Introduction
Lung cancer is one of the most prevalent cancers in
Taiwan and also plays a leading role in cancer-related mortality.
The major histological cell types include adenocarcinoma, squamous
cell carcinoma, small cell carcinoma and large cell carcinoma.
Advanced lung cancer may spread to extrathoracic sites, the most
frequent sites being the liver, adrenal glands, bones and brain.
Gastrointestinal (GI) metastasis from primary lung cancer is rare,
although it is a recognized phenomenon in the literature. Certain
case reports have been published (1–5),
including cases of symptomatic GI metastasis as well as
asymptomatic cases discovered unintentionally. When patients have
malignant lung and GI lesions at the same time, the primary site
among these lesions is often difficult to establish, particularly
when both the lung and GI lesions have the same histologic cell
type. It is essential to make a differential diagnosis of the
primary origin among these lesions since these results may lead to
a different choice of treatment.
Case report
A 41-year-old female fish vendor with a smoking
history presented at Chang Gung Memorial Hospital, Keelung, Taiwan,
with diffused abdominal pain and fullness. Written informed consent
was obtained from the patient prior to the study. The patient had
also had a chronic cough for 5 months. An abdominal computed
tomography (CT) scan was performed to rule out peritonitis, and
this revealed mild ascites and peritoneal carcinomatosis.
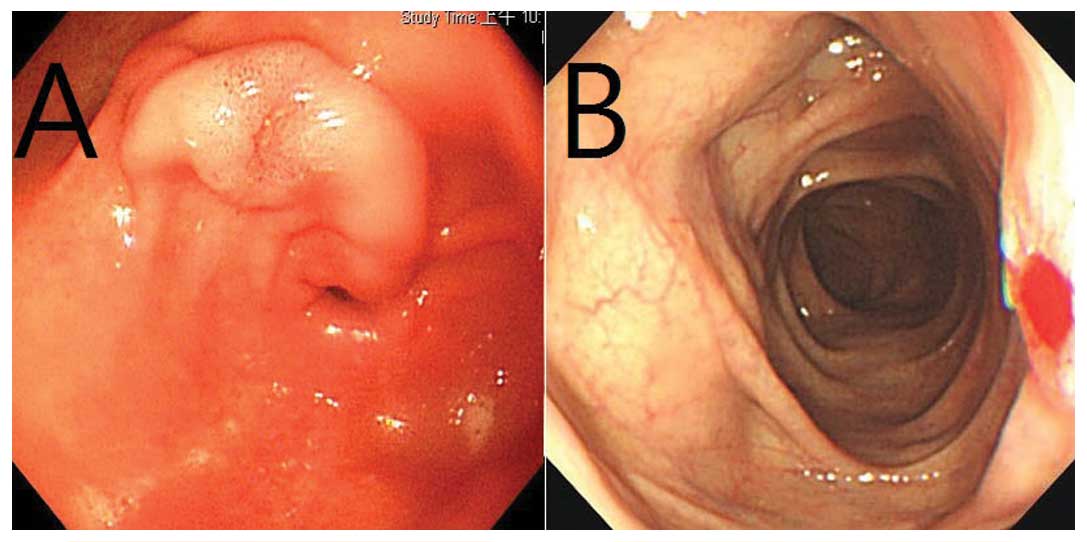
Panendoscopy revealed a 1.5-cm tumor with slight central ulceration
in the patient’s stomach (Fig. 1A).
Colonfibroscopy was performed which revealed a 1-cm tumor with
central ulceration in the transverse colon (Fig. 1B). Both gastric and colonic biopsies
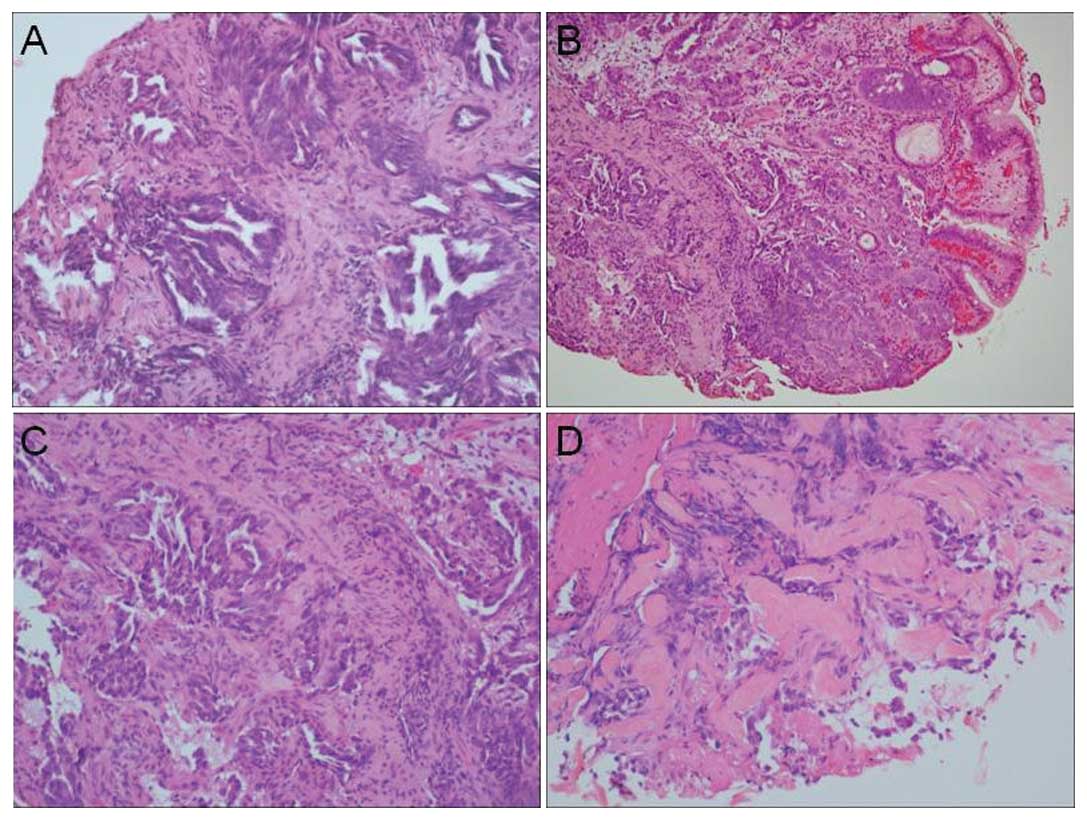
revealed an adenocarcinoma pattern using hematoxylin and eosin
(H&E) staining (Fig. 2B-D). CT
of the chest was later performed which revealed a left lower lobe
tumor. Bronchoscopy was arranged and transbronchial biopsy was
performed from LB 8 and 9. The transbronchial biopsy revealed
adenocarcinoma (Fig. 2A). Tissue
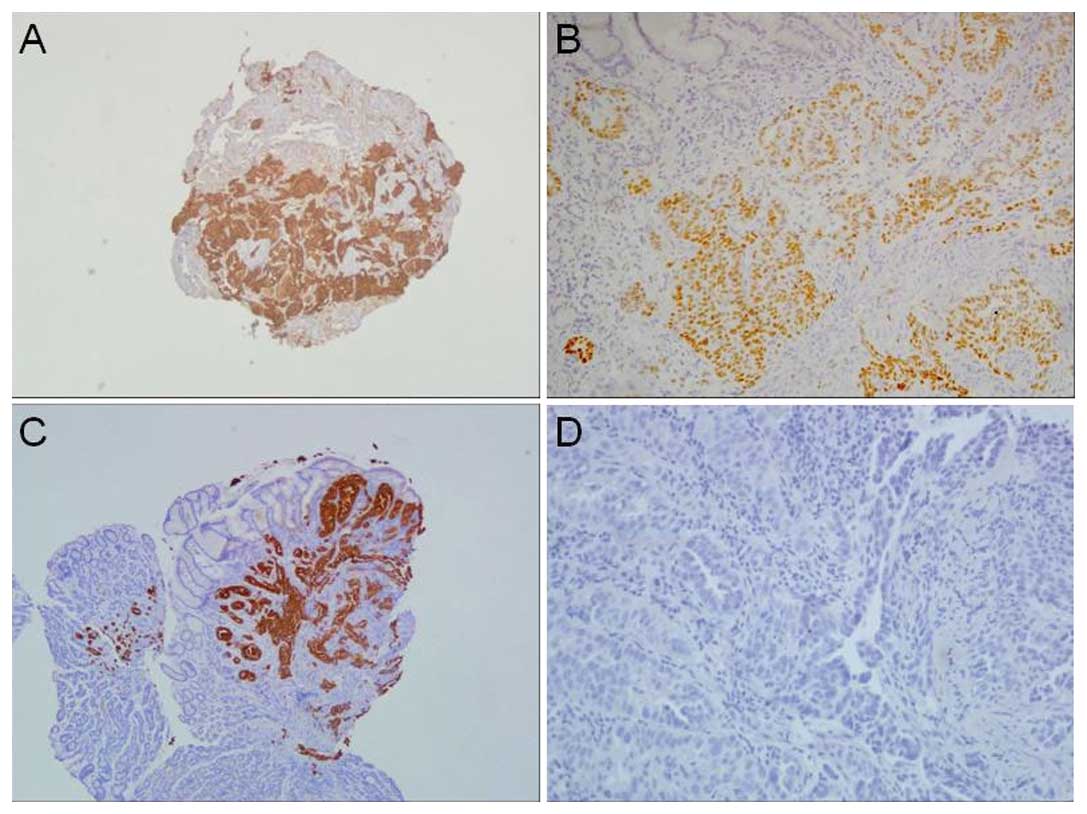
from biopsies including the stomach, colon and lung underwent
immunohistochemical (IHC) staining (Fig. 3), from which lung cancer origin was
diagnosed. An EGFR mutation test including L858R, exon 19 deletion,
T790M, G719A, G719C and L861Q was negative. A bone scan also
revealed multiple active bone lesions.
Following the diagnosis, the patient received
chemotherapy with cisplatin (70 mg/m2) and docetaxol (70
mg/m2) on two occasions then switched to cisplatin (80
mg/m2) and vinorelbine (30 mg/m2). A brain
MRI was performed when the patient complained of a headache which
revealed both hematogenous and leptomengeal brain metastasis with
mild obstructive hydrocephalus. The cytology of CSF also
demonstrated positivity for malignant cells. The patient was
administered targeted therapy with erlotinib 150 mg QD. A
subsequent chest/abdominal CT scan revealed that the tumor was
decreasing in size. A brain MRI revealed complete regression after
6 months of erlotinib treatment. However, after 11 months of
erlotinib treatment, the patient complained of nausea, vomiting and
headaches. A brain MRI then revealed marked progression of
hydrocephalus and for this reason a ventriculoperitoneal (VP) shunt
was implanted by a neurosurgeon. Unfortunately, the patient
developed respiratory distress 2 months later due to the
progression of lung cancer. She succumbed to the disease 15 months
after diagnosis.
Discussion
In women with ascites and peritoneal carcinomatosis,
the most common origin is ovarian, colon, gastric or pancreatic
cancer. In the case described above, three sites of adenocarcinoma
were identified. Therefore a differential diagnosis including
colon, gastric and lung adenocarcinoma should be considered.
Synchronous lung and colon cancer has previously been reported
(6). The possibility of synchronous
lung, gastric and colon cancer should also be kept in mind.
Different anti-malignancy strategies, including chemotherapy or
targeted therapy, should be used depending on the different origins
of the cancer cell line. The regimen for treating these three types
of cancer is quite different. The response and prognosis are also
different.
In the H&E-stained sections of biopsied tissue
in the present case, both the primary lung tumor and metastatic
gastric tumor revealed similar glandular patterns infiltrating in
the stroma. However, only a few cauterized tumor cells were
observed in the colonic biopsy (Fig.
2).
TTF-1 is a 38–40 kD transcription factor member of
the NFx2 family which is normally expressed in thyroid and
pulmonary epithelial cells (7). It
is expressed in the nuclei of 60–75% of lung adenocarcinoma cases
but seldom expressed in gastric and colonic adenocarcinoma
(8). CK7 is expressed in both
pulmonary and intestinal adenocarcinoma (9); but CK20 is expressed in 80% of gastric
adenocarcinoma and 95% of colonic adenocarcinoma (10). In this case, the tumor cells in the
gastric biopsy showed positive TTF-1 nuclear and CK7 staining in
the IHC study, which is consistent with pulmonary origin. The
possibility of gastrointestinal origin was excluded following the
negative results of CK20 and CDX2 staining. The tumor cells of the
lung and colonic biopsy both demonstrated positive TTF-1 staining.
According to the above IHC results, the final diagnosis of the
neoplastic glands was pulmonary origin. Therefore, the patient was
treated as having non-small cell lung cancer. Initially, she had a
positive response following targeted therapy with erlotinib. Her
time to progression reached 6 months. Clinically, the patient’s
prognosis was also compatible with lung cancer.
GI tract metastasis from primary lung cancer has
been described in certain previous case reports. The metastatic
sites described have included the stomach (1–5), small
bowel (11–13), appendix (14), colon (15) and anus (16). The clinical findings have included
epigastric pain (9), anemia
(17,18), upper gastrointestinal bleeding
(7,19), bowel obstruction (11,13,20),
bowel perforation (5,11), peritonitis (17) and polyp formation (16). These symptoms may require surgical
intervention or palliative therapy, including chemotherapy or
targeted therapy. Unfortunately, the prognosis was poor in these
patients.
Certain patients were asymptomatic and the finding
of GI tract metastasis was incidental. The actual incidence of
non-small cell lung cancer metastasizing to the GI tract is
uncertain. Autopsy reports have suggested that the prevalence is
approximately 4.7–14% (12,21). Studies from Italy (22) and Taiwan (23) have suggested that 0.5–1.7% of
patients with primary cancer developed GI tract metastasis. The
cell type in Taiwan was squamous cell carcinoma (3/6) in the
majority of cases, while large cell carcinoma (10/18) was dominant
in Italy. The average time between the discovery of GI metastasis
and mortality was only 130.3 days (range, 23–371 days). This may be
due to multiple metastases. Compared with other lung cancer
patients who developed GI tract metastasis, our patient lived
considerably longer. In this patient, the survival time following
diagnosis was 461 days. We presume that her anti-malignant therapy
was effective during the first 12 months. Erlotinib demonstrated a
definite significant benefit for this patient although the EGFR
mutation test was negative. In cases such as this, the differential
diagnosis of metastasis of lung cancer origin or GI malignancy is
essential. Adequate treatment is dependent on a correct
diagnosis.
Positron emission tomography (PET)-CT has been
proven useful in the diagnosis of GI cancer (24). It is effective for the detection of
distant metastasis except in the case of brain and liver metastasis
(25). Certain studies have also
revealed asymptomatic GI metastasis from lung cancer by PET-CT
(26). A preoperative PET-CT may
give a clinical indication of GI metastasis from lung cancer and
improve the correctness of staging and further treatment.
Therefore, PET-CT is crucial prior to the treatment of primary lung
cancer.
In conclusion, GI tract metastasis of lung cancer is
rare but well-documented. The prevalence rate is 0.5–14% according
to clinical and autopsy reports. The common metastatic sites are
the stomach, small intestine or colon. For the patient, both
gastric and colon metastasis of lung cancer is very rare.
Symptomatic GI metastasis should be treated by earlier surgical
intervention or medical treatment. PET-FDG may provide the
potential to increase the diagnosis of occult distant metastasis.
If appropriate treatment can be provided earlier, these patients
may have a better quality of life and also longer survival. The
present case study is a good example of this. We hope that by
sharing our experience we increase the confidence in the treatment
of GI metastasis of primary lung cancer for both physicians and
patients.
References
|
1
|
Fletcher MS: Gastric perforation secondary
to metastatic carcinoma of the lung: a case report. Cancer.
46:1879–1882. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barrio J, Arriola JA, San Vicente MT, et
al: Bleeding of the upper digestive tract due to gastric metastasis
of squamous lung carcinoma. Gastroenterol Hepatol. 22:405–407.
1999.(In Spanish).
|
|
3
|
Ishii T, Kida K, Katsura H, et al: Large
cell carcinoma of the lung with metastasis of the gastric
submucosa. Nihon Ronen Igakkai Zasshi. 36:416–419. 1999.(In
Japanese).
|
|
4
|
Yamamoto M, Matsuzaki K, Kusumoto H, et
al: Gastric metastasis from lung carcinoma. Case report
Hepatogastroenterology. 49:363–365. 2002.
|
|
5
|
Casella G, Di Bella C, Cambareri AR, et
al: Gastric metastasis by lung small cell carcinoma. World J
Gastroenterol. 12:4096–4097. 2006.PubMed/NCBI
|
|
6
|
Peng YF and Gu J: Synchronous colorectal
and lung cancer: report of three cases. World J Gastroenterol.
14:969–973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lau SK, Luthringer DJ and Eisen RN:
Thyroid transcription factor-1: a review. Appl Immunohistochem Mol
Morphol. 10:97–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matoso A, Resnick MB and Wang LJ:
Comparison of 2 monoclonal TTF-1 antibodies. Appl Immunohistochem
Mol Morphol. 19:3842011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang PJ, Shah M, Spiegel GW and Brooks
JJ: Cytokeratin 7 immunoreactivity in rectal adenocarcinomas. Appl
Immunohistochem Mol Morphol. 11:306–310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen ZM and Wang HL: Alteration of
cytokeratin 7 and cytokeratin 20 expression profile is uniquely
associated with tumorigenesis of primary adenocarcinoma of the
small intestine. Am J Surg Pathol. 28:1352–1359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joyce WP, Huddy SP, Corbishley C and
Wright NL: Small bowel complications of metastatic lung carcinoma.
Ir J Med Sci. 159:149–150. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McNeill PM, Wagman LD and Neifeld JP:
Small bowel metastases from primary carcinoma of the lung. Cancer.
59:1486–1489. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Renault PA, Arotçarena R, Calès V, et al:
Metastatic obstruction of the small bowel revealing or complicating
squamous-cell lung cancer. Two cases and a review of the
literature. Rev Pneumol Clin. 59:161–165. 2003.(In French).
|
|
14
|
Miyazaki K, Satoh H and Sekizawa K:
Metastasis to appendix from lung adenocarcinoma. Int J Gastrointest
Cancer. 36:59–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bastos I, Gomes D, Gouveia H and de
Freitas D: Colonic metastasis of a lung carcinoma with ileocolic
fistula. J Clin Gastroenterol. 26:3481998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawahara K, Akamine S, Takahashi T, et al:
Anal metastasis from carcinoma of the lung: report of a case. Surg
Today. 24:1101–1103. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Locher C, Grivaux M, Jeandel R and
Blanchon F: Intestinal metastases from lung cancer. Rev Mal Respir.
23:273–276. 2006.(In French).
|
|
18
|
Sugiyama M, Sato A, Oguri S, Sumi K,
Tsuboi T and Kurasawa T: A case of squamous cell lung carcinoma
with gastric metastasis diagnosed by gastroendoscopic biopsy. Nihon
Kokyuki Gakkai Zasshi. 48:668–671. 2010.(In Japanese).
|
|
19
|
Park SW, Cho HJ, Choo WS, et al: A case of
intestinal hemorrhage due to small intestinal metastases from
primary lung cancer. Korean J Intern Med. 6:79–84. 1991.PubMed/NCBI
|
|
20
|
Papaziogas B, Koutelidakis I,
Christopoulos P, et al: Intestinal metastasis of a primary lung
carcinoma presenting as mechanical small bowel obstruction. J
Gastrointest Cancer. Jul 1–2011.PubMed/NCBI
|
|
21
|
Antler AS, Ough Y, Pitchumoni CS, Davidian
M and Thelmo W: Gastrointestinal metastases from malignant tumors
of the lung. Cancer. 49:170–172. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rossi G, Marchioni A, Romagnani E, et al:
Primary lung cancer presenting with gastrointestinal tract
involvement: clinicopathologic and immunohistochemical features in
a series of 18 consecutive cases. J Thorac Oncol. 2:115–120. 2007.
View Article : Google Scholar
|
|
23
|
Yang CJ, Hwang JJ, Kang WY, et al:
Gastro-intestinal metastasis of primary lung carcinoma: clinical
presentations and outcome. Lung Cancer. 54:319–323. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hustinx R: PET imaging in assessing
gastrointestinal tumors. Radiol Clin North Am. 42:1123–1139.
ix2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gamez C, Rosell R, Fernandez A, et al:
PET/CT fusion scan in lung cancer: current recommendations and
innovations. J Thorac Oncol. 1:74–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shiono S, Masaoka T, Sato T and Yanagawa
N: Positron emission tomography (PET)-computed tomography (CT)
suggesting small intestinal metastasis from lung cancer; report of
a case. Kyobu Geka. 59:426–429. 2006.(In Japanese).
|