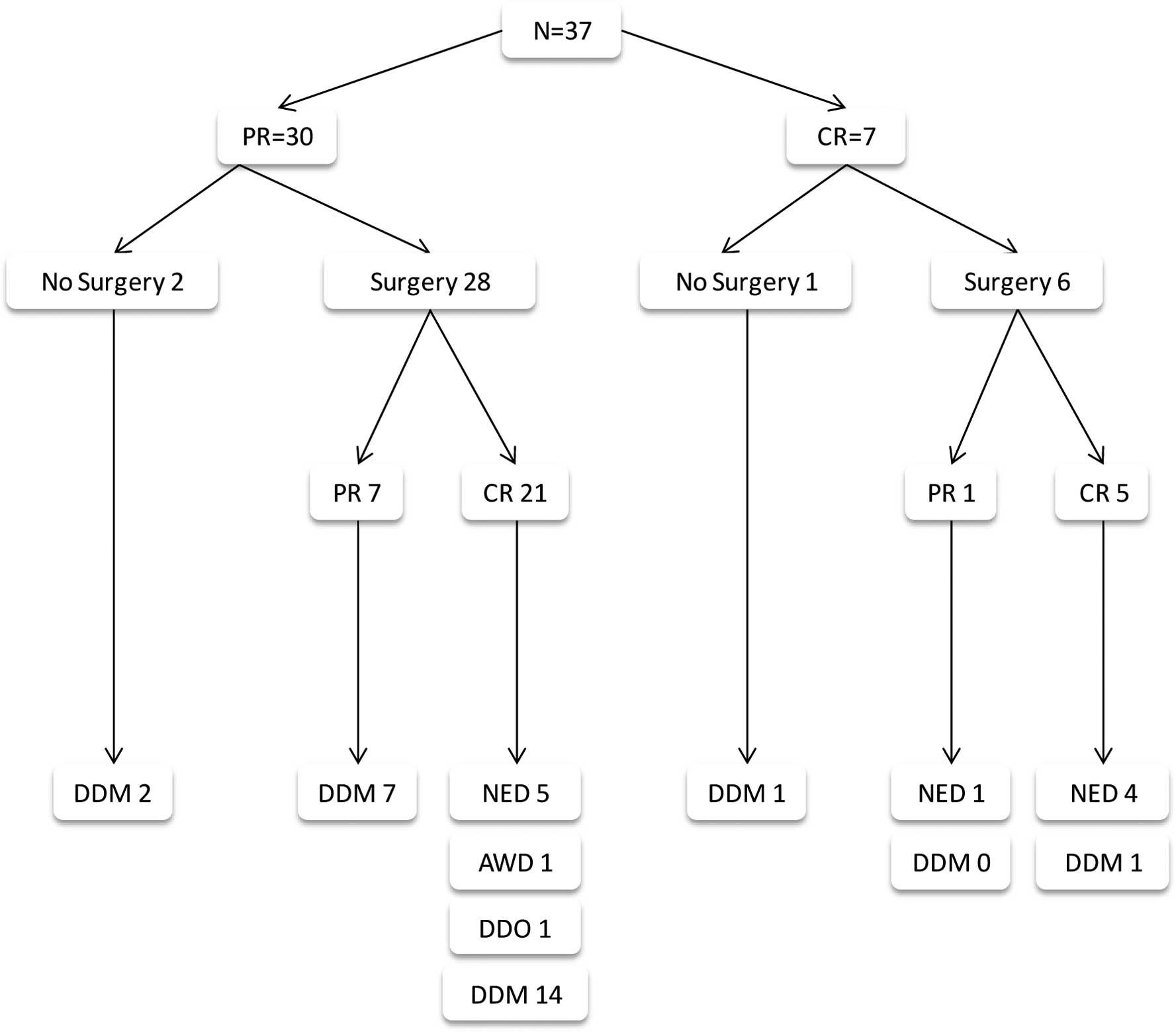
Thirty-seven patients were included in this analysis
(Table I, Fig. 1), of whom 4 did not undergo surgery.
Of these 4 patients, 2 patients never became resectable, and
succumbed to their disease. One patient achieved temporary CR
(PET-CT), but had a recurrence prior to consenting to undergo
surgery. The fourth patient is alive with NED 108 months following
emergency surgical removal of large metastatic bleeding ulcerating
axillary disease of unknown primary malignancy and the
disappearance of biopsy proven liver and prostate metastases.
Thirty-four patients underwent surgery following a median of 4 CBT
courses, and 27 achieved CR. Patients who did not have surgery
(N=3), and those in whom surgery failed to remove all residual
disease (N=7), succumbed to melanoma. Similarly, 16 patients who
were rendered disease-free by CBT and surgery combined succumbed to
the disease.
Of the 37 patients, 11 patients (30%) did not
succumb to melanoma during the median follow-up period of 72
(14–156) months following the last intervention. One of these 11
patients succumbed to non-small-cell lung cancer 87 months
following the last intervention for melanoma. Another patient was
alive with diminishing disease and expanding vitiligo 25 months
from the last intervention, at the time of data analysis. The
patient remains alive with no active disease over 30 months
later.
Five of 8 patients with unknown primary survived
with NED for a median of 89 (30–156) months since the last
intervention.
Discussion
CBT regimens have been employed to treat melanoma
patients. Although high response rates were documented (2–4,6), phase
III studies failed to show survival advantage to CBT over the
standard single agent DTIC, and were associated with significant
toxicity (7). Furthermore, the
exact role of each component of these multi-agent regimens has not
been well defined. Thus, these regimens have not matured into a
standard first-line treatment. A number of clinical studies are
ongoing to find the best regimen. By applying modern targeted
approaches, two of these new studies yielded two new highly
effective, life-prolonging, drugs in metastatic melanoma.
The high response rates of CBT regimens have also
been the focus of other studies. Hwu et al rendered 3 of 35
stage IV patients NED by surgery following temozolomide and
interferon induction treatment (8).
In their study, Lewis et al reported a 26% response rate
using preoperative and postoperative CBT (9). Even peri-operative interferon alone
was able to achieve up to 55% PR with 15% pCR (10). However, a more recent combination
containing cisplatin, paclitaxel and sorafenib failed to induce a
response rate of this magnitude (11).
The traditional CBT regimens created the best
opportunity for a significant response during the study period
(7). Thus, it seemed reasonable to
regard these patients as resectable and attempt surgery to render
the responders NED in order to secure long-term remission.
The role of surgery is not well defined in those
patients rendered cCR by the induction therapy alone. The mere
presence of microscopic residual disease places the patients at a
potential risk of recurrence, thus, surgery appears crucial to
secure CR and long-term remission. The present study may serve as a
proof-of-principle, since 30% long term remission achieved is not
perceived as typical natural history of advanced stage III and IV
melanoma.
The use of a priori CBT is significant for
various reasons. Quality of life indices are better in responders
compared to supportive care, and responders are more likely to
benefit from further treatments (12), as demonstrated in the present
series. Surgery for responders may be of a lesser extent and less
mutilating. Surgery may be omitted for non-responders.
Hypothetically, the induction therapy also ‘sterilizes’ the
periphery of the tumor mass, thus reducing the surgical field and
intravascular tumor cell shedding (13). Although direct evidence to support
this hypothesis is scarce, the overall results of this study may
be, in part, due to such an effect. Response to CBT facilitates and
encourages surgical attempts. Disease progression is an indicator
that CBT treatment is more beneficial than surgery. Surgery for
responders results in clinically meaningful prolongation of
remission in an, admittedly low, but significant percentage of
these advanced stage patients.
Numerous studies in favor of surgical resection of
metastatic melanoma in selected populations have been published and
reviewed (14,15). Adding induction CBT may improve
patient selection for surgery, reduce its extent, and improve
quality of life. Patients with reasonable performance status, whose
metastatic disease may become resectable, should be offered the
option of this multidisciplinary approach, which is currently the
only one with up to 30% chance of an unmaintained remission.
Recently, extensive clinical experience has been
gained with the use of new technologies for advanced melanoma such
as B-RAF inhibitors and anti CTLA-4 antibodies. The group of the
B-RAF inhibitors has shown a notable, albeit short clinical
response of more than 50% and is potentially applicable to the
above neoadjuvant multidisciplinary approach (Chapman et al,
American Society of Clinical Oncology 2011 meeting, abs. LBA4,
2011).
References
|
1
|
National Cancer Institute. SEER Cancer
Statistics Review, 1975–2006. http://seer.cancer.gov/csr/1975_2006/.
Accessed: April 12, 2012
|
|
2
|
Buzaid AC, Colome M, Bedikian A, Eton O,
Legha SS, Papadopoulos N, Plager C, Ross M, Lee JE, Mansfield P, et
al: Phase II study of neoadjuvant concurrent biochemotherapy in
melanoma patients with local-regional metastases. Melanoma Res.
8:549–556. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein ME, Bernstein Z, Drumea K, Zalik M,
Shklar Z, Steiner M and Haim N: Sustained complete remission after
chemobiohormonal therapy for metastatic melanoma. Am J Clin Oncol.
22:62–64. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gibbs P, Anderson C, Pearlman N, LaClaire
S, Becker M, Gatlin K, O’Driscoll M, Stephens J and Gonzalez R: A
phase II study of neoadjuvant biochemotherapy for stage III
melanoma. Cancer. 94:470–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sasson HN, Poo WJ, Bakas MH and Ariyan S:
Prolonged survival in patients with advanced melanoma treated with
neoadjuvant chemotherapy followed by resection. Ann Plast Surg.
37:286–292. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bar J, Yerushalmi R, Shapira-Frummer R,
Kutchuk I, Sulkes A, Gutman H, Catane R and Schachter J: Concurrent
chemobio- therapy with cisplatin, dacarbazine, decrescendo
interleukin-2 and interferon α2b in patients with metastatic
melanoma. Oncol Rep. 20:1533–1538. 2008.PubMed/NCBI
|
|
7
|
O’Day S and Boasberg P: Management of
metastatic melanoma 2005. Surg Oncol Clin N Am. 15:419–437.
2006.
|
|
8
|
Hwu WJ, Panageas KS, Menell JH, Lamb LA,
Aird S, Krown SE, Williams LJ, Chapman PB, Livingston PO, Wolchok
JD and Houghton AN: Phase II study of temozolomide plus pegylated
interferon-alpha-2b for metastatic melanoma. Cancer. 106:2445–2451.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis KD, Robinson WA, McCarter M,
Pearlman N, O’Day SJ, Anderson C, Amatruda TT, Baron A, Zeng C,
Becker M, et al: Phase II multicenter study of neoadjuvant
biochemotherapy for patients with stage III malignant melanoma. J
Clin Oncol. 24:3157–3163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moschos SJ, Edington HD, Land SR, Rao UN,
Jukic D, Shipe-Spotloe J and Kirkwood JM: Neoadjuvant treatment of
regional stage IIIB melanoma with high-dose interferon alfa-2b
induces objective tumor regression in association with modulation
of tumor infiltrating host cellular immune responses. J Clin Oncol.
24:3164–3171. 2006. View Article : Google Scholar
|
|
11
|
Hauschild A, Agarwala SS, Trefzer U, Hogg
D, Robert C, Hersey P, Eggermont A, Grabbe S, Gonzalez R, Gille J,
et al: Results of a phase III, randomized, placebo-controlled study
of sorafenib in combination with carboplatin and paclitaxel as
second-line treatment in patients with unresectable stage III or
stage IV melanoma. J Clin Oncol. 27:2823–2830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarnaik AA, Zager JS and Sondak VK:
Multidisciplinary management of special melanoma situations:
oligometastatic disease and bulky nodal sites. Curr Oncol Rep.
9:417–427. 2007. View Article : Google Scholar
|
|
13
|
Stephens FO: Induction chemotherapy: to
downgrade aggressive cancers to improve curability by surgery
and/or radiotherapy. Eur J Surg Oncol. 27:672–688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gutman H, Hess KR, Kokotsakis JA, Ross MI,
Guinee VF and Balch CM: Surgery for abdominal metastases of
cutaneous melanoma. World J Surg. 25:750–758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mosca PJ, Teicher E, Nair SP and Pockaj
BA: Can surgeons improve survival in stage IV melanoma? J Surg
Oncol. 97:462–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schachter J, Rakowsky E, Sulkes A and
Adler A: A sequential four-drug chemotherapy and biotherapy with
interferon alpha and GM-CSF - an innovative protocol for the
treatment of metastatic melanoma. Cancer Biother Radiopharm.
13:155–164. 1998. View Article : Google Scholar : PubMed/NCBI
|