Introduction
Patients with obstructive jaundice (OJ) are at high
risk of postoperative complications (1,2), such
as endotoxemia, renal failure, and even mortality. OJ is associated
with dysfunction of liver uptake function (3). Several previous studies have shown
that the liver uptake function was severely suppressed in patients
and rats with biliary obstruction (4–6).
Usually OJ is depressed by internal drainage (ID) or external
drainage (ED), but it is indeterminate whether there is any
difference between the effects of ID and ED on liver uptake
function.
Superparamagnetic iron oxide (SPIO) is a
liver-specific magnetic resonance imaging (MRI) contrast agent. The
technique relies on the ability of the liver to take up SPIO
particles (7,8). The current clinical application of
SPIO is mainly to detect hepatocellular carcinoma (9–11), and
few studies are available on the application of SPIO in OJ
(12).
This study investigated whether the SPIO-enhanced
MRI could be effectively used to evaluate the liver uptake function
in OJ rats, and consequently revealed the effect of ID and ED on
liver uptake function.
Materials and methods
Animals
In total, 40 male Sprague Dawley rats weighing
300–400 g were used in this study. The animals were housed in a
specific-pathogen-free environment in the Laboratory Animal Service
Center of Capital Medical University, and fed with standard rat
chow. The animals were randomly divided into four groups: Sham
surgery (SH, n=10), OJ (n=10), ID (n=10), ED (n=10). The laparotomy
in all groups was made through a 4–5 cm upper middle abdominal
incision. The following protocol was applied: i) SH group: Common
bile duct was only divided. ii) OJ group: The common bile duct was
ligated twice with 5/0 silk. iii) ED group: A 19G (approximately 1
mm in diameter) epidural catheter was inserted into the common bile
duct, and the other end of the catheter exteriorized at the nape of
the neck through the subcutaneous channel. The outlet of the
catheter was ligated for 14 days and then drained for 14 days. iv)
ID group: One catheter was inserted into the common bile duct.
Another catheter was inserted into the jejunum, and the outer end
of the catheter was also exteriorized at the nape of the neck. The
outlets of the common bile duct catheter and the jejunum catheter
were connected by an elastic tube. The tube was ligated for 14
days. On the 15th day, the ligation was removed and drainage lasted
for 14 days. Two of the 40 animals died during the study. One was
in the ID group, and the other was in the ED group.
The SH and OJ group were scanned by SPIO-MRI, and
sacrificed for liver and blood collection 14 days following
surgery. The same procedure was applied in the ID and ED groups
after 14 days of drainage. The study was approved by the Ethics
Committee of Beijing Chaoyang Hospital
SPIO contrast agent
Resovist (Schering Ag, Germany) was used as the SPIO
contrast agent, and is a hydrophilic colloidal solution of SPIO
coated with carboxydextran, which forms ferucarbotran. The diameter
of the nanoparticle is 62 nm. The contrast agent was administered
via a tail vein or femoral vein (30 μmol Fe/kg).
MRI scan
Anesthesia was induced with an intraperitoneal
injection of 10% chloral hydrate (3.5 ml/kg) (made by Beijing
Chaoyang Hospital). The rats were placed in a plastic container to
restrict the respiratory movement. The MRI was performed with a
1.5-T superconducting imaging system (Signa HDx 1.5T; GE
Healthcare, Waukesha, WI, USA) 1 h following the administration of
SPIO. Scout images were obtained first. The T2 value was acquired
by the T2 mapping sequence with 15 readout echoes: TR = 1025 ms,
first TE/ΔTE = 7.8/7.8 ms, flip angle = 30°, FOV = 80×80 mm, scan
matrix = 160×128, slice thickness = 3.0 mm, bandwidth = 31.25 Hz,
NEX = 4. The scan time was 8 min 44 sec. The T2* value was obtained
by the T2* mapping sequence with 15 readout echoes: TR = 150 ms,
first TE/ΔTE = 2.6/4.4 ms, flip angle = 20°, FOV = 80×80 mm, scan
matrix = 128×128, slice thickness = 3 mm, bandwidth = 31.25 Hz, NEX
= 2. The scan time was 2 min 38 sec.
Analysis of MRI data
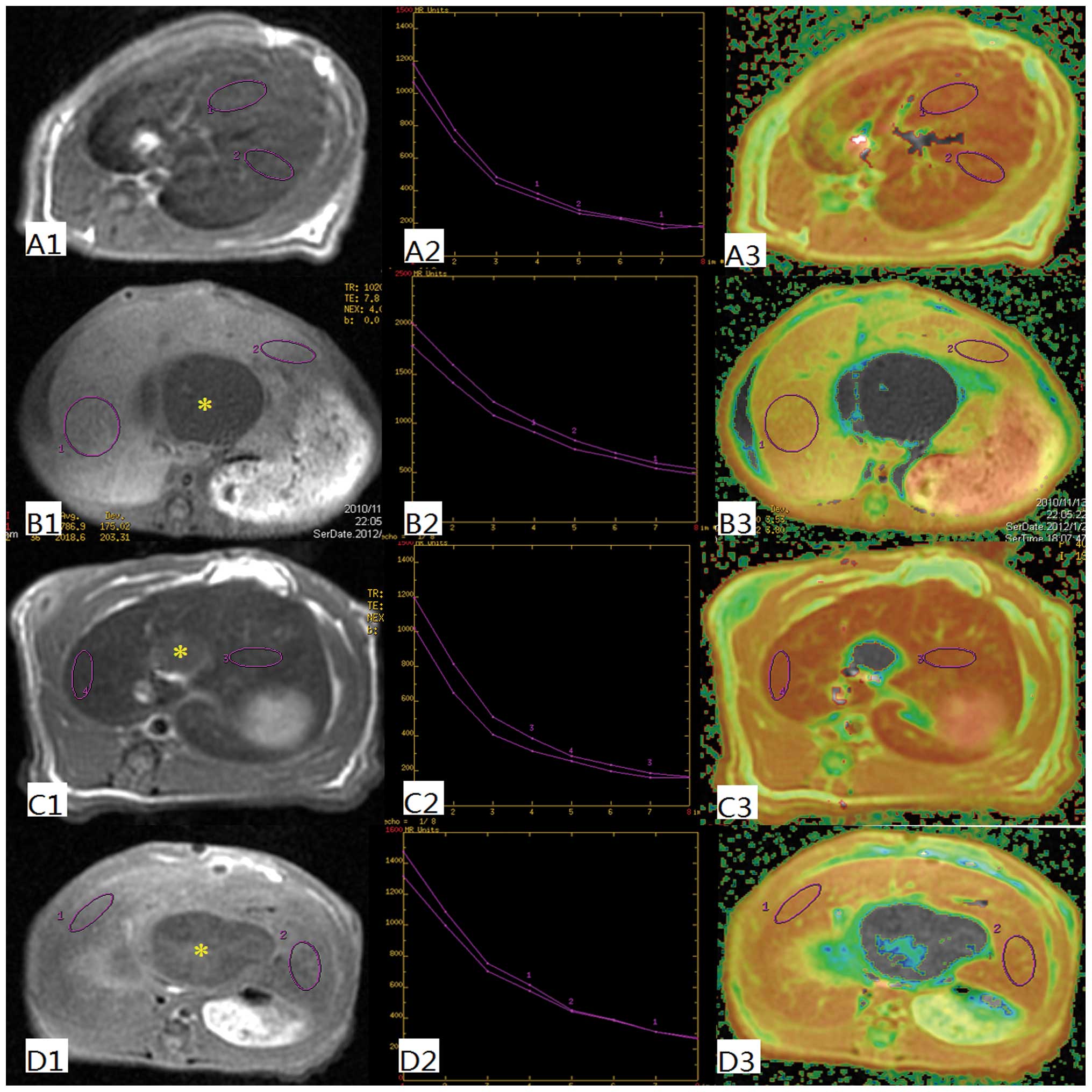
The T2 and T2* value analysis was performed with MRI
workstation packaged software: functool-T2MAP. The region of
interest (ROI) boxes were oval and placed in both the right and
left lobes of the liver. The ROIs were selected to be as large as
possible, with exclusion of any focal hepatic lesions and major
branches of the portal and hepatic veins. The ROI boxes were placed
on three slices through the center of the liver, and the mean T2
and T2* values of the right and left lobe were calculated,
respectively.
Kupffer cells in liver sections
Each liver was fixed with 10% formalin. Liver
sections were stained with hematoxylin and eosin (H&E) and
Perl’s Prussian blue. Hepatic lobule structure and SPIO
nanoparticles were observed and imaged under a microscope (Olympus
microscope, BX41). The number of SPIO-nanoparticle clusters was
counted manually in three high-power fields (x200), and the mean
was calculated.
Statistical analysis
Data were shown as the means ± standard deviation
(SD). Statistical analysis was performed using SPSS version 17.0
for Windows (Chicago, IL, USA). One-way ANOVA followed by Tukey’s
honestly significant difference (HSD) test, Kruskal-Wallis test and
Mann-Whitney U test were used to evaluate statistical significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
T2 and T2* values
In order to determine the uptake function of the
liver, T2 and T2* values were evaluated (Fig. 1). These values are quantitative
parameters for liver parenchyma, which can be reduced by SPIO
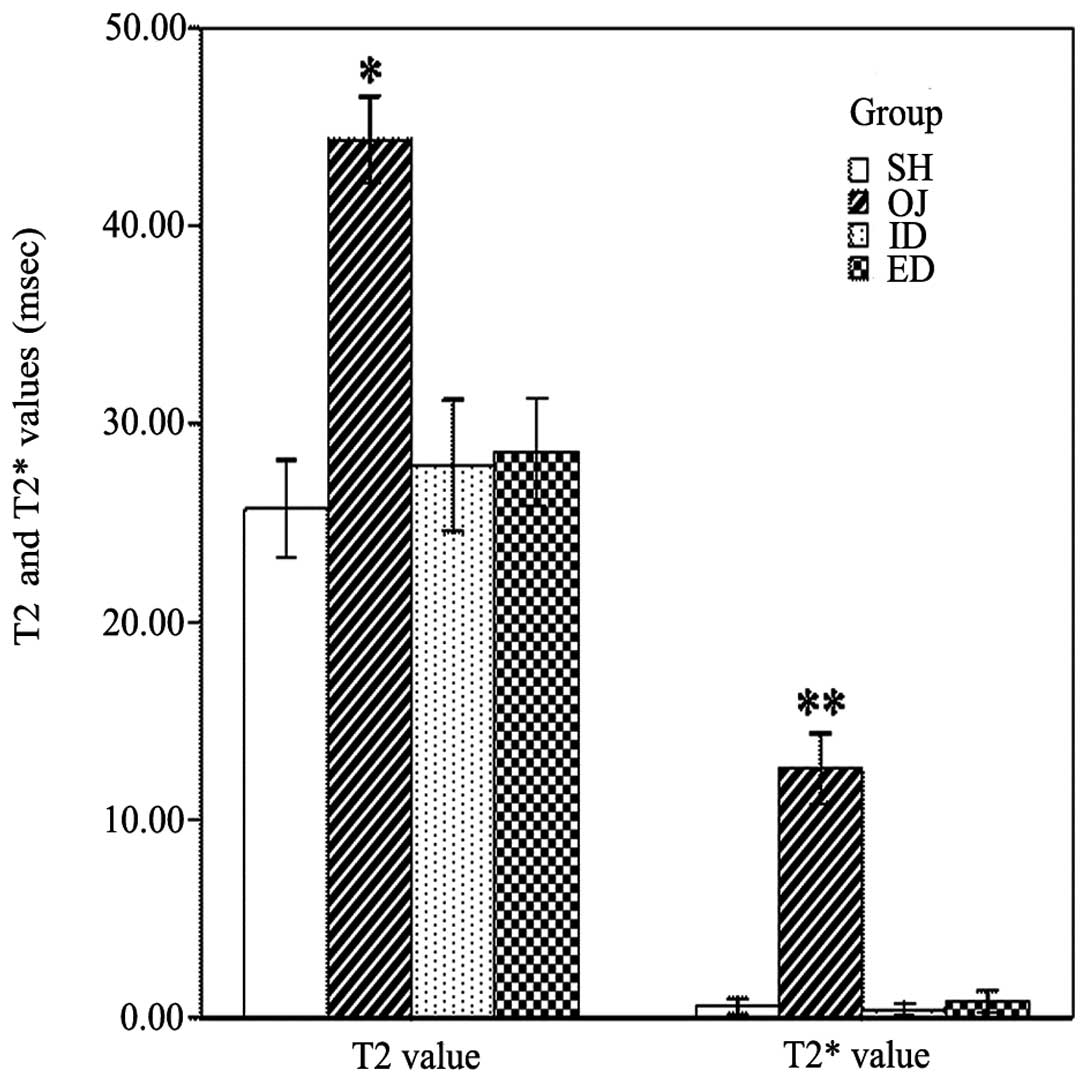
nanoparticles. The T2 values in the OJ group are significantly
higher (44.3±2.2) than in the other groups. There was a significant
decrease of the T2 values in the SH (25.7±2.4), ID (27.9±3.3) and
ED (28.5±2.7) groups (P<0.001). In a similar manner the T2*
value in the SH (0.6±0.4), ID (0.4±0.3) and ED (0.8±0.5) groups
decreased significantly, whereas the T2* value in the OJ group was
significantly higher (12.6±1.8; P<0.001) (Fig. 2).
Liver section and SPIO nanoparticle
clusters
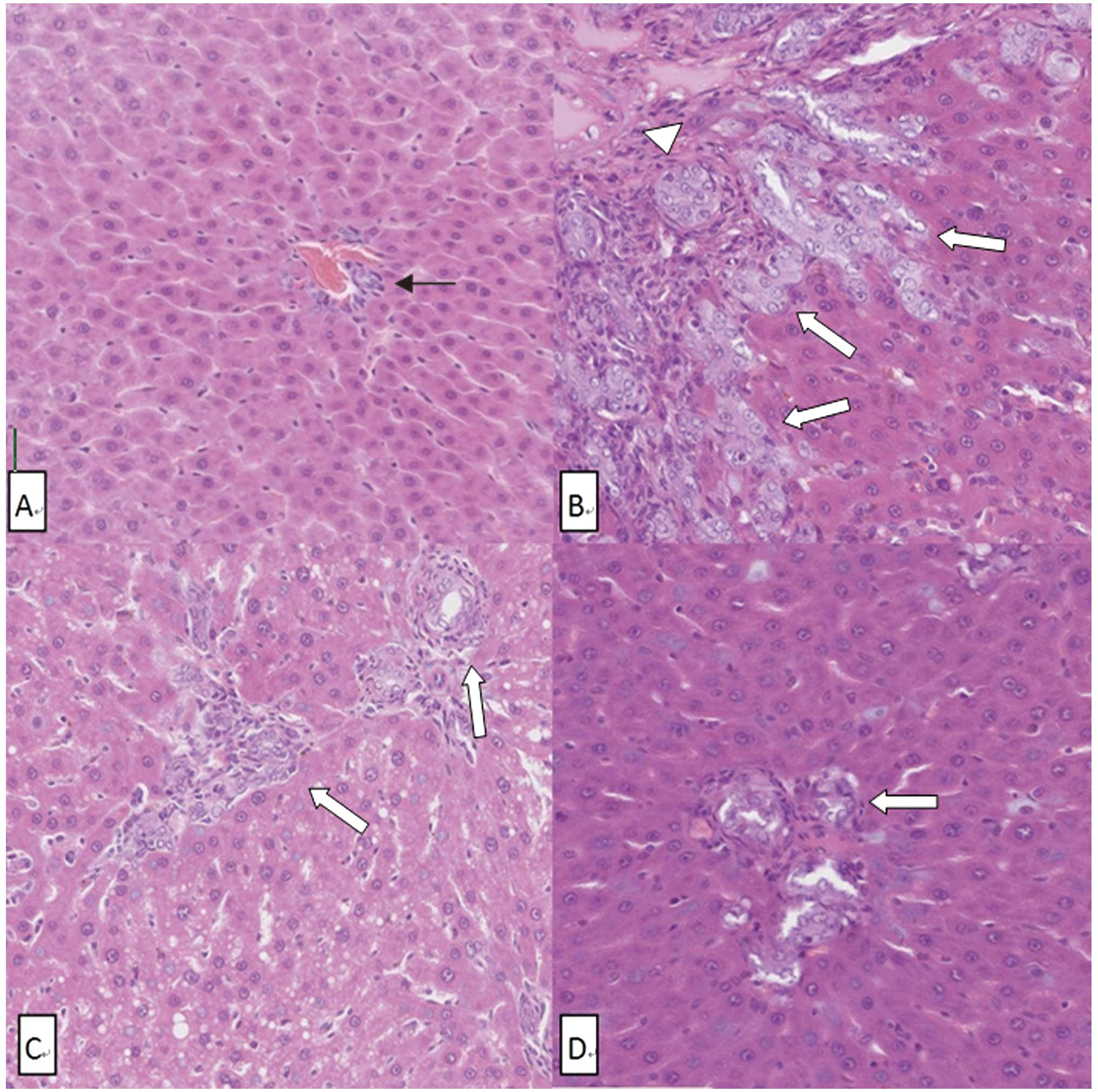
Photomicrographs of liver tissue stained by H&E
revealed the histological structure of the liver. The OJ group
showed the typical obstructive jaundice histological changes:
proliferation of bile ductules, neutrophil infiltration in
sinusoid, hepatic lobule reconstruction and intracellular bile
retention. The histological changes in the ID and ED groups were
much milder than in the OJ group (Fig.
3).
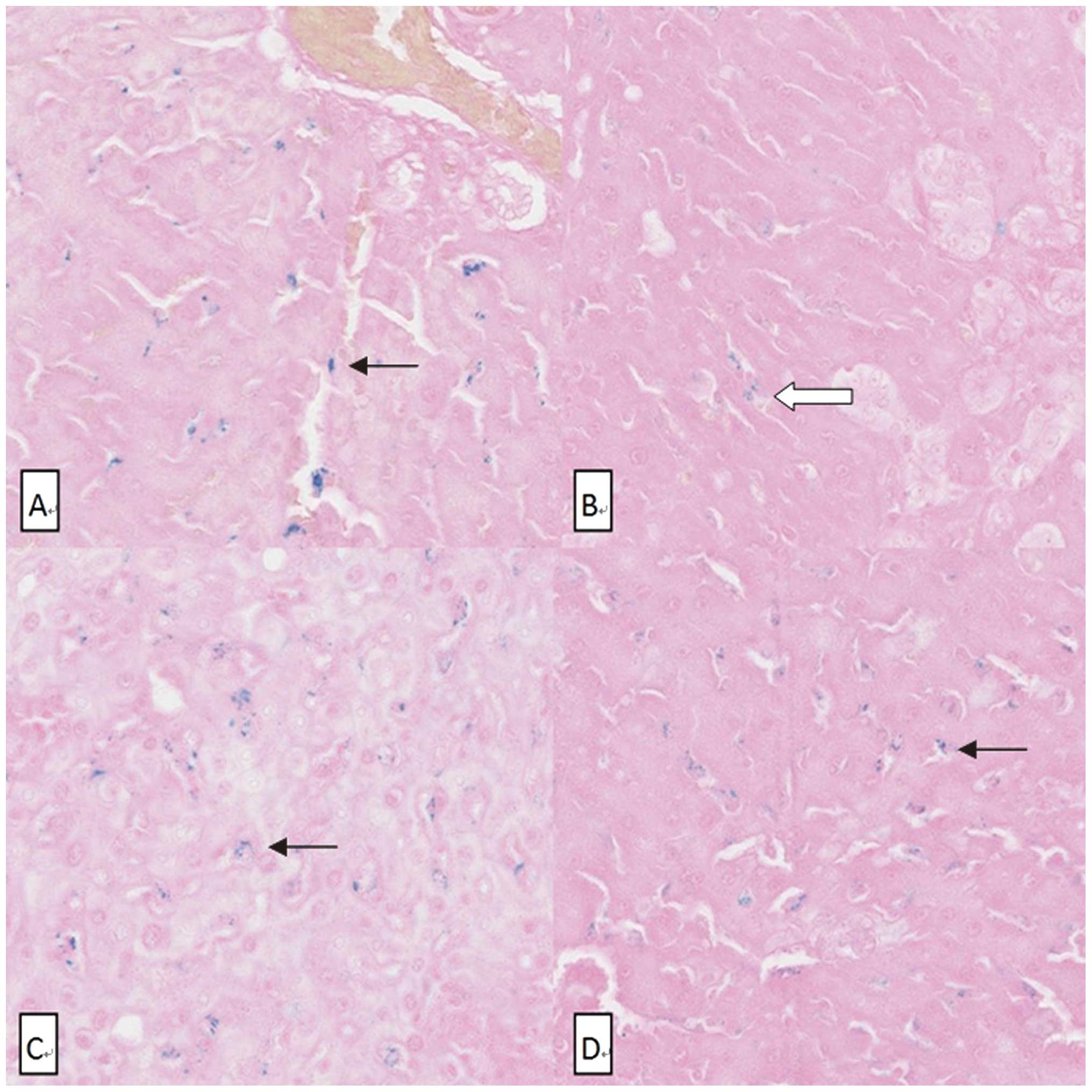
The liver section stained by Perls’ Prussian blue
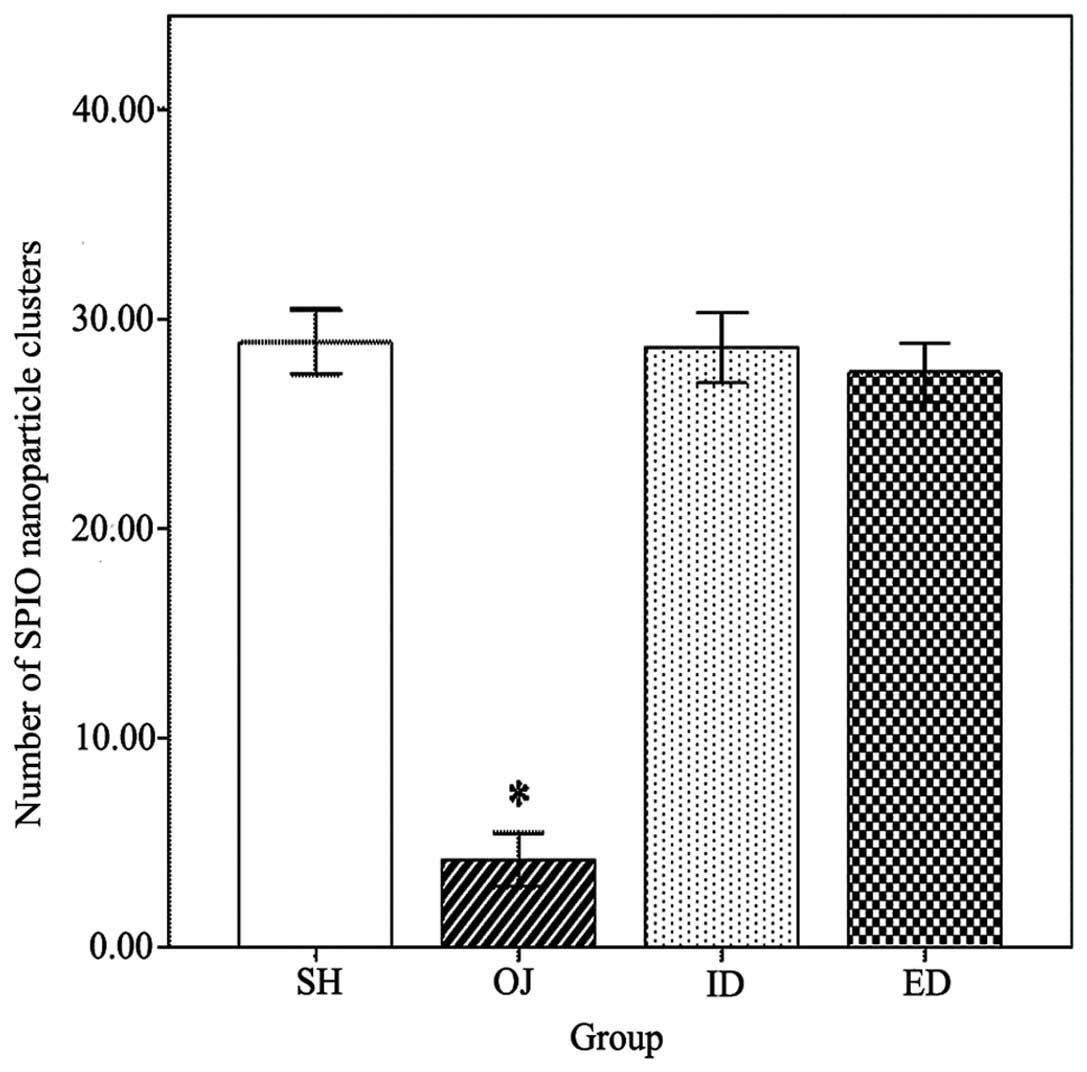
revealed the number of SPIO nanoparticle clusters (Fig. 4). The number of SPIO nanoparticle
clusters in the OJ group (4.2±1.3) was significantly lower than in
the other three groups (P<0.001) (Fig. 5). No SPIO-nanoparticles were
identified in certain OJ group sections.
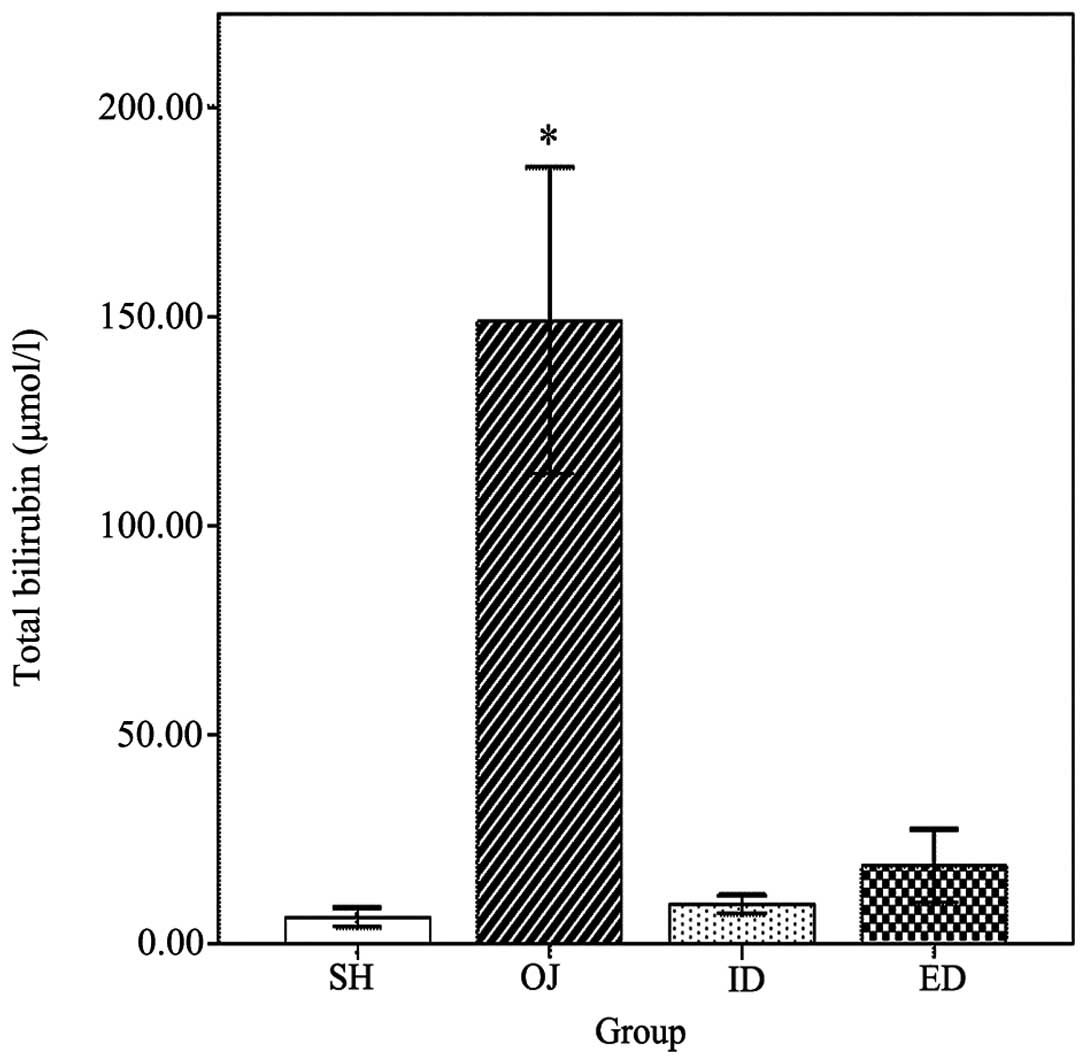
Total bilirubin
Total bilirubin was used to assess the severity of
OJ (Fig. 6). The total bilirubin in
OJ (149.1±36.7 μmol/l) rats was significantly higher (P<0.001)
than in the other groups. No significant difference was found among
the SH (6.4±2.3 μmol/l), ID (9.4±2.3 μmol/l) and ED (18.6±8.8
μmol/l) groups.
Discussion
OJ patients have an increased risk of complications
and mortality. Liver uptake dysfunction is important in these
processes. It is responsible for the clearance of bacteria and
lipopolysaccharides from the intestines. Several studies in the
past have shown that the uptake function of the liver was severely
suppressed in patients and rats with biliary obstruction (4–6). In
the above-mentioned studies, the vascular clearance method was used
to evaluate liver uptake function. A series of different test
particles, including, synthetic dyes, colloidal carbon and
radiolabelled bacteria have been used. However, these methods are
invasive or radiologically harmful, and cannot be applied
clinically. Therefore, this study was designed to use SPIO-enhanced
MRI to assess the liver uptake function.
SPIO is a liver-specific MRI contrast agent. The
agent is capable of shortening the T2 transverse relaxation time
and T2* relaxation time of liver, so that the T2 and T2* values
decrease. Previously, SPIO was mainly used to differentiate
hepatocellular carcinoma from benign focal lesions. Recently, some
investigators have assessed the application of SPIO in certain
diffuse diseases, such as non-alcoholic fatty liver disease, liver
cirrhosis or fibrosis (13–15). These studies showed that following
the administration of SPIO, the signal in the model group exhibited
a significantly lower reduction compared to the normal group. The
signal in the severe disease group showed a lower reduction
compared to the mild group. This phenomenon was repeated in animal
models and patients. These studies indicated that diffuse liver
diseases impair liver uptake function, which may be identified by
SPIO-enhanced MRI.
T2 and T2* values of the liver in the OJ group are
significantly higher than in the other groups. This suggested that
there were less SPIO-nanoparticles in the liver parenchyma due to
the depression of liver uptake function (16,17).
There were much fewer SPIO-nanoparticles observed in the OJ group
liver sections than in the other three groups. Additionally, no
SPIO-nanoparticle clusters were observed in many areas of the OJ
group section. There was no significant difference between the ID
group and ED group when the T2 value, T2* value and the number of
SPIO-nanoparticle clusters were compared. No significant
differences were found between the therapeutic effect of ID and ED
on liver uptake function.
In Perls’ Prussian blue staining of liver sections,
SPIO- nanoparticles taken up by liver are shown as blue dots. Thus,
the number of blue dots in liver sections reflects the liver uptake
function. The number of blue dots was counted and normalized for
each sample, which was used as the standard method to assess the
uptake function of the liver in our study.
The total bilirubin and histological changes of
liver sections showed that the OJ model and the drainage model were
successful. The total bilirubin of the OJ group was significantly
higher than that of the SH group. No significant difference was
observed among the total bilirubin of the ID, ED and SH groups. The
photomicrographs of the H&E-stained tissue slices showed
typical obstructive jaundice histological characteristics. In the
ID and ED groups, the histological findings were much milder. The
obstructive jaundice historical changes in the ID and ED groups
partially reversed, indicating histological reverse following
functional recovery.
Previous studies have proven that biliary
decompression improved liver uptake function (4–6). In
the present study, SPIO-enhanced MRI also confirmed this
phenomenon. No significant difference was found between the ID and
ED groups when the T2 and T2* values were compared. Clements et
al reported that rats undergoing internal biliary drainage had
a significantly increased liver uptake function compared with the
rats treated by ED (18). We
attributed the difference between the two studies to the different
drainage models used. In our study, rats in the ID and ED groups
underwent a single surgical procedure. The biliary obstruction and
decompression were obtained by obstructing and releasing the
catheter placed outside the rats. This procedure minimized the
effect of surgery on rats. In Clements et al’s study, the
biliary obstruction and decompression were obtained by laparotomy
performed twice. There were two control groups in their study:
sham/ED and sham/ID. The rats of these two groups underwent ED or
ID procedures, respectively, without OJ. Their results showed that
the liver uptake function of the sham/ED group was lower than the
sham/ID group. Therefore, we assumed that their ED surgery may
depress the liver uptake function.
In this study, we found that: i) SPIO-enhanced MRI
could be used to assess the liver uptake function in certain liver
conditions, such as OJ, and patients underwent biliary relief; ii)
OJ suppresses liver’s uptake function in rats. Internal and
external biliary drainage is capable of reversing the change in the
uptake function of Kupffer cells. Our study did not show a
significant difference between the therapeutic effect of ID and ED
on liver uptake function. This suggests that ED may be enough for
OJ patients clinically.
Acknowledgements
This study was supported by the Chinese National Key
Technology R&D Program No. 2007BAI05B06, and the Chinese
National Scientific Research Foundation No. 30900364.
References
|
1
|
Tomioka M, Iinuma H and Okinaga K:
Impaired Kupffer cell function and effect of immunotherapy in
obstructive jaundice. J Surg Res. 92:276–282. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ljungdahl M, Osterberg J, Ransjo U,
Engstrand L and Haglund U: Inflammatory response in patients with
malignant obstructive jaundice. Scand J Gastroenterol. 42:94–102.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scott-Conner CE and Grogan JB: The
pathophysiology of biliary obstruction and its effect on phagocytic
and immune function. J Surg Res. 57:316–336. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Charlier N, Neyrinck AM, Beghein N,
Delzenne NM and Gallez B: Assessment of liver phagocytic activity
using EPR spectrometry and imaging. Magn Reson Imaging. 27:565–569.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding JW, Andersson R, Stenram U,
Lunderquist A and Bengmark S: Effect of biliary decompression on
reticuloendothelial function in jaundiced rats. Br J Surg.
79:648–652. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Megison SM, Dunn CW, Horton JW and Chao H:
Effects of relief of biliary obstruction on mononuclear phagocyte
system function and cell mediated immunity. Br J Surg. 78:568–571.
1991. View Article : Google Scholar
|
|
7
|
Nishie A, Yoshimitsu K, Nakayama T, et al:
In vitro imaging of human monocytic cellular activity using
superparamagnetic iron oxide. Comput Med Imaging Graph. 31:638–642.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Briley-Saebo K, Bjornerud A, Grant D,
Ahlstrom H, Berg T and Kindberg GM: Hepatic cellular distribution
and degradation of iron oxide nanoparticles following single
intravenous injection in rats: implications for magnetic resonance
imaging. Cell Tissue Res. 316:315–323. 2004. View Article : Google Scholar
|
|
9
|
Kim T, Murakami T, Hori M, Onishi H,
Tomoda K and Nakamura H: Effect of superparamagnetic iron oxide on
tumor-to-liver contrast at T2*-weighted gradient-echo MRI:
comparison between 3.0T and 1.5T MR systems. J Magn Reson Imaging.
29:595–600. 2009.
|
|
10
|
Namkung S, Zech CJ, Helmberger T, Reiser
MF and Schoenberg SO: Superparamagnetic iron oxide (SPIO)-enhanced
liver MRI with ferucarbotran: efficacy for characterization of
focal liver lesions. J Magn Reson Imaging. 25:755–765. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanimoto A and Kuribayashi S: Application
of superparamagnetic iron oxide to imaging of hepatocellular
carcinoma. Review Eur J Radiol. 58:200–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito T, Abe T, Tsuchiya T, et al:
Sequential magnetic resonance imaging for evaluation of Kupffer
cell function. Hepatogastroenterology. 55:596–599. 2008.PubMed/NCBI
|
|
13
|
Lucidarme O, Baleston F, Cadi M, et al:
Non-invasive detection of liver fibrosis: Is superparamagnetic iron
oxide particle-enhanced MR imaging a contributive technique. Eur
Radiol. 13:467–474. 2003.PubMed/NCBI
|
|
14
|
Tomita K, Tanimoto A, Irie R, et al:
Evaluating the severity of nonalcoholic steatohepatitis with
superparamagnetic iron oxide-enhanced magnetic resonance imaging. J
Magn Reson Imaging. 28:1444–1450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanabe M, Ito K, Shimizu A, et al:
Hepatocellular lesions with increased iron uptake on
superparamagnetic iron oxide-enhanced magnetic resonance imaging in
cirrhosis or chronic hepatitis: comparison of four magnetic
resonance sequences for lesion conspicuity. Magn Reson Imaging.
27:801–806. 2009. View Article : Google Scholar
|
|
16
|
Kalber TL, Smith CJ, Howe FA, et al: A
longitudinal study of R2* and R2 magnetic resonance imaging
relaxation rate measurements in murine liver after a single
administration of 3 different iron oxide-based contrast agents.
Invest Radiol. 40:784–791. 2005.
|
|
17
|
Liu W, Dahnke H, Rahmer J, Jordan EK and
Frank JA: Ultrashort T2* relaxometry for quantitation of highly
concentrated superparamagnetic iron oxide (SPIO) nanoparticle
labeled cells. Magn Reson Med. 61:761–766. 2009.
|
|
18
|
Clements WD, McCaigue M, Erwin P, Halliday
I and Rowlands BJ: Biliary decompression promotes Kupffer cell
recovery in obstructive jaundice. Gut. 38:925–931. 1996. View Article : Google Scholar : PubMed/NCBI
|