Introduction
Voltage-dependent potassium channels (Kv) contribute
to the myogenic regulation of vascular tone (1). In addition, Kv channels act in the
cell cycle progression and differentiation of smooth muscle cells
(2,3). In this context, Kv channels govern
cell proliferation by controlling specific checkpoints during the
cell cycle that are crucial for further cycle progression (4–7).
During the G1/S phase of the cell cycle a temporary
hyperpolarization occurs, in which Kv channels are involved
(8). The voltage-dependent Kv1.3
and Kv1.5 channels are involved in cell proliferation, and
expression remodeling has been described during neoplastic growth
(5,9). A number of tissues, such as brain,
muscle and the immune system, co-express the two channels (10–12).
Kv1.3 abundance is generally downregulated, whereas Kv1.5
expression is enhanced in several types of human cancer (5,9,13).
Kv1.3 and Kv1.5 increase during myoblast
proliferation (11). Kv1.5 is the
main subunit in skeletal myoblasts, whereas Kv1.3 is critical in
smooth muscle (2,3). Thus, Kv1.5 exhibits a cycle-dependent
regulation in skeletal muscle myoblasts (11) and Kv1.3, which controls
proliferation in leukocytes (14,15),
is also involved in vascular cell proliferation and migration
(2,3). In a preliminary report, it was noted
that Kv1.3 and Kv1.5 undergo remodeling in human skeletal muscle
sarcomas (13), which was recently
demonstrated in depth (16),
whereas there are no studies addressing Kv1.3 and Kv1.5 during
neoplastic smooth muscle proliferation. The aim of the present
study was to investigate, for the first time, the expression of
Kv1.3 and Kv1.5 in human smooth muscle neoplasms. Leiomyoma (LM)
and leiomyosarcoma (LMS) are benign and malignant soft tissue
neoplasms, respectively, that arise from smooth muscle cells. LM is
the most common type of uterine neoplasm occurring in women older
than 35 years. Retroperitoneal LMS, a common primary
retroperitoneal neoplasm, arises from smooth muscle within
arteries, veins or the bowel.
In the present study, we observed a positive
correlation between the malignancy of the smooth muscle neoplasm
and the expression of Kv1.3 and Kv1.5. The results were notable for
Kv1.3, which seems to be crucial in smooth myoblasts (2,3). The
results indicate that Kv1.5 and Kv1.3 are potential tumorigenic
targets for aggressive LMS.
Materials and methods
Patients, tissue characteristics and
sample processing
A total of 6 smooth muscle tumor samples, 3 LM
samples and 3 LMS samples were obtained from the Department of
Pathology of Vall d’Hebron University Hospital and the Department
of Pathology of Bellvitge Hospital (Barcelona, Spain) between 2008
and 2009. A summary of patient and sample information is shown in
Table I. Four non-tumoral uterine
smooth muscle samples were added to the series as controls.
Patients were informed and gave their consent for sample
collection. The research investigations were approved by the
University and the hospital’s ethics committee.
 | Table ISample characteristics and diagnostic
markers. |
Table I
Sample characteristics and diagnostic
markers.
| Diagnosis | n | Mean age | Gender | Diagnostic
method/markers |
|---|
| Retroperitoneal
leiomyosarcoma (LMS) | 3 | 67 | M | Vimentin, desmin,
smooth muscle actin |
| Uterine leiomyoma
(LM) | 3 | 43 | F | Blinded
morphometry |
Control and tumor samples were fixed in neutral
formalin and embedded in paraffin for immunohistochemistry. Blinded
diagnosis was performed independently by two pathologists using
light microscopy and conventional hematoxylin and eosin staining.
Tissue microarrays were constructed, including 3 cores of 2-mm
diameter from each case; two cores were from the central area of
the tumor and the third was obtained from the invasive front. The
results from representative cores were recorded.
For LMS, markers such as smooth-muscle actin,
vimentin and desmin were used. LM was diagnosed with simple
hematoxylin and eosin staining and light microscopy evaluation.
Antibodies and immunohistochemistry
Immunohistochemical staining using the
avidin-biotin-peroxidase technique was performed for each antibody.
Tissue microarrays were generated on poly-L-lysine-coated glass
slides. Sections were deparaffinized in xylene and rehydrated in
graded alcohol. For antigen retrieval, the microarrays were heated
either in a pressure cooker in 10 mM citric acid monohydrate, pH
6.0, for 5 min (Kv1.5), or in 10 mM citric acid monohydrate, pH 9.0
for 40 min in a 98°C water bath (Kv1.3). Endogenous peroxidase was
blocked by incubating the sections in 3% hydrogen peroxidase
blocking solution for 10 min. Arrays were immunoblotted with
anti-Kv1.3 (1:70) and anti-Kv1.5 (1:100) polyclonal antibodies
(Alomone) for 1 h, followed by an HRP-labeled polymer anti-rabbit
antibody (DakoCytomation, Glostrup, Denmark) for 30 min.
Immunohistochemical detection was performed with the EnVision
system (DakoCytomation). The samples were counterstained with
hematoxylin, dehydrated and mounted. Negative controls were
prepared in all cases by omitting the primary antibody as described
in previous studies (16,17). Antibody staining has been
extensively validated previously (13,16,17).
Additionally, as previously reported, but not yet understood, some
Kv1.5 nuclear staining was observed (13,16–18);
therefore, only cytoplasm staining was evaluated.
Immunohistochemistry evaluation and
statistical analysis
Cases were evaluated by pathologists who assessed
the percentage of positively stained cells and semi-quantitatively
described the staining intensity. Kv1.3 and Kv1.5 staining was
evaluated by calculating a histoscore (Hscore). The Hscore = (1 × %
weakly stained cells) + (2 × % moderately stained cells) + (3 × %
strongly stained cells), with results ranging from 0 to 300, as
previously described (16,19). Weakly stained cells were marked (+),
moderately stained cells (++) and strongly stained cells (+++). In
randomly chosen cases, the immunostaining was performed in complete
tissue sections to evaluate the consistency of the results.
Values were expressed as the means ± SEM and
analyzed by Student’s t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Immunohistochemistry of Kv1.3 and Kv1.5
in smooth muscle tumors
In vascular smooth muscle (VSMC), Kv1.3 and Kv1.5
expression remodels during VSMC proliferation (2,3). Kv1.5
downregulates, whereas Kv1.3 increases (2). However, the expression of Kv1.3 and
Kv1.5 in other smooth muscle tissues, such as the bladder or
uterus, is under debate (3). Since
the two channels are involved in cell proliferation, we
investigated the expression of Kv1.3 and Kv1.5 in human smooth
muscle neoplasms. Two types of human smooth muscle tumors were
studied: an indolent uterus smooth muscle LM and an aggressive
retroperitoneal LMS. Healthy specimen counterparts were also
included.
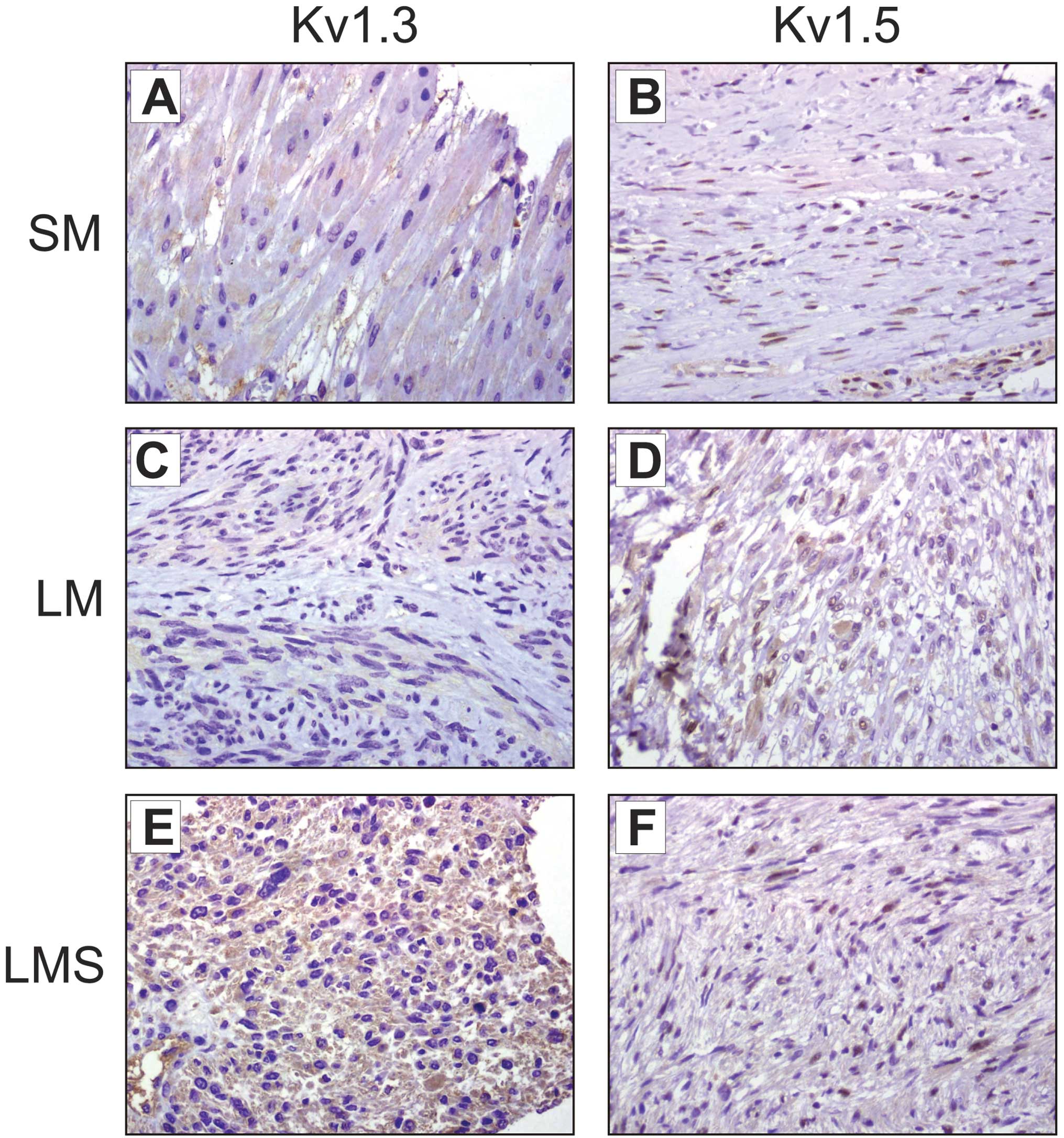
Fig. 1 shows
representative images of Kv1.3 and Kv1.5 staining in LM and LMS
compared with healthy myometrial samples. Remodeling of Kv1.3 was
detected in uterine smooth muscle biopsies. Kv1.3 staining in the
control sample (Fig. 1A) was faint
(+, 90%) and extremely low (−/+, 50%) in the less aggressive LM
(Fig. 3C). However, the expression of Kv1.3 was clearly notable
(++, 100%) in LMS (Fig. 3E). By contrast, Kv1.5 staining was almost
absent (−, 98%) in the healthy smooth muscle (Fig. 1B). A heterogeneous faint Kv1.5
expression was detected (+, 50%) in LM samples (Fig. 1D), whereas LMS showed a homogenous
but poor expression (−/+, 100%) of Kv1.5 (Fig. 1F).
Histoscore evaluation for smooth muscle
tumors
Hscore has been validated as a semi-quantitative
analysis for evaluation of the protein expression with
immunohistochemistry (16,19). Therefore, to analyze Kv1.3 and Kv1.5
staining, an Hscore was calculated as described in Materials and
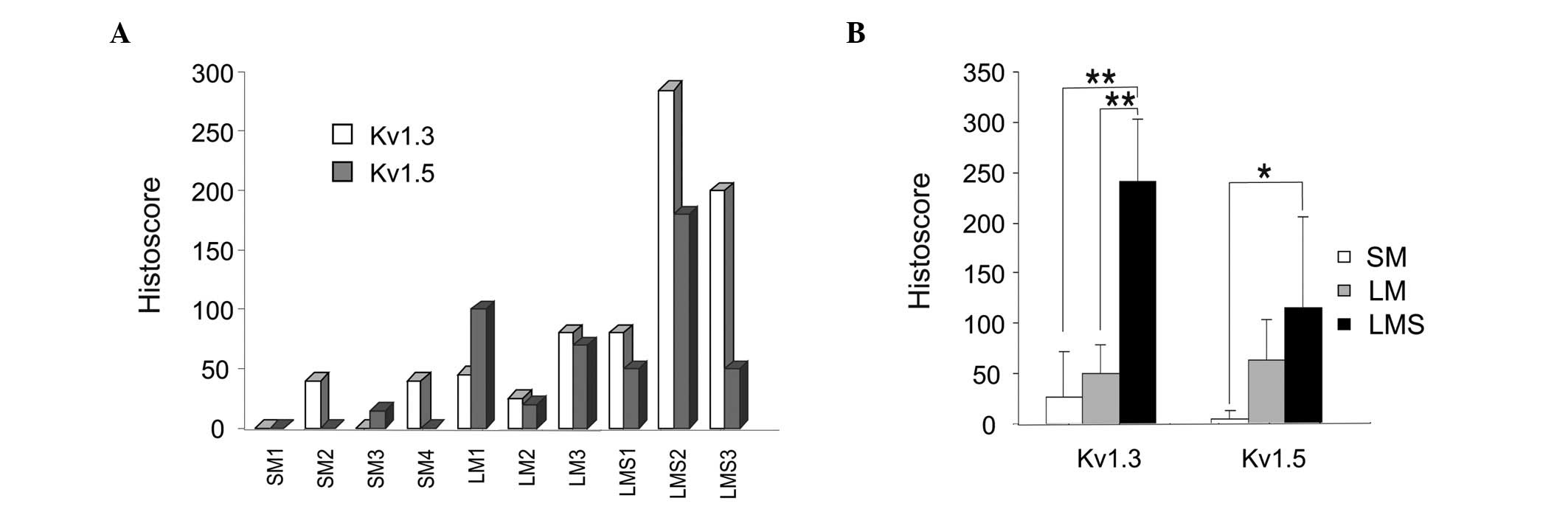
methods (Fig. 2). Fig. 2A shows a graphical representation of
the Hscore for individual samples of smooth muscle tumors and
healthy specimens. Fig. 2B shows
the means ± SEM for different groups. Kv1.3 appeared to be the
dominant channel in smooth muscle tumors. The expression of Kv1.3
in healthy smooth muscle was low or null, similar to that of the
indolent LM, but its expression was notably increased in LMS
(Fig. 2A and B). Kv1.3 expression
in this aggressive sarcoma was 4-fold higher than that in the
control samples (Fig. 2B), whereas
Kv1.5 staining was slightly increased in the two types of tumors
(Fig. 2A and B).
Thus, although the expression of the two channels
showed a similar correlation with malignancy (Fig. 2B), the results were clear for Kv1.3.
Kv1.3 staining was similar in the indolent LM and healthy
specimens, but was significantly elevated in the aggressive LMS. A
similar pattern was observed for Kv1.5, although to a lesser
extent.
Discussion
Kv channels, which control cell excitability in
muscle, also contribute to myoblast proliferation (2,11). In
this study, we have shown for the first time that the expression of
Kv1.3 and Kv1.5 increased in smooth muscle tumorigenesis in a close
correlation with malignancy. Kv1.3, which governs smooth muscle
proliferation and migration (2,3),
increased notably in LMS. In addition, Kv1.5 expression, which
steadily increases with aggressiveness, was shown to be almost
absent in human myometrial smooth muscle.
Kv1.5 is involved in skeletal muscle cell
proliferation (11). Unlike Kv1.5,
Kv1.3 governs macrophage cell growth (14), but plays no substantial role in
skeletal myoblast proliferation (11). In a recent study, we observed a
correlation of Kv1.3 and Kv1.5 expression with tumor malignancy in
rhabdomyosarcomas (16). However,
Kv1.3 staining revealed no major differences between tumors and
healthy samples (16). Therefore,
the role of Kv1.3 in skeletal myoblasts is uncertain. By contrast,
Kv1.3 action on smooth muscle proliferation seems defined and,
similar to leukocytes, recent evidence supports the involvement of
Kv1.3 in vascular smooth muscle cell (VMSC) proliferation and
migration (2,3).
Kv1.3 and Kv1.5 remodel in a large variety of human
cancers (13). In addition, their
expression correlates with rhabdomyosarcoma aggressiveness
(16). Much evidence supports a
role for ion channels in cancer development, progression and
metastasis, and Kv1.3 and Kv1.5 may serve as potential biomarkers
and/or anti-cancer therapeutic targets (9). In our study, aggressive LMS exhibited
Kv1.3 staining higher than that of indolent LM and control smooth
muscle biopsies. In addition, Kv1.5 expression also increased in
LMS. Both results are in agreement with previous reports
demonstrating that Kv1.5 and Kv1.3 play a role in skeletal and
smooth muscle cell proliferation (2,3,11).
VSMCs are responsible for the correct contraction
and dilatation of blood vessels, which play a crucial role in
hypertension. In addition, VSMCs are capable of changing their
phenotype from contractile to proliferative (2,3). This
is an important feature during wound healing, but it may also
become pathological in neointimal hyperplasia. The expression
pattern of Kv undergoes a marked change during this switch. While
contractile murine VSMCs express almost all isoforms of the Kv1
family (Kv1.1 – Kv1.6), which control the vascular tone (3,20–22),
proliferating cells lose the expression of the majority of the
VSMCs, solely upregulating Kv1.3 (2,3). Thus,
proliferating human vein smooth muscle cells undergoing intimal
neoplasia express high levels of Kv1.3, which further supports a
putative role in VSMC proliferation. In this situation, Kv1.3
blockers inhibit growth and migration of venous cells (2,3). In
this context, our results support findings of previous reports,
suggesting Kv1.3 as a potential target for pathologies involving
the excessive proliferation of smooth muscle cells (2,3).
In conclusion, these results demonstrate for the
first time that Kv1.3 and Kv1.5 are specifically remodeled during
smooth muscle carcinogenesis. A notable Kv1.3 expression is
associated with smooth muscle cancer aggressiveness and a
correlation of Kv1.5 expression is observed with tumorigenesis.
Furthermore, our study argues in favor of Kv channels as potential
therapeutic targets in pathologies involving excessive cell
proliferation.
Acknowledgements
This study was supported by grants from the
Ministerio de Ciencia e Innovación (MICINN), Spain (BFU2008-00431,
BFU2011-23268 and CSD2008-00005) to A.F. J.B. holds a fellowship
from the MICINN. The editorial assistance of the American Journal
Experts is also acknowledged.
References
|
1
|
Hille B: Ion Channels of Excitable
Membranes. 3rd edition. Sinauer; Sunderland, MA: xviii. pp.
8142001
|
|
2
|
Cidad P, Moreno-Dominguez A, Novensa L, et
al: Characterization of ion channels involved in the proliferative
response of femoral artery smooth muscle cells. Arterioscler Thromb
Vasc Biol. 30:1203–1211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheong A, Li J, Sukumar P, et al: Potent
suppression of vascular smooth muscle cell migration and human
neointimal hyperplasia by KV1.3 channel blockers. Cardiovasc Res.
89:282–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conti M: Targeting K+ channels
for cancer therapy. J Exp Ther Oncol. 4:161–166. 2004.
|
|
5
|
Felipe A, Vicente R, Villalonga N, et al:
Potassium channels: new targets in cancer therapy. Cancer Detect
Prev. 30:375–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kunzelmann K: Ion channels and cancer. J
Membr Biol. 205:159–173. 2005. View Article : Google Scholar
|
|
7
|
Pardo LA: Voltage-gated potassium channels
in cell proliferation. Physiology (Bethesda). 19:285–292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wonderlin WF and Strobl JS: Potassium
channels, proliferation and G1 progression. J Membr Biol.
154:91–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Felipe A, Bielanska J, Comes N, et al:
Targeting the voltage-dependent K+ channels Kv1.3 and
Kv1.5 as tumor biomarkers for cancer detection and prevention. Curr
Med Chem. 9:661–674. 2011.
|
|
10
|
Vicente R, Escalada A, Villalonga N, et
al: Association of Kv1.5 and Kv1.3 contributes to the major
voltage-dependent K+ channel in macrophages. J Biol
Chem. 281:37675–37685. 2006. View Article : Google Scholar
|
|
11
|
Villalonga N, Martinez-Marmol R,
Roura-Ferrer M, et al: Cell cycle-dependent expression of Kv1.5 is
involved in myoblast proliferation. Biochim Biophys Acta.
1783:728–736. 2008. View Article : Google Scholar
|
|
12
|
Swanson R, Marshall J, Smith JS, et al:
Cloning and expression of cDNA and genomic clones encoding three
delayed rectifier potassium channels in rat brain. Neuron.
4:929–939. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bielanska J, Hernandez-Losa J,
Perez-Verdaguer M, et al: Voltage-dependent potassium channels
Kv1.3 and Kv1.5 in human cancer. Curr Cancer Drug Targets.
9:904–914. 2009. View Article : Google Scholar
|
|
14
|
Vicente R, Escalada A, Coma M, et al:
Differential voltage-dependent K+ channel responses
during proliferation and activation in macrophages. J Biol Chem.
278:46307–46320. 2003.PubMed/NCBI
|
|
15
|
Villalonga N, Escalada A, Vicente R, et
al: Kv1.3/Kv1.5 heteromeric channels compromise pharmacological
responses in macrophages. Biochem Biophys Res Commun. 352:913–918.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bielanska J, Hernandez-Losa J, Moline T,
et al: Differential expression of Kv1.3 and Kv1.5 voltage-dependent
K+ channels in human skeletal muscle sarcomas. Cancer
Invest. 30:203–208. 2012. View Article : Google Scholar
|
|
17
|
Bielanska J, Hernandez-Losa J, Moline T,
et al: Voltage-dependent potassium channels Kv1.3 and Kv1.5 in
human fetus. Cell Physiol Biochem. 26:219–226. 2010. View Article : Google Scholar
|
|
18
|
Lan M, Shi Y, Han Z, et al: Expression of
delayed rectifier potassium channels and their possible roles in
proliferation of human gastric cancer cells. Cancer Biol Ther.
4:1342–1347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castellvi J, Garcia A, Ruiz-Marcellan C,
et al: Cell signaling in endometrial carcinoma: phosphorylated
4E-binding protein-1 expression in endometrial cancer correlates
with aggressive tumors and prognosis. Hum Pathol. 40:1418–1426.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Archer SL, Souil E, Dinh-Xuan AT, et al:
Molecular identification of the role of voltage-gated K+
channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction
and control of resting membrane potential in rat pulmonary artery
myocytes. J Clin Invest. 101:2319–2330. 1998.PubMed/NCBI
|
|
21
|
Chen TT, Luykenaar KD, Walsh EJ, Walsh MP
and Cole WC: Key role of Kv1 channels in vasoregulation. Circ Res.
99:53–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nelson MT and Quayle JM: Physiological
roles and properties of potassium channels in arterial smooth
muscle. Am J Physiol. 268:C799–C822. 1995.PubMed/NCBI
|